Abstract
Objective
We aim to explain the correlation among IL‐18 gene polymorphism, its protein expression and LEDVT in the Chinese Han population.
Methods
A total of 138 LEDVT patients and 150 healthy people volunteered as LEDVT and control groups. All the data, including the gender, age, BMI, levels of TG, LDL/HDL, TC, GLU, APTT, BUN, Cr, ALT, AST, ApoA1, ApoB, and Fg was detected. IL‐18 level, IL‐18 −137G/C and −607C/A polymorphism, and risk factors of LEDVT were detected using ELISA, PCR‐RFLP and multivariate logistic regression analysis, respectively.
Results
Increased BMI, GLU, Fg, BUN, ApoB and IL‐18 and decreased APTT were found in the LEDVT group. The GC + CC genotype and C allele in −137G/C polymorphism was elevated in the control group when compared to that in the LEDVT group. The IL‐18 level was elevated in the case group when compared to the control group with respect to the same genotype in −607C/A and −137G/C polymorphisms, and in the LEDVT group, IL‐18 level was higher in the GG genotype than that in the GC + CC genotype of −137G/C polymorphism. BUN, GG genotype and IL‐18 level were independent risk factors, but APTT was a protective factor of LEDVT.
Conclusion
On the basis of our results, we concluded that the GG genotype of −137G/C polymorphism and IL‐18 level are independent risk factors of LEDVT, and IL‐18 gene polymorphism affects the level of IL‐18 in LEDVT patients.
Keywords: −137G/C, −607C/A, interleukin‐18, lower extremity deep venous thrombosis, polymorphism
1. INTRODUCTION
Venous thromboembolism (VT), including pulmonary embolism (PE) and deep vein thrombosis (DVT), is a widely‐recognized disorder with a high incidence rate of ~131.5/100 000 people every year.1 DVT and PE are the two different stages of VT, which are associated with common risk factors caused by environmental, behavioral, and genetic interactions.2, 3 DVT is the fundamental reason for cardiovascular‐related mortality and its treatment such as anticoagulation therapy and mechanical leg compression only has a function of palliation.4 Systemic or oral anticoagulation therapy remains ineffective in eliminating thrombus burden and it cannot prevent the post‐thrombotic syndrome as well.5 Lower extremity deep vein thrombosis (LEDVT), is predominantly a disease of older age which is a now more prevalent in clinical studies and it typically starts below the knee, and yet may extend proximally, leading to PE.6 It is a prevailing disease with an incidence rate of 1/1000 adults annually.7 Acute DVT may result in PE, thrombus progression, phlegmasia cerulea dolens, venous gangrene, phlegmasia alba dolens, and even death.8 The standard treatment for DVT is oral anticoagulation, aiming to restrict the thrombus propagation and reduce PE risk.9 As a prevalent disorder, various genetic factors have been indicated to affect the susceptibility toward thrombosis as well as DVT incidence.10
Interleukin‐18 (IL‐18), a member of the IL‐1 superfamily, can enhance both acquired and innate immune responses, which are highly expressed in synovial fluids, sera, and synovial tissues of patients having rheumatoid arthritis.11 IL‐18 is also a pro‐inflammatory cytokine which plays an important role in the Th1 response, and is capable of inducing interferon (IFN)‐γ production in natural killer cells and T cells.12 Moreover, gene polymorphism of IL‐18 plays a significant role in the hepatitis C virus infection among Americans, Europeans, Indians and also in the Chinese Han population.13 Due to its important role in the immune response, pathophysiology and pathogenesis of various infectious diseases, IL‐18 gene polymorphism is known to affect expression levels and also the outcome of the infection.14 IL‐18 promoter polymorphisms were involved in different inflammatory diseases, among which three single nucleotide polymorphisms at positions of −607C/A, −656G/T and −137G/C among promoters of the IL‐18 gene were found.15 Moreover, a study also explained the possible relationship between two different promoter polymorphisms, −607C/A (rs1946518) and −137G/C (rs187238) in IL‐18 gene and prognosis and occurrence of prostate cancer in the Han Chinese population.16 IL‐18, an immune response modulator, plays an important role in the pathogenesis of various inflammatory‐related disorders.17 The serum IL‐18 level is elevated in the DVT, which might damage the venous endothelial cells, leading to venous thrombosis.18 Thus, our study aims to explain the association of IL‐18 gene polymorphism and its protein expression with LEDVT in the Han population.
2. MATERIALS AND METHODS
2.1. Ethics statement
All experiments in this study were approved by the Ethics Committee of Huzhou Central Hospital and all patients signed the informed consent.
