Abstract
Background
The current methods for detecting Mycobacterium tuberculosis (Mtb) are not clinically optimal. Standard culture methods (SCMs) are slow, costly, or unreliable, and loop‐mediated isothermal amplification (LAMP) cannot differentiate live Mtb.
Methods
This study compared reverse transcription (RT)‐LAMP, LAMP, and an SCM for detecting Mtb. A first experiment tested the sensitivity and specificity of primers for 9 species of Mycobacterium (H37Rv, M. intracellulare, M. marinum, M. kansasii, M. avium, M. flavescens, M. smegmatis, M. fortuitum, and M. chelonae); and 3 non‐Mycobacterium species (Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae). A second experiment tested sputum specimens for the presence of Mtb, from 100 patients with tuberculosis (clinical) and 22 from patients without tuberculosis (control), using Roche solid culture (SCM), LAMP, and RT‐LAMP. In the clinical samples.
Results
The rates of positivity for Mtb of the SCM, LAMP, and RT‐LAMP methods were 88%, 92%, and 100%, respectively. The difference in detection rate was significant between RT‐LAMP and SCM, but RT‐LAMP and LAMP were comparable. In the control group, the detection rates were nil for all three methods.
Conclusion
The specificities of the methods were similar. The sensitivity of RT‐LAMP was ~10‐fold higher than that of LAMP for detecting Mtb. Unlike LAMP, RT‐LAMP could identify viable bacteria, and was able to detect a single copy of Mtb. Among SCM, LAMP, and RT‐LAMP, the latter is the most suitable for wide use in the lower‐level hospitals and clinics of China for detecting Mtb in sputum samples.
Keywords: 16S rRNA, loop‐mediated isothermal amplification, Mycobacterium tuberculosis, reverse transcription loop‐mediated isothermal amplification
1. INTRODUCTION
Tuberculosis (TB) is a serious chronic infectious disease, caused by the obligate pathogenic bacterial species Mycobacterium tuberculosis (Mtb). TB is one of the oldest diseases that still harm mankind, with specimens isolated from ancient Egyptian mummies. Currently, about 9‐10 million people contract tuberculosis each year,1, 2, 3, 4, 5, 6 even as the emergence of drug‐ and multidrug‐resistant tuberculosis strains continue to emerge.7, 8, 9 The prevention and treatment of TB requires detection methods for Mtb that are quick to perform and with high sensitivity and specificity,10, 11 so that patients may be treated early.12, 13
At present, the main methods to diagnosis tuberculosis depend on detection of Mtb. These methods include the following: Acid‐fast bacillus smear, culture, and nucleic acid amplification. Each of these has disadvantages in clinical medicine. Although acid‐fast sputum smear staining is simple and easy to perform, it has low sensitivity and a low detection rate,14, 15, 16 and it cannot identify between live and dead bacteria. The Roche solid culture method requires a long culture cycle, 4‐8 weeks.15, 17 The turnaround time of the improved liquid rapid culture system is 10 days, but it detects only viable bacteria18 and is costly and susceptible to contamination.19, 20
Techniques that rely on nucleic acid amplification include polymerase chain reaction (PCR) and loop‐mediated isothermal amplification (LAMP).21, 22, 23, 24 PCR is rapid, sensitive, and specific, but requires high investments in equipment and operator skills, which create obstacles for their use in settings with limited clinical resources, which create obstacles for their use in settings with limited clinical resources.25 LAMP overcomes these shortcomings, with advantages such as rapid reaction (30‐50 minutes) and simple operation. Personnel require only simple training, and the amplification results can be determined by the naked eye. Thus, LAMP is suitable for primary medical care institutions or peripheral laboratory,26, 27, 28 In August 2016, WHO recommends that the basic medical units in developing countries use the new detection method TB‐LAMP, which can replace the sputum smear method.29 However, the target of PCR, LAMP, and other molecular detection methods is Mtb DNA, and results are positive for both viable and dead bacteria. Thus, these techniques cannot be used to test the efficacy of anti‐tuberculosis treatment in clinical medicine.
The ribosomal RNA (rRNA) 16S rRNA is a housekeeping gene with a high copy number and short half‐life, accounting for 80% of the total RNA. Because rRNA molecules are transcribed only in metabolically active cells and are rapidly degraded upon the cessation of metabolism, the direct analysis of rRNA molecules can reveal the diversity and, to certain extent, the quantity of metabolically active organisms.30, 31, 32, 33 Therefore, the selection of reverse transcription (RT) combined with LAMP to amplify 16S rRNA not only helps determine viable bacteria, but also improves the sensitivity. RT‐LAMP and LAMP are similar methods except that the template for RT‐LAMP is RNA during amplification, in which the reverse transcriptase and RNase inhibitor are included.
This study investigated the viability of reverse transcription (RT)‐LAMP for detecting Mtb, relative to LAMP and a Roche culture method. The 16S rRNA gene of Mtb was used as the target gene. The Roche culture method was used, as a gold standard SCM.
2. MATERIALS AND METHODS
2.1. Experiment one: Validation of primers used in the study
2.1.1. Strains and reagents
For primers sensitivity and specificity test, nine standard strains of H37Rv, Mycobacterium intracellulare, Mycobacterium marinum, Mycobacterium kansasii, Mycobacterium avium, Mycobacterium flavescens, Mycobacterium smegmatis, Mycobacterium fortuitum, and Mycobacterium chelonae and three non‐Mycobacterium strains of Staphylococcus aureus, pseudomonas aeruginosa, and Klebsiella pneumoniae, collected in our hospital, are preserved by this clinical laboratory. The modified Roche culture medium is prepared in our laboratory, the Bacterial DNA Extraction Kit was purchased from Shenggong (Shanghai), and the RNeasy Mini Kit was purchased from Qiagen (Germany).
2.1.2. Processing of sputum specimen
Bacterial RNA and DNA samples were prepared for RT‐LAMP and LAMP. In short, first add 2 volumes of RNAprotect Bacteria Reagent into 1 volume of sputum sample to protect RNA and then add 2 volumes of 2% N‐acetyl‐L‐cysteine‐NaOH solution to liquefy the sputum sample.34 vortex for 2 minutes until fully liquefied. Then plating 0.1 mL of specimen on to modified acid Roche medium to identify the bacteria and the remaining was stored at −80°C to be used to extract RNA and DNA. RNA was isolated, using the RNeasy Mini Kit (Qiagen, Germany) in accordance with the manufacturer's instructions and treated with DNase I. DNA was isolated using the Bacterial DNA Extraction Kit (Shenggong Shanghai).
2.1.3. RT‐LAMP primer design
The 16S rRNA sequence of the Mtb standard strain (NR_102810.1) and various non‐tuberculosis Mycobacterium species were compared, using Clustal Omega: M. intracellulare (NR_074661.1); M. marinum (NR_025214.1); M. kansasii (NR_121712.1); M. avium (NR_102855.1); M. flavescens (NR_044815.1); M. smegmatis (NR_074718.1); M. fortuitum (X65529.1) and M. chelonae (AF480594.1). The 16S rRNA sequences located at 170‐210 bp and 430‐490 bp were selected for the specific primers using primer design software Primer Explorer V5. Several specific primer sets were designed for the above region and their specificity at the 3' end of each primer was compared and verified, using BLAST (Figure 1, Table 1). Primers F3 and B3 are the external primers, FIP and BIP are internal primers. FIP is composed of F1c and F2, BIP is composed of B1c and B2. All primers were synthesized by Shanghai Biotechnology.
Figure 1.
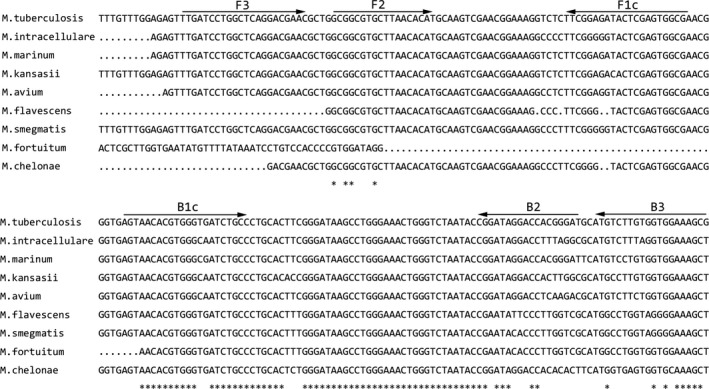
Specific amplification region of RT‐LAMP
Table 1.
RT‐LAMP primers used in this study
| Sequence (5′→3′) | Length, bp | |
|---|---|---|
| F3 | TCCTGGCTCAGGACGAAC | 18 |
| B3 | CGCTTTCCACCACAAGACAT | 20 |
| FIP (F1c + F2) | TCGCCACTCGAGTATCTCCGAA‐GCGGCGTGCTTAACACAT | 40 |
| BIP (B1c + B2) | AGTAACACGTGGGTGATCTGCC‐ATCCCGTGGTCCTATCCG | 40 |
2.1.4. RT‐LAMP reaction system
The 25 μL of reaction mix comprised the following: 1.6 μM of inner primers (FIP and BIP); 0.2 μM of outer primers (F3 and B3); 0.8 M of betaine (Sigma, Saint Louis, MO, USA); 8 mM of MgSO4; 1.4 mM of dNTPs; 8 U of Bst DNA polymerase (New England BioLabs, Ipswich, MA, USA); 20 U of recombinant ribonuclease Inhibitor (Invitrogen, Shanghai, China); 100 U of M‐MLV reverse transcriptase (Vazyme, Shanghai, China); 2.5 μL of 10× buffer; 1 μL of template RNA; 1 μL of mixture of calcium chlorophyll and MnCl2 which concentrations are 0.05 mM and 0.6 mM or HNB (150 μM). Deionized water was added up to 25 μL. A reaction tube without RNA template was the negative control. The tubes were incubated in a water bath at 60−°C for 50 minutes followed by a quenching at 90°C for 2 minutes. The completion of amplification was indicated by the color in the tube, wherein green is considered positive and orange is negative or sky blue is considered positive and purple is negative. The reaction temperature and time of RT‐LAMP and LAMP were also compared. The amplicon was confirmed by 2% agarose gel electrophoresis.