2.2. Study subjects
Between October 2014 and December 2015, a total of 138 LEDVT patients of the Chinese Han population treated in the Vasculocardiology Deparment of Huzhou Central Hospital volunteered as the LEDVT group in our study, among those 73 were male and 65 were female with a mean age of (58.19 ± 12.71) and a mean weight of (69.3 ± 9.8) kg. The included criteria were as follows: according to the LEDVT diagnosis criteria revised in 1995 by the Committee Specialized in Peripheral Vascular Disease of the Society of Integrated Traditional Chinese and Western Medicine19; (i) acute stage of LEDVT: patients have an abrupt onset of LEDVT (often within 7 days), accompanied by severe distending pain in affected limb, tenderness in the shank and femoral triangle, maroon skin color, increased body temperature, extensive swelling, dilatation of the superficial vein of the affected limb and a “positive Homans” sign; (ii) In chronic stage of LEDVT, patients have an onset period lasting over 1 month, accompanied by obstruction to the venous return, vein blood reverse flow in the late stage, varicose veins and vein engorgement, limb swelling and pain, hollowness and alogotrophy after activity. All the patients were detected with DVT using lower limb deep vein angiography and were later diagnosed with LEDVT. The excluded criteria were as follows: patients had acute arterial embolism, erysipelas, acute lymphangitis, primary pelvic tumor, leg fibrositis, and leg injury hematoma. Later, in the corresponding period another 150 healthy people (Han population) with no history of LEDVT underwent physical examination in Huzhou Central Hospital and were enrolled as control group, among which 79 were male and 71 were female with a mean age of (55.92 ± 13.22) years old and a mean weight of (68.4 ± 10.6) kg.
2.3. Clinical data collection
All the data, including the gender, age, body mass index (BMI), triglycerides (TG), low density lipoprotein (LDL), high density lipoprotein (HDL), total cholesterol (TC), fasting blood‐glucose (GLU), partial thromboplastin time (APTT), blood urea nitrogen (BUN), Cr, alanine transaminase (ALT), aspartate transaminase (AST), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), and fibrinogen (Fg) was detected in both the groups.
2.4. Evaluating the Serum IL‐18 level
A total of 2 mL of peripheral venous blood was collected from all the subjects on an empty stomach, later it was put in an acid‐citrate dextrose (ACD) anticoagulant tube (Shanghai Qian Ling Chemical Co., Ltd., Shanghai, China), for centrifugation (3000 r/min) for 5 minutes, along with the serum preserved at a temperature of −20°C in the refrigerator for further use. An enzyme‐linked immunosorbent kit (ELISA, R&D System, Minneapolis, Minn., USA) was used to determine the serum IL‐18 level.
2.5. Polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP)
The PCR‐RFLP was used to detect the polymorphism of IL‐18 gene promoters −137G/C and −607C/A. The modified salting out method was used to extract the leukocyte DNA and based on the Primer 5.0 software (Shanghai Sangon Biological Engineering Technology & Services Co., Ltd, Shanghai, China), the primer sequences were designed for the target gene. After annealing, temperature and cycle times were continuously changed and later tested by the polyacrylamide gel electrophoresis (PAGE). The final primer sequences were obtained as follows: −607C/A: forward primer, 5′‐TTGTAACATTGTAGGAATTACC‐3′; reverse primer, 5′‐ATGTAATATCACTATTTTCATGAGA‐3′; −137G/C: forward primer, 5′‐ATGCTTCTAATGGACTAAGGA‐3′; reverse primer, 5′‐GTAATATCACTATTTTCATGAATT‐3′. The PCR reaction conditions of −607C/A were as follows: predenaturation at 95°C for 5 minutes, denaturation at 94°C for 30 seconds, annealing at 64°C for 30 seconds, extension at 72°C for 30 seconds, and final extension at 72°C for 7 minutes. The PCR reaction conditions of −137G/C were as below: predenaturation at 95°C for 5 minutes, denaturation at 94°C for 30 seconds, annealing at 50°C for 1 minute, extension at 72°C for 5 seconds, and final extension at 72°C for 7 minutes. Each PCR had 35 cycles. Enzyme digestion and electrophoresis detection were as follows: After enzyme digestion, the PCR amplification products were obtained and digested by the MseI restriction enzyme (Shanghai Yubo Biotech Co., Ltd, Shanghai, China) and EcoRI restriction enzyme (Shanghai Yubo Biotech Co., Ltd, Shanghai, China), followed by a gel electrophoresis by 4% agarose (Genetech, Vacaville, CA, USA) to detect the genotype and the mutant sites.
2.6. Statistical analysis
The SPSS 21.0 software (IBM Corp., Armonk, NY, USA) was used for the data analysis. The measured data was presented as mean ± standard deviation and the t‐test was used to compare the data between the two groups. The data calculated was also demonstrated in percentages and ratios and the chi‐square test was adapted for group comparisons. The Hardy‐Weinberg equilibrium (HWE) test was performed to detect the group representation, genotype and allele frequency using the chi‐square test and multivariate analysis of variance using binary logistic regression analysis. P < .05 meant there was a statistically significant difference.
3. RESULTS
3.1. Clinical data of the subjects in the LEDVT and control groups
According to the comparison the LEDVT group had increased levels of BMI, GLU, Fg, BUN, and ApoB but decreased APTT when compared to that in the control group (all P < .05). There were no significant difference in the gender, age, TG, LDL, HDL, CHOL, Cr, ALT, AST, and ApoA1 between the two groups (P > .05) (Table 1).
Table 1.
The clinical data between the LEDVT and control groups
| LEDVT (n = 138) | Control (n = 150) | P | |
|---|---|---|---|
| Gender (male/female) | 74/64 | 79/71 | .906 |
| Mean age | 58.19 ± 12.71 | 55.92 ± 13.22 | .139 |
| BMI (kg/m2) | 24.58 ± 2.16 | 23.00 ± 2.49 | <.0001 |
| Affected limb | |||
| Left LEDVT | 75 | – | |
| Right LEDVT | 29 | – | |
| Left‐ and right LEDVT | 34 | – | |
| TG (mmol/L) | 1.69 ± 0.79 | 1.64 ± 0.73 | .577 |
| LDL (mmol/L) | 3.13 ± 0.86 | 3.04 ± 0.72 | .335 |
| HDL (mmol/L) | 1.10 ± 0.24 | 1.14 ± 0.34 | .253 |
| TC (mmol/L) | 4.72 ± 1.00 | 4.74 ± 0.79 | .850 |
| GLU (mmol/L) | 5.25 ± 0.88 | 4.98 ± 0.71 | .004 |
| APTT (min) | 34.18 ± 5.54 | 37.33 ± 7.44 | <.0001 |
| BUN (mmol/L) | 5.84 ± 1.69 | 5.37 ± 1.38 | .010 |
| Cr (mmol/L) | 80.40 ± 18.62 | 78.33 ± 16.29 | .315 |
| ALT (IU/L) | 32.06 ± 12.08 | 29.85 ± 9.56 | .085 |
| AST (IU/L) | 31.98 ± 10.50 | 29.78 ± 9.31 | .061 |
| ApoA1 (g/L) | 1.30 ± 0.38 | 1.33 ± 0.40 | .515 |
| ApoB (g/L) | 0.99 ± 0.49 | 0.80 ± 0.42 | .001 |
| Fg (g/L) | 3.83 ± 1.11 | 3.25 ± 0.78 | <.0001 |
ALT, alanine transaminase; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; APTT, partial thromboplastin time; AST, aspartate transaminase; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; Fg, fibrinogen; GLU, fasting blood‐glucose; HDL, high density lipoprotein; LDL, low density lipoprotein; LEDVT, lower extremity deep venous thrombosis; TC, total cholesterol; TG, riglycerides.
3.2. Identification of PCR restriction enzyme electrophoresis
As shown in the Figure 1, the PCR restriction enzyme electrophoresis images showed that IL‐18 −607C/A (PCR product length: 196 bp) polymorphism had CC, AA and CA genotypes, but the IL‐18 −137G/C (PCR product length: 261 bp) polymorphism had GG, CC and GC genotypes after the restriction enzyme digestion.
Figure 1.

Electrophoresis Photo images for the polymorphisms of IL‐18 gene promoters −607C/A and −137G/C. A, Electrophoresis images for the IL‐18 −607C/A polymorphism; Lane 1 and 2, CC genotype; Lane 3 and 4, CA genotype; Lane 5 and 6, AA genotype; B, Electrophoresis images for the IL‐18 −137G/C polymorphism; Lane 1 and 2, GG genotype; Lane 3 and 4, GC genotype; Lane 5 and 6, CC genotype; IL‐18, interleukin‐18
3.3. Frequency distribution of IL‐18 genotypes and its alleles in the LEDVT and control groups
The HWE demonstrated that both IL‐18 −137G/C polymorphism and −607C/A polymorphism were in accordance with the HWE, which verified that all the selected data were of group representation.