2.1.5. Sensitivity of RT‐LAMP
The sensitivity of RT‐LAMP was tested in triplicate using DNA and RNA extracted from 2 mL of Mtb sputum samples diluted in a 10‐fold series.
2.1.6. Specificity of RT‐LAMP
Positive Mtb controls of H37Rv NR and negative control of pulmonary non‐Mtb bacteria were used to compare the specificity between RT‐LAMP and LAMP.
2.1.7. Detection limit of RT‐LAMP
A detection limit of RT‐LAMP was calculated, using the following formula:
Y (copies/μL) = [X (g/μL) RNA × 6.02 × 10²³]/[target gene length (basic group number) × 340], in which 1 ng is approximately equal to 109 copies RNA, 1 ag approximately equal to 1 copy RNA.
2.1.8. Restriction enzyme digestion of RT‐LAMP and LAMP products
After the RT‐LAMP reaction, the restriction enzyme Xho I was used for restriction fragment length polymorphism (RFLP) analysis.
3. EXPERIMENT TWO
3.1. Clinical samples
Between November 2015 and July 2016, sputum specimens were collected from 100 TB suspected patients who were not given anti‐tuberculosis treatment with tuberculosis (clinical) and 22 from patients without tuberculosis (control). All individuals were patients at Chinese People's Liberation Army Bethune International Peace Hospital Infection Branch. Patients with tuberculosis included 54 men and 46 women, aged 15‐80 years. The 22 non‐tuberculosis patients had received diagnoses of other pulmonary disease, according to bacteriological examination.
3.2. Statistical analysis
The sensitivities of the SCM, LAMP, and RT‐LAMP tests for detecting Mtb were determined, using the chi‐squared test. P < .05 was considered statistically significant.
4. RESULTS
4.1. Optimization of reaction conditions
The higher reaction temperature may decrease the activity of reverse transcriptase activities; the best reaction temperature was 60°C having a specific clear band (Figure 2A). The optimum temperature for Bst DNA Polymerase generally is 63‐65°C.35, 36 The DNA reaction occurred at 60‐65°C for LAMP (Figure 2B). RT‐LAMP reaction starts at 15 minutes and the specific amplification occurred at 30 minutes. LAMP reaction starts at 30 minutes and the amplicon was observed only at 40 minutes. (Figure 3A,B)
Figure 2.
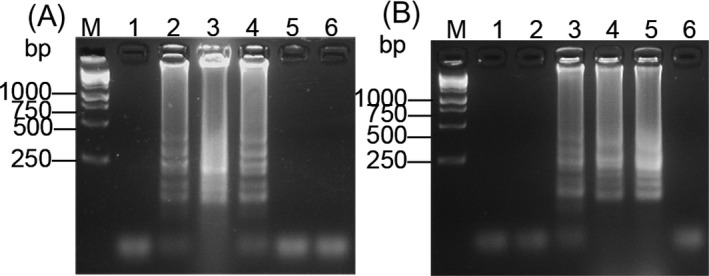
The electrophoresis for different temperature amplifications of (A) RT‐LAMP and (B) LAMP. M: 250 bp DNA marker. Lanes 1‐5: 56, 58, 60, 63, and 65°C. Lane 6: negative control
Figure 3.
Amplification starting times of (A) RT‐LAMP and (B) LAMP. M: 250 bp DNA marker. Lanes 1‐5: 5, 15, 30, 40, and 50 min. Lane 6: Negative control
4.2. The analytical sensitivity of RT‐LAMP and LAMP
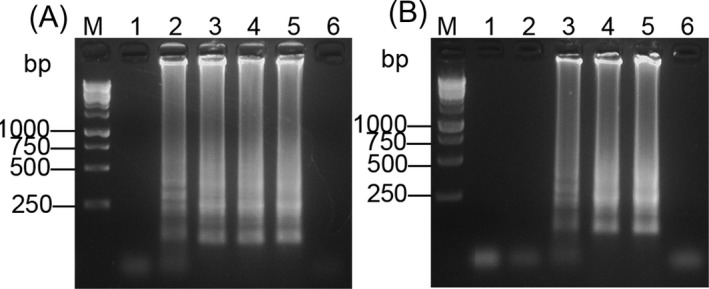
The sensitivity of RT‐LAMP assay was 1.0 × 100 CFU/mL (Figure 4A), and the sensitivity of the LAMP method was 1.0 × 101 CFU/mL (Figure 4B). The sensitivity of RT‐LAMP was 10‐fold higher than that of the LAMP.