The GC + CC genotype and C allele in −137G/C polymorphism significantly increased in the control group compared to those in the LEDVT group (all P < .05), suggesting that GC + CC genotype and C allele in −137G/C polymorphism were the protective gene for the LEDVT (OR = 0.826, 95% CI = 0.586‐0.996, P = .008; OR = .743, 95% CI = 0.047‐0.989, P = .011). There was no significant difference in the genotype and allele frequency of −607C/A polymorphism between the LEDVT and the control group (P > .05) (Table 2).
Table 2.
Frequency distribution of IL‐18 genotype and allele between the LEDVT and control groups
| LEDVT (n, %) | Control (n, %) | OR | 95% CI | χ2 | P | |
|---|---|---|---|---|---|---|
| −607C/A | ||||||
| CC | 41 (29.71) | 40 (26.67) | 1 | |||
| CA | 69 (50.00) | 72 (48.00) | 1.070 | 0.619‐1.848 | 0.058 | .809 |
| AA | 28 (20.29) | 38 (25.33) | 1.391 | 0.723 ‐ 2.676 | 0.980 | .322 |
| CA + AA | 97 (70.29) | 110 (73.33) | 1.162 | 0.695‐1.944 | 0.329 | .566 |
| C | 151 (54.71) | 152 (50.67) | 1 | |||
| A | 125 (45.29) | 148 (49.33) | 1.176 | 0.848‐1.632 | 0.943 | .332 |
| −137G/C | ||||||
| GG | 120 (86.96) | 108 (72.00) | 1 | |||
| GC | 16 (11.59) | 33 (22.00) | 0.436 | 0.228‐0.837 | 6.441 | .011 |
| CC | 2 (1.45) | 9 (6.00) | 0.200 | 0.042‐0.947 | 4.984 | .026 |
| GC + CC | 18 (13.04) | 42 (28.00) | 0.386 | 0.210‐0.710 | 9.749 | .002 |
| G | 256 (92.75) | 249 (83.00) | 1 | |||
| C | 20 (7.25) | 51 (17.00) | 0.381 | 0.221‐0.658 | 12.650 | <.001 |
LEDVT, lower extremity deep venous thrombosis; CI, combination index; OR, odd ratio; IL‐18, interleukin‐18.
3.4. Changes of serum IL‐18 level in different genotypes of IL‐18 −137G/C polymorphism and −607C/A polymorphism
As shown in Figure 2, the IL‐18 levels in the LEDVT and control groups were (268.74 ± 37.91) pg/mL and (165.28 ± 28.15) pg/mL, respectively, indicating that the serum IL‐18 level increased significantly in the LEDVT group compared to the control group (P < .05). IL‐18 level was high in the LEDVT group when compared to the control group with respect to the same genotype in −607C/A and −137G/C polymorphisms (P < .05), and within the LEDVT group, IL‐18 level was higher in the GG genotype than that in the GC + CC genotype of −137G/C polymorphism (P < .05), while no such significant difference was found in the control group (P > .05). Hence, this experiment was not able to establish any association between genotypes of −607C/A polymorphism and serum IL‐18 level in the LEDVT and control groups (P > .05) (Table 3).
Figure 2.
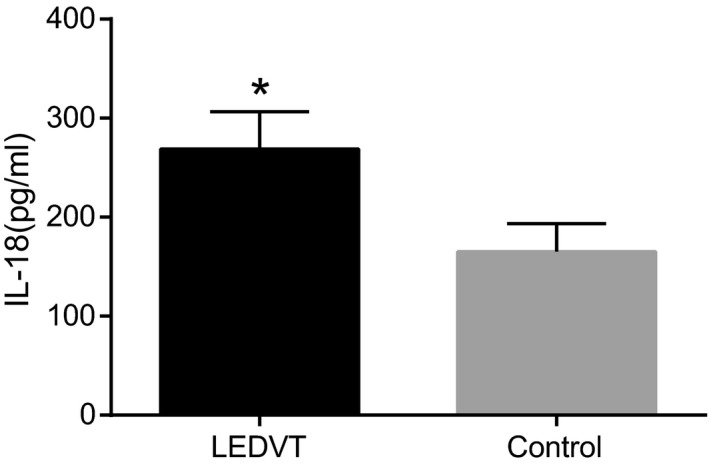
Serum IL‐18 levels between the LEDVT and control groups. *P < .05, compared with the control group; LEDVT, lower extremity deep venous thrombosis; IL‐18, interleukin‐18
Table 3.