Figure 4.
Sensitivity assay of RT‐LAMP and LAMP. (A) Electrophoresis and dye pattern of calcein and HNB of RT‐LAMP. (B) Electrophoresis and dye pattern of calcein and HNB of LAMP. M: 250 bp DNA marker. Lanes 1‐9: 1.0 × 107, 1.0 × 106, 1.0 × 105, 1.0 × 104, 1.0 × 103, 1.0 × 102, 1.0 × 101, 1.0 × 100, 1.0 × 10−1 CFU/mL. Lane 10: Negative control
4.3. The specificity of RT‐LAMP
As shown in Figure 5, RT‐LAMP and LAMP specifically amplified Mycobacterium rRNA, but not the non‐Mycobacterium species, indicating that the RT‐LAMP primers used in this study specifically targeted Mycobacterium rRNA.
Figure 5.

Specificity assay of RT‐LAMP. M: 250 bp DNA marker. (A) Electrophoresis and dye pattern of calcein and HNB, Lanes 1‐4: Sputum samples of Mtb, Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae. (B) Electrophoresis and dye pattern of calcein and HNB, Lanes 1‐9: H37Rv, Mycobacterium intracellulare, M. marinum, M, kansasii, M. avium, M. flavescens, M. smegmatis, M. fortuitum, and M. chelonae
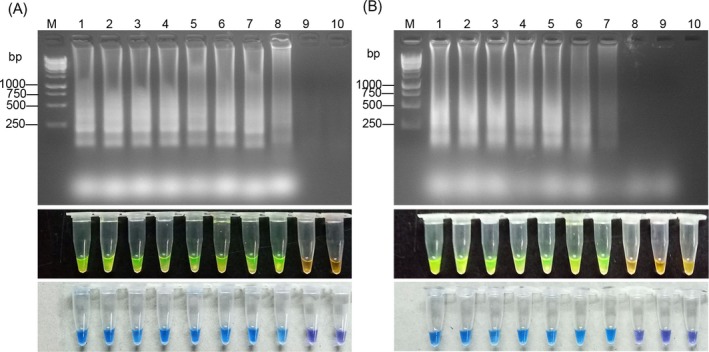
4.4. Detection limit of RT‐LAMP
The detection limit was determined, using the H37Rv standard in triplicate. Figure 6 showed that the detection limit of RT‐LAMP was 1 ag, which equals about 1 copy of the RNA.
Figure 6.
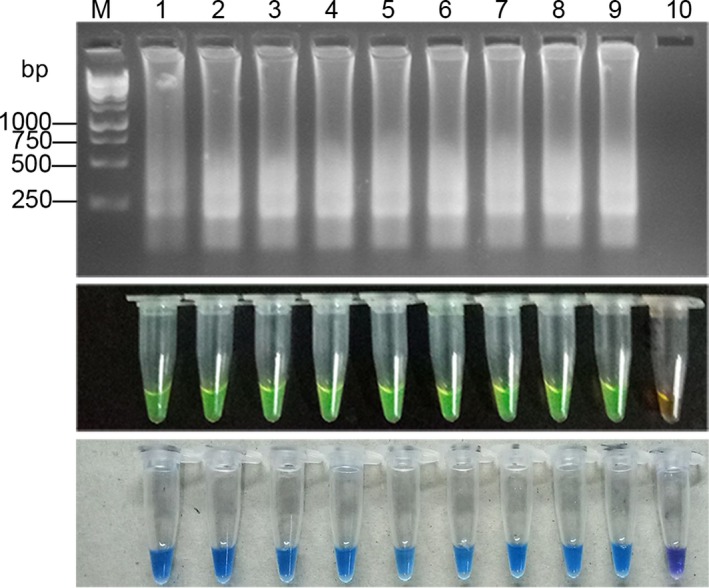
RT‐LAMP detection limit assay. Electrophoresis and dye pattern of calcein and HNB of RT‐LAMP, M: 250 bp DNA marker. Lanes 1‐9:100 pg,10 pg, 1 pg, 100 fg, 10 fg, 1 fg, 100 ag, 10 ag, 1 ag/μL. Lane 10: Negative control
4.5. Restriction enzyme digestion of RT‐LAMP and LAMP products
The amplified product was digested by Xho I, the fragments were showed in Figure 7.
Figure 7.
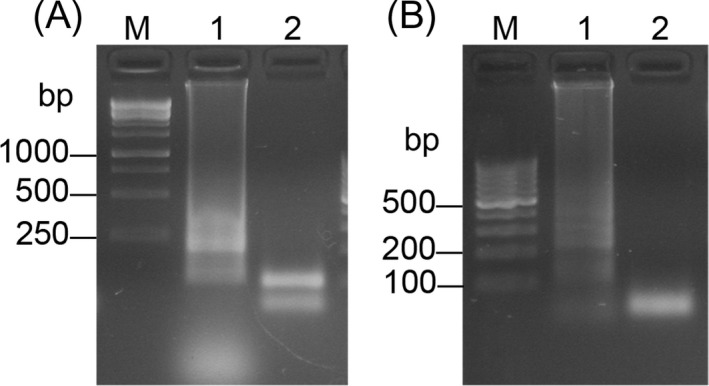
RFLP analysis. Lane 1, before the digestion. Lane 2, RFLP pattern. (A) RT‐LAMP. M: 250 bp DNA marker. (B) LAMP. M: 100 bp DNA marker
4.6. Clinical samples assay of RT‐LAMP and LAMP
The results of RT‐LAMP and LAMP in the clinical sputum specimen are shown in the Figure 8. RT‐LAMP correctly identified some Mtb samples that were shown to be negative by the SCM and LAMP methods. This shows that RT‐LAMP has higher sensitivity than SCM or LAMP (Figure 4).
Figure 8.
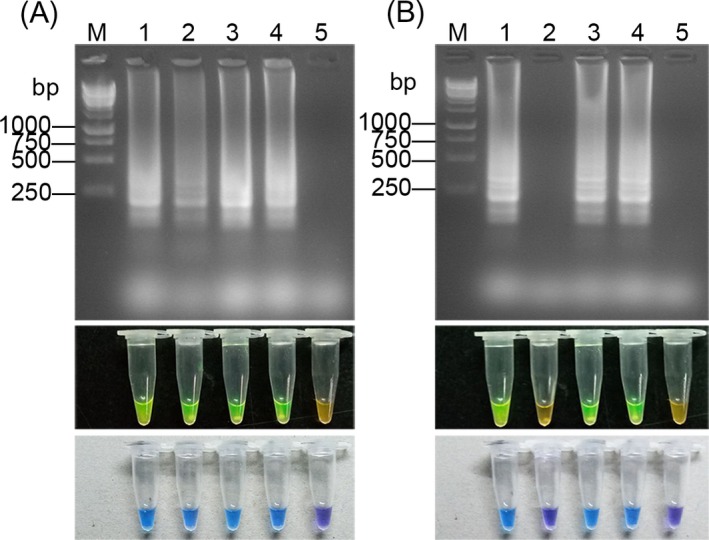
Electrophoresis and dye pattern of calcein and HNB in clinical samples. M: 250 bp DNA marker. Lanes 1‐4: Sputum samples from patients with tuberculosis. Lane 5: Negative control. (A) RT‐LAMP. (B) LAMP
For the tuberculosis patient group of 122 clinical samples, the sensitivity was 88% for SCM, 100% for RT‐LAMP, and 92% for LAMP. The detection rate of sensitivity is significantly different between RT‐LAMP and SCM (P < .01, Table 2), but not differentiate between LAMP and SCM (P > .05, Table 2). RT‐LAMP was significantly more sensitive for detecting Mtb compared with SCM (P < .01), but LAMP and SCM were statistically similar (P > .05, Table 2). The rate of positive identification by RT‐LAMP was significantly higher than that of LAMP (P < .05, Table 2).
Table 2.
Test results of clinical samples
| Detection | Mtb | Non‐Mtb | ||
|---|---|---|---|---|
| SCM | + | 88a | 0 | — |
| − | 12a | 22 | — | |
| RT‐LAMP | + | 100 | 0 | <0.01b |
| − | 0 | 22 | — | |
| LAMP | + | 92 | 0 | >0.05c |
| − | 8 | 22 | <0.05d |
Mtb patients, n = 100; non‐Mtb patients, n = 22;
The sensitivity of RT‐LAMP compared to SCM;
The sensitivity of LAMP compared to SCM;
The sensitivity of RT‐LAMP compared to LAMP.
4.7. Follow‐up samples assay of RT‐LAMP and LAMP
To explore the relationship between RT‐LAMP test results and anti‐tuberculosis treatment, we divided the same patient into three groups according to the anti‐tuberculosis treatment cycle. The results of RT‐LAMP and LAMP were shown in Table 3. The results showed that in patients without treatmen, the positive rate of RT‐LAMP was higher than LAMP, in patients with extended treatment (>6 months), the positive rate of RT‐LAMP was lower than LAMP. That is because viable bacteria are less after treatment, and more dead bacteria remain in the body. So RT‐LAMP can evaluate the efficacy of anti‐tuberculosis whether there is drug resistance.
Table 3.