Changes of serum IL‐18 level in different genotypes of IL‐18 −137G/C polymorphism and −607C/A polymorphism between the LEDVT and control groups
| Genotype | LEDVT | Control | ||
|---|---|---|---|---|
| Case (n) | IL‐18 level (pg/mL) | Case (n) | IL‐18 level (pg/mL) | |
| −607C/A | ||||
| CC | 41 | 278.34 ± 38.97a | 40 | 173.85 ± 38.93 |
| CA + AA | 97 | 271.25 ± 43.62a | 110 | 159.25 ± 43.62 |
| −137G/C | ||||
| GG | 120 | 258.65 ± 32.30a, * | 108 | 154.65 ± 32.30 |
| GC + CC | 18 | 239.34 ± 29.13a | 42 | 151.34 ± 29.13 |
P < .05, compared with the same genotype between the LEDVT group and the control group.
*P < .05, GG genotype compared with the GC + CC genotype; LEDVT, lower extremity deep venous thrombosis; IL‐18, interleukin‐18.
3.5. Multivariate logistic regression analysis for the risk factor of LEDVT
The different genotypes of −137G/C polymorphism and levels of IL‐18, BMI, GLU, APTT, BUN, ApoB and Fg, were considered as the potential risk factors in multivariate logistic regression analysis of our study. The input method was used for the analysis and BUN, IL‐18‐GG polymorphism and IL‐18 level were independent risk factors, but APTT was a protective factor of LEDVT (all P < .05); whereas the BMI, GLU, ApoB, and Fg made no significant difference (P > .05) (Table 4).
Table 4.
Multivariate logistic regression analysis for the risk factor of LEDVT
| Factor | B | SE | Wals | Sig. | Exp (B) | 95% CI |
|---|---|---|---|---|---|---|
| 137G/C | −2.66 | 1.21 | 4.82 | 0.028 | 0.07 | 0.01‐0.75 |
| BMI | 0.31 | 0.17 | 3.17 | 0.075 | 1.36 | 0.97‐1.90 |
| GLU | −0.35 | 0.51 | 0.47 | 0.492 | 0.71 | 0.26‐1.91 |
| APTT | −0.12 | 0.05 | 5.31 | 0.021 | 0.88 | 0.8‐0.98 |
| BUN | 0.53 | 0.25 | 4.64 | 0.031 | 1.70 | 1.05‐2.77 |
| ApoB | 0.49 | 0.80 | 0.37 | 0.543 | 1.63 | 0.34‐7.76 |
| Fg | 0.45 | 0.36 | 1.54 | 0.214 | 1.57 | 0.77‐3.19 |
| IL‐18 | 0.1 | 0.02 | 35.24 | <0.001 | 1.11 | 1.07‐1.14 |
LEDVT, lower extremity deep venous thrombosis; BMI, body mass index; GLU, fasting blood‐glucose; APTT, partial thromboplastin time; BUN, blood urea nitrogen; ApoB, apolipoprotein B; Fg, fibrinogen; CI, combination index; IL‐18, interleukin‐18.
4. DISCUSSION
LEDVT, along with its complications still has a high incidence among the hospitalized patients.20 It is a multifactorial disease with acquired and genetic risk factors playing a significant role in its pathogenesis.21, 22, 23, 24 Some genetic factors were mentioned in the etiology of LEDVT.25, 26 Thus, in this study we aim to demonstrate the association of IL‐18‐GG polymorphism and IL‐18 level with LEDVT. In the end, our study provided evidence that GG genotype of −137G/C polymorphism and IL‐18 level were independent risk factors of LEDVT and IL‐18 gene polymorphism can influence the serum IL‐18 level in LEDVT patients.
Initially, the LEDVT group had increased levels of BMI, GLU, Fg, BUN, ApoB and IL‐18 but decreased APTT when compared to the control group. BMI was demonstrated as a biomarker for improvement in proximal DVT disease.27 GLU and FBG levels were also a main criterion used to diagnose the glucose intolerance.28 Fg, being a multifunctional plasma protein, plays a crucial role in several biological processes.29 A study examined the effect of Fg on intracranial hemorrhage and concluded that Fg concentrate was a suitable therapy for enhancing plasma Fg for the treatment of intracranial hemorrhage along with hematoma expansion.30 The BUN, which is a broadly available, easily determinable and inexpensive marker, could be adapted to confirm patients who are at the risk of cardiovascular endpoints, and the elevated BUN was inconsistency with increased mortality.31, 32 The apoB to apoA1, an indicator to balance the atheroprotective and atherogenic cholesterol transport,33 was found to be independently related to cardiovascular diseases.34 IL‐18‐stimulated macrophages triggered endothelial cell apoptosis, indicating that, in vivo, excess IL‐18 suppressed tumor blood vessel formation.35 The APTT is widely used to monitor therapeutic anticoagulation with standard heparin.36 Together, BUN, and IL‐18 level were independent risk factors, but APTT was a protective factor of LEDVT, which was also further proved by the multivariate logistic regression analysis.