The results of untreatment and treatment of RT‐LAMP and LAMP (cases)
Treatment for less than 6 mo;
Treatment for more than 6 mo.
5. DISCUSSION
It is predicted that TB will continue to be one of the world's major infectious diseases by 2020,37 which indicates that TB is still spreading worldwide. In recent years, the infection rate of Mtb has been increasing gradually.38, 39 It is difficult to identify based on morphology, especially for AIDS patients infected with non‐Mtb that were often misdiagnosed as tuberculosis39, 40, 41 leading to delayed treatment. The RT‐LAMP technique in this study is highly specific, and can be used to differentiate Mtb from non‐tuberculosis Mycobacteria. Traditional nucleic acid amplification to detect TB is based on the DNA of Mtb,42, 43, 44 which is unable to differentiate viable bacteria or evaluate the clinical efficacy of chemotherapy. However, 16S rRNA with its short half‐life exists only in the metabolic period of live bacteria. Therefore, RT‐LAMP technology to detect Mtb, using RNA, avoids the positive results caused by residual Mtb DNA. LAMP technology, with its simple, fast, and economical operation, has high sensitivity and specificity. The present study showed that RT‐LAMP had a significantly higher Mtb detection rate compared with SCM, but the detection rates of LAMP and SCM were similar. The rate of positive detection of RT‐LAMP was significantly higher than that of the LAMP method.
The negative result, but showed by RT‐LAMP, obtained by LAMP from sputum specimens of patients with Mtb may be due to the low copy number of bacteria, which limited detection. Using RNA, the detection sensitivity of RT‐LAMP was higher, since the 16S rRNA copy number is 103‐105 times higher than that of DNA.31 and RNA is single‐chain structure, which is fragile and easy to degrade, and is not easy to cause contamination. In addition, the sensitivity of RT‐LAMP is ~10‐fold higher than that of LAMP. The short reaction time (15 min) and high sensitivity of RT‐LAMP should be useful clinically for evaluating the effectiveness of anti‐tuberculosis drugs. The follow‐up patients show that with the extension of treatment time, the negative detection of the RT‐LAMP significantly decreased, 16S rRNA negative patients increased, suggest that it can be used to assess drug efficacy and identify drug resistance. RT‐LAMP can distinguish between latent TB infection (LTBI) and TB disease. Most patients with untreated LTBI will never develop TB disease. Lee et al30 reported that combines reverse transcription, loop‐mediated isothermal amplification, and enzyme‐linked immunosorbent assay (RT‐LAMP‐ELISA) for the rapid detection of viable M. tuberculosis have a higher cost ($10) and is more time consuming (5 hours) and less useful in primary health care.
In conclusion, the RT‐LAMP technique evaluated in this study is simple, rapid, specific, and sensitive. It can be used to detect viable Mtb and is practical for use in primary medical care institutions or peripheral laboratory. If connected to a more convenient quantitative device, this technology should be used in daily diagnostic and epidemiological investigations for Mtb.
ACKNOWLEDGMENTS
Not applicable.
Wu D, Kang J, Li B, Sun D. Evaluation of the RT‐LAMP and LAMP methods for detection of Mycobacterium tuberculosis . J Clin Lab Anal. 2018;32:e22326 10.1002/jcla.22326
Funding information
Scientific Research Foundation for Medical Science and Public Health of PLA (No.CBJ14C010)
REFERENCES
- 1. Eurosurveillance editorial team . WHO publishes Global tuberculosis report 2013. Euro Surveill 2013;18:1‐1. [PubMed] [Google Scholar]
- 2. Aung WW, Ei PW, Nyunt WW, et al. Phenotypic and genotypic analysis of anti‐tuberculosis drug resistance in Mycobacterium tuberculosis isolates in Myanmar. Ann Lab Med. 2015;35:494‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zumla A, George A, Sharma V, Herbert RH, Oxley A, Oliver M. The WHO 2014 global tuberculosis report‐further to go. Lancet Glob Health. 2015;3:e10‐e12. [DOI] [PubMed] [Google Scholar]
- 4. Ntoumi F, Kaleebu P, Macete E, et al. Taking forward the World TB Day 2016 theme ‘Unite to End Tuberculosis’ for the WHO Africa Region. Int J Infect Dis. 2016;46:34‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ansumana R, Keitell S, Roberts GM, et al. Impact of infectious disease epidemics on tuberculosis diagnostic, management, and prevention services: experiences and lessons from the 2014‐2015 Ebola virus disease outbreak in West Africa. Int J Infect Dis. 2017;56:101‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Global tuberculosis report 2016. World Health Organization. 2017.