The IL‐18 level elevated remarkably in the LEDVT group when compared to the control group with respect to the same genotype in −607C/A and −137G/C, and in the LEDVT group IL‐18 level was higher in the GG genotype of −137G/C than that in the GC + CC genotype. A study also revealed that IL‐18 gene −137G/C polymorphism as well as −137C/‐607A haplotype is related to the colorectal cancer.37 Another study indicated that IL‐18 −137G/C and −607A/C polymorphisms could control the IL‐18 protein levels and also influence an individual's sensitivity to oral cancer.38 Vairaktaris et al. and Asefi et al. have detected the impact of IL‐18 −607A/C and −137G/C polymorphisms on clinical parameters and the occurrence of oral cancer.39, 40 Also, Saenz‐Lopez et al41 demonstrated that IL‐18 −137 GG genotype was remarkably associated with a high tumor grade, size, and stage in renal cell carcinoma patients. And it was further proved in a study that GG genotype of the −137G/C polymorphism was 2.165‐times more risky than the GC genotype for the prostate cancer progression and lowered the rate of progression‐free survival.16 Importantly, IL‐18‐607A/C and also −137G/C promoter polymorphisms were associated with susceptibility to penicillin allergy and in particular, the −137G/C position plays an important role in IL‐18 expression.42 Serum IL‐18 level increased in the DVT, which potentially impaired venous endothelial cells, leading to venous thrombosis, hence IL‐18 could be a new potential target for the prevention of DVT.18 In addition, interleukin polymorphisms have been reported to be involved in similar diseases along with LEDVT: IL‐6 and its promoter polymorphism at −572G/C are associated with the risk of venous thromboembolisms (VTE) and IL‐10 −1082A/G polymorphism is correlated with the risk of DVT.43, 44 IL‐22 and IL‐17, which are secreted from Th17 cells, have been considered as the new diagnostic markers of DVT, and they both might provide the novel molecular target for accelerating thrombus resolution in patients with DVT.45 All the data mentioned above was consistent with our findings, and we reached to the conclusion that LEDVT patients with GG genotype of the IL‐18 −137G/C gene had elevated IL‐18 level.
In conclusion, our study demonstrated that the GG genotype of IL‐18 −137G/C polymorphism and IL‐18 level are independent risk factors of LEDVT, and IL‐18 gene polymorphism can influence the serum IL‐18 level in LEDVT patients. However, due to the limited data and experimental conditions, more improvements in the study could be done in the future.
COMPETING INTERESTS
The authors have declared that no competing interests exist.
ACKNOWLEDGMENTS
This study was supported by Huzhou Science and Technology Bureau and Huzhou Central Hospital (2014GYB14). We thank the reviewers for their critical comments.
Chen Y‐L, Shou L‐H, Zhang Z‐X. Association of interleukin‐18 gene polymorphism and its protein expression with the lower extremity deep venous thrombosis in the chinese han population: A case‐control study. J Clin Lab Anal. 2018;32:e22345 10.1002/jcla.22345
REFERENCES
- 1. Martinez C, Cohen AT, Bamber L, Rietbrock S. Epidemiology of first and recurrent venous thromboembolism: a population‐based cohort study in patients without active cancer. Thromb Haemost. 2014;112:255‐263. [DOI] [PubMed] [Google Scholar]
- 2. Monreal M, Barba R, Tolosa C, et al. Deep vein thrombosis and pulmonary embolism: the same disease? Pathophysiol Haemost Thromb. 2006;35:133‐135. [DOI] [PubMed] [Google Scholar]
- 3. Nielsen JD. The incidence of pulmonary embolism during deep vein thrombosis. Phlebology. 2013;28(Suppl 1):29‐33. [DOI] [PubMed] [Google Scholar]
- 4. Blattler W, Partsch H. Leg compression and ambulation is better than bed rest for the treatment of acute deep venous thrombosis. Int Angiol. 2003;22:393‐400. [PubMed] [Google Scholar]
- 5. Lin PH, Ochoa LN, Duffy P. Catheter‐directed thrombectomy and thrombolysis for symptomatic lower‐extremity deep vein thrombosis: review of current interventional treatment strategies. Perspect Vasc Surg Endovasc Ther. 2010;22:152‐163. [DOI] [PubMed] [Google Scholar]
- 6. Ho VB, van Geertruyden PH, Yucel EK, et al. ACR Appropriateness Criteria((R)) on suspected lower extremity deep vein thrombosis. J Am Coll Radiol. 2011;8:383‐387. [DOI] [PubMed] [Google Scholar]
- 7. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4‐I8. [DOI] [PubMed] [Google Scholar]
- 8. Sundar G, Keshava SN, Moses V, et al. Outcomes of catheter‐directed treatment of lower extremity deep vein thrombosis of patients presenting to a tertiary care hospital. Indian J Radiol Imaging. 2016;26:73‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buller HR, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:401S‐428S. [DOI] [PubMed] [Google Scholar]
- 10. Li G, Han ZL, Dong HG, et al. Platelet endothelial cell adhesion molecule‐1 gene 125C/G polymorphism is associated with deep vein thrombosis. Mol Med Rep. 2015;12:2203‐2210. [DOI] [PubMed] [Google Scholar]
- 11. Ji JD, Lee WJ. Interleukin‐18 gene polymorphisms and rheumatoid arthritis: a meta‐analysis. Gene. 2013;523:27‐32. [DOI] [PubMed] [Google Scholar]
- 12. Tavares NA, Santos MM, Moura R, et al. Interleukin 18 (IL18) gene promoter polymorphisms are associated with type 1 diabetes mellitus in Brazilian patients. Cytokine. 2013;62:286‐289. [DOI] [PubMed] [Google Scholar]
- 13. Yue M, Wang JJ, Tang SD, et al. Association of interleukin‐18 gene polymorphisms with the outcomes of hepatitis C virus infection in high‐risk Chinese Han population. Immunol Lett. 2013;154:54‐60. [DOI] [PubMed] [Google Scholar]
- 14. Taheri M, Hashemi‐Shahri SM, Hamzehnejadi M, et al. Lack of association between interleukin‐18 ‐607 C/A gene polymorphism and pulmonary tuberculosis in Zahedan, Southeast Iran. Prague Med Rep. 2012;113:16‐22. [DOI] [PubMed] [Google Scholar]
- 15. Kim HL, Cho SO, Kim SY, et al. Association of interleukin‐18 gene polymorphism with body mass index in women. Reprod Biol Endocrinol. 2012;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu JM, Liu JN, Wei MT, et al. Effect of IL‐18 gene promoter polymorphisms on prostate cancer occurrence and prognosis in Han Chinese population. Genet Mol Res. 2013;12:820‐829. [DOI] [PubMed] [Google Scholar]
- 17. Dziedziejko V, Kurzawski M, Paczkowska E, Machalinski B, Pawlik A. The impact of IL18 gene polymorphisms on mRNA levels and interleukin‐18 release by peripheral blood mononuclear cells. Postepy Hig Med Dosw (Online). 2012;66:409‐414. [DOI] [PubMed] [Google Scholar]
- 18. Mo J, Bai B, Li Y, et al. Expression of interleukin‐18 in a rat model of deep vein thrombosis. J Cardiovasc Surg (Torino). 2012;53:625‐630. [PubMed] [Google Scholar]
- 19. Shackford SR, Cipolle MD, Badiee J, et al. Determining the magnitude of surveillance bias in the assessment of lower extremity deep venous thrombosis: a prospective observational study of two centers. J Trauma Acute Care Surg 2016;80:734‐739; discussion 740‐731. [DOI] [PubMed] [Google Scholar]
- 20. Casella IB, Bosch MA, Sabbag CR. Incidence and risk factors for bilateral deep venous thrombosis of the lower limbs. Angiology. 2009;60:99‐103. [DOI] [PubMed] [Google Scholar]
- 21. Muller‐Buhl U, Leutgeb R, Engeser P, et al. Varicose veins are a risk factor for deep venous thrombosis in general practice patients. Vasa. 2012;41:360‐365. [DOI] [PubMed] [Google Scholar]
- 22. Chandrakasan S, Sood S, Ham S, et al. Risk factors and management of deep venous thrombosis in children following post‐surgical hypopituitarism in craniopharyngioma. Pediatr Blood Cancer. 2011;57:175‐177. [DOI] [PubMed] [Google Scholar]
- 23. Niki Y, Matsumoto H, Hakozaki A, Mochizuki T, Momohara S. Rheumatoid arthritis: a risk factor for deep venous thrombosis after total knee arthroplasty? Comparative study with osteoarthritis. J Orthop Sci. 2010;15:57‐63. [DOI] [PubMed] [Google Scholar]
- 24. Koopman K, Uyttenboogaart M, Vroomen PC, et al. Risk factors for cerebral venous thrombosis and deep venous thrombosis in patients aged between 15 and 50 years. Thromb Haemost. 2009;102:620‐622. [DOI] [PubMed] [Google Scholar]