- 7. Zhang L, Meng Q, Chen S, et al. Treatment outcomes of multidrug‐resistant tuberculosis patients in Zhejiang, China, 2009‐2013. Clin Microbiol Infect 2017. 10.1016/j.cmi.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 8. Cafe Oliveira LN, Muniz‐Sobrinho Jda S, Viana‐Magno LA, Oliveira Melo SC, Macho A, Rios‐Santos F. Detection of multidrug‐resistant Mycobacterium tuberculosis strains isolated in Brazil using a multimarker genetic assay for katG and rpoB genes. Braz J Infect Dis. 2016;20:166‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Millan‐Lou MI, Olle‐Goig JE, Tortola MT, Martin C, Samper S. Mycobacterial diversity causing multi‐ and extensively drug‐resistant tuberculosis in Djibouti, Horn of Africa. Int J Tuberc Lung Dis. 2016;20:150‐153. [DOI] [PubMed] [Google Scholar]
- 10. Horvat RT. Gamma Interferon Assays Used in the Diagnosis of Tuberculosis. Clin Vaccine Immunol. 2015;22:845‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yokoyama T, Kinoshita T, Okamoto M, et al. High Detection Rates of Urine Mycobacterium tuberculosis in Patients with Suspected Miliary Tuberculosis. Intern Med. 2017;56:895‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaib un N, Javed H, Zafar A, Qayyum A, Rehman A, Ejaz H. Comparison of fluorescence microscopy and Ziehl‐Neelsen technique in diagnosis of tuberculosis in paediatric patients. J Pak Med Assoc. 2015;65:879‐881. [PubMed] [Google Scholar]
- 13. Oxlade O, Sugarman J, Alvarez GG, Pai M, Schwartzman K. Xpert(R)MTB/RIF for the Diagnosis of Tuberculosis in a Remote Arctic Setting: Impact on Cost and Time to Treatment Initiation. PLoS ONE. 2016;11:e0150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lombardi G, Di Gregori V, Girometti N, Tadolini M, Bisognin F, Dal Monte P. Diagnosis of smear‐negative tuberculosis is greatly improved by Xpert MTB/RIF. PLoS ONE. 2017;12:e0176186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agrawal M, Bajaj A, Bhatia V, Dutt S. Comparative Study of GeneXpert with ZN Stain and Culture in Samples of Suspected Pulmonary Tuberculosis. J Clin Diagn Res. 2016;10:DC09‐DC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Q, Zhou C. Comparison of laboratory testing methods for the diagnosis of tuberculous pleurisy in China. Sci Rep. 2017;7:4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhirud P, Joshi A, Hirani N, Chowdhary A. Rapid laboratory diagnosis of pulmonary tuberculosis. Int J Mycobacteriol. 2017;6:296‐301. [DOI] [PubMed] [Google Scholar]
- 18. Wang HY, Uh Y, Kim S, Shim TS, Lee H. Evaluation of the Quantamatrix Multiplexed Assay Platform System for simultaneous detection of Mycobacteirum tuberculosis and rifampicin resistance gene using culture‐positive Mycobacteria. Int J Infect Dis 2017;61:107‐113. [DOI] [PubMed] [Google Scholar]
- 19. Feyzioglu B, Dogan M, Sanli OO, Ozdemir M, Baykan M. Comparison of the performance of TK system with LJ and MGIT methods in the diagnosis of tuberculosis. Int J Clin Exp Med. 2014;7:1084‐1088. [PMC free article] [PubMed] [Google Scholar]
- 20. Yu FL, Lee JC, Wang MS, et al. Evaluation of a modified direct agar proportion method for testing susceptibility of Mycobacterium tuberculosis from MGIT samples.. J Microbiol Immunol Infect. 2016;49:60‐65. [DOI] [PubMed] [Google Scholar]
- 21. Siala M, Smaoui S, Taktak W, et al. First‐time detection and identification of the Mycobacterium tuberculosis Complex members in extrapulmonary tuberculosis clinical samples in south Tunisia by a single tube tetraplex real‐time PCR assay. PLoS Negl Trop Dis. 2017;11:e0005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aryan E, Makvandi M, Farajzadeh A, et al. A novel and more sensitive loop‐mediated isothermal amplification assay targeting IS6110 for detection of Mycobacterium tuberculosis complex. Microbiol Res. 2010;165:211‐220. [DOI] [PubMed] [Google Scholar]
- 23. Barletta F, Vandelannoote K, Collantes J, Evans CA, Arevalo J, Rigouts L. Standardization of a TaqMan‐based real‐time PCR for the detection of Mycobacterium tuberculosis‐complex in human sputum. Am J Trop Med Hyg. 2014;91:709‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. N'Guessan K, Horo K, Coulibaly I, et al. Rapid detection of Mycobacterium tuberculosis complex in sputum Samples using PURE TB‐LAMP assay. Int J Mycobacteriol. 2016;5(Suppl 1):S164‐S165. [DOI] [PubMed] [Google Scholar]
- 25. Kouzaki Y, Maeda T, Sasaki H, et al. A Simple and Rapid Identification Method for Mycobacterium bovis BCG with Loop‐Mediated Isothermal Amplification. PLoS ONE. 2015;10:e0133759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moon SH, Kim EJ, Tomono J, et al. Detection of Mycobacterium tuberculosis complex in sputum specimens using a loop‐mediated isothermal amplification assay in Korea. J Med Microbiol. 2015;64:1335‐1340. [DOI] [PubMed] [Google Scholar]
- 27. Bentaleb EM, Abid M, El Messaoudi MD, et al. Development and evaluation of an in‐house single step loop‐mediated isothermal amplification (SS‐LAMP) assay for the detection of Mycobacterium tuberculosis complex in sputum samples from Moroccan patients. BMC Infect Dis. 2016;16:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sethi S, Dhaliwal L, Dey P, Kaur H, Yadav R, Sethi S. Loop‐mediated isothermal amplification assay for detection of Mycobacterium tuberculosis complex in infertile women. Indian J Med Microbiol. 2016;34:322‐327. [DOI] [PubMed] [Google Scholar]
- 29. World Health Organization . 2016. World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals: World Health Organization.