- 25. Varga EA, Kujovich JL. Management of inherited thrombophilia: guide for genetics professionals. Clin Genet. 2012;81:7‐17. [DOI] [PubMed] [Google Scholar]
- 26. Rosendaal FR, Reitsma PH. Genetics of venous thrombosis. J Thromb Haemost. 2009;7(Suppl 1):301‐304. [DOI] [PubMed] [Google Scholar]
- 27. Ogren M, Eriksson H, Bergqvist D, Sternby NH. Subcutaneous fat accumulation and BMI associated with risk for pulmonary embolism in patients with proximal deep vein thrombosis: a population study based on 23 796 consecutive autopsies. J Intern Med. 2005;258:166‐171. [DOI] [PubMed] [Google Scholar]
- 28. Martins AS, Jansen AK, Rodrigues LO, et al. Lower fasting blood glucose in neurofibromatosis type 1. Endocr Connect. 2016;5:28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Oliveira S, Vitorino de Almeida V, Calado A, Rosario HS, Saldanha C. Integrin‐associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochim Biophys Acta. 2012;1818:481‐490. [DOI] [PubMed] [Google Scholar]
- 30. McBride D, Tang J, Zhang JH. Maintaining plasma fibrinogen levels and fibrinogen replacement therapies for treatment of intracranial hemorrhage. Curr Drug Targets 2015;999:53‐78. [DOI] [PubMed] [Google Scholar]
- 31. Gary T, Pichler M, Schilcher G, et al. Elevated blood urea nitrogen is associated with critical limb ischemia in peripheral arterial disease patients. Medicine (Baltimore). 2015;94:e948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beier K, Eppanapally S, Bazick HS, et al. Elevation of blood urea nitrogen is predictive of long‐term mortality in critically ill patients independent of “normal” creatinine. Crit Care Med. 2011;39:305‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med. 2006;259:437‐446. [DOI] [PubMed] [Google Scholar]
- 34. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937‐952. [DOI] [PubMed] [Google Scholar]
- 35. Xing Y, Tian Y, Kurosawa T, et al. Inhibition of blood vessel formation in tumors by IL‐18‐polarized M1 macrophages. Genes Cells. 2016;21:287‐295. [DOI] [PubMed] [Google Scholar]
- 36. Hurzeler C. von Felten A [Is the activated partial thromboplastin time suitable for monitoring of thrombosis therapy with high‐dose standard heparin?]. Schweiz Med Wochenschr. 1994;124:712‐719. [PubMed] [Google Scholar]
- 37. Guo JY, Qin AQ, Li RK, et al. [Association of the IL‐18 gene polymorphism with susceptibility to colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15:400‐403. [PubMed] [Google Scholar]
- 38. Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL‐18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112:146‐152. [DOI] [PubMed] [Google Scholar]
- 39. Vairaktaris E, Serefoglou ZC, Yapijakis C, et al. The interleukin‐18 ‐607A/C polymorphism is not associated with risk for oral cancer. Anticancer Res. 2007;27:4011‐4014. [PubMed] [Google Scholar]
- 40. Asefi V, Mojtahedi Z, Khademi B, Naeimi S, Ghaderi A. Head and neck squamous cell carcinoma is not associated with interleukin‐18 promoter gene polymorphisms: a case‐control study. J Laryngol Otol. 2009;123:444‐448. [DOI] [PubMed] [Google Scholar]
- 41. Saenz‐Lopez P, Carretero R, Vazquez F, et al. Impact of interleukin‐18 polymorphisms‐607 and ‐137 on clinical characteristics of renal cell carcinoma patients. Hum Immunol. 2010;71:309‐313. [DOI] [PubMed] [Google Scholar]
- 42. Ming L, Wen Q, Qiao HL, Dong ZM. Interleukin‐18 and IL18 ‐607A/C and ‐137G/C gene polymorphisms in patients with penicillin allergy. J Int Med Res. 2011;39:388‐398. [DOI] [PubMed] [Google Scholar]
- 43. Mahemuti A, Abudureheman K, Aihemaiti X, et al. Association of interleukin‐6 and C‐reactive protein genetic polymorphisms levels with venous thromboembolism. Chin Med J (Engl). 2012;125:3997‐4002. [PubMed] [Google Scholar]
- 44. Tang B, Chen YK, Luo WJ, Fu J, Sun JM. Association between interleukin‐10 ‐1082A/G, ‐819C/T and ‐592C/A polymorphisms with deep venous thrombosis. Hum Immunol. 2014;75:203‐207. [DOI] [PubMed] [Google Scholar]
- 45. Huang LG, Jin X, Wu XJ, et al. Increased levels of cytokines interleukin‐17 (IL‐17) and IL‐22 in deep vein thrombosis. J Comput Theor Nanosci. 2016;13:2763‐2766. [Google Scholar]