- 30. Lee MF, Chen YH, Peng CF. Evaluation of reverse transcription loop‐mediated isothermal amplification in conjunction with ELISA‐hybridization assay for molecular detection of Mycobacterium tuberculosis. J Microbiol Methods. 2009;76:174‐180. [DOI] [PubMed] [Google Scholar]
- 31. Choi Y, Hong SR, Jeon BY, et al. Conventional and real‐time PCR targeting 16S ribosomal RNA for the detection of Mycobacterium tuberculosis complex. Int J Tuberc Lung Dis. 2015;19:1102‐1108, i‐ii. [DOI] [PubMed] [Google Scholar]
- 32. Yu G, Fadrosh D, Goedert JJ, Ravel J, Goldstein AM. Nested PCR Biases in Interpreting Microbial Community Structure in 16S rRNA Gene Sequence Datasets. PLoS ONE. 2015;10:e0132253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lau SC, Liu WT. Recent advances in molecular techniques for the detection of phylogenetic markers and functional genes in microbial communities. FEMS Microbiol Lett. 2007;275:183‐190. [DOI] [PubMed] [Google Scholar]
- 34. Kurtoglu MG, Ozdemir M, Kesli R, Baysal B. Comparison of the GenoType((R)) MTBC Molecular Genetic Assay with culture methods in the diagnosis of tuberculosis. Arch Med Sci. 2014;10:315‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Becker S. LAMP ‐ An innovative POC tool for diagnosing pulmonary TB in remote areas. Indian J Tuberc. 2017;64:72‐76. [DOI] [PubMed] [Google Scholar]
- 36. Fallahi S, Mazar ZA, Ghasemian M, Haghighi A. Challenging loop‐mediated isothermal amplification (LAMP) technique for molecular detection of Toxoplasma gondii. Asian Pac J Trop Med. 2015;8:366‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haldar S, Chakravorty S, Bhalla M, De Majumdar S, Tyagi JS. Simplified detection of Mycobacterium tuberculosis in sputum using smear microscopy and PCR with molecular beacons. J Med Microbiol. 2007;56(Pt 10):1356‐1362. [DOI] [PubMed] [Google Scholar]
- 38. Osei FA, Enimil A, Ansong D, et al. Review of Organism Density and Bacteriologic Conversion of Sputum among Tuberculosis Patients. Int Sch Res Notices. 2017;2017:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu X, Liu P, Liu G, et al. The prevalence of non‐tuberculous mycobacterial infections in mainland China: systematic review and meta‐analysis. J Infect. 2016;73:558‐567. [DOI] [PubMed] [Google Scholar]
- 40. Engelbrecht MC, Kigozi NG, Chikobvu P, Botha S, Rensburg HCJV. Unsuccessful TB treatment outcomes with a focus on HIV co‐infected cases: a cross‐sectional retrospective record review in a high‐burdened province of South Africa. BMC Health Serv Res. 2017;17:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shahraki AH, Heidarieh P, Bostanabad SZ, et al. “Multidrug‐resistant tuberculosis” may be nontuberculous mycobacteria. Eur J Intern Med. 2015;26:279‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ross A, Somssich IE. A DNA‐based real‐time PCR assay for robust growth quantification of the bacterial pathogen Pseudomonas syringae on Arabidopsis thaliana. Plant Methods. 2016;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chae H, Han SJ, Kim SY, et al. Development of a one‐step multiplex PCR assay for differential detection of major Mycobacterium species. J Clin Microbiol 2017;55:2736‐2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Biswas J, Kazi MS, Agarwal VA, Alam MS, Therese KL. Polymerase chain reaction for Mycobacterium tuberculosis DNA detection from ocular fluids in patients with various types of choroiditis in a referral eye center in India. Indian J Ophthalmol. 2016;64:904‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]


