Abstract
Background
Four automated hemoglobin separation devices are compared in their ability to detect hemoglobinopathies, both in HbA1c and in hemoglobinopathy mode.
Methods
Quality control material and 58 samples, including one heterozygous α‐thalassemia sample, six heterozygote β‐thalassemia samples and 32 samples with a known hemoglobin variant, were used to assess imprecision of HbF and HbA2 measurements, correlation with the gold standard and sensitivity for detecting β‐thalassemia and Hb variants on D‐100 (Bio‐Rad Laboratories), HA 8180T (Menarini), HLC‐723G8 (Tosoh Bioscience) and Capillarys 2 Flex Piercing (Sebia).
Results
Imprecision was <10% for both HbF and HbA2 in all modes of all analyzers. Correlation studies for HbF and HbA2 demonstrated statistically significant but small biases when compared to the gold standard. All six β‐thalassemia samples but one were detected on all analyzers using a HbA2 cut‐off value of 3.5%. D‐100, HA8180T and the Hb‐pathy mode of the HLC‐723G8 and the Capillarys are able to detect the most common important Hb variants (Hb C, D, E and S), but more seldom variants can be missed as they co‐elute with HbA0. The HbA1c mode of the Capillarys correctly detected all measured hemoglobin variants and can therefore be used as a hemoglobinopathy screening device. This was also the case for the most common important Hb variants on the HbA1c mode of the HLC‐723G8, but two rare variants were not detected.
Conclusion
This study stresses the importance for individual laboratories to know the advantages and drawbacks of their hemoglobin separation analyzer and its different modes in the diagnosis of hemoglobinopathies.
Keywords: Bio‐Rad Laboratories D‐100, hemoglobinopathy screening, Menarini HA 8180T, Sebia Capillarys 2 Flex Piercing, Tosoh Bioscience HLC‐723G8
1. Introduction
Hemoglobinopathies encompass all genetic diseases of hemoglobin (Hb). These genetic disturbances either affect the structure (hemoglobin variants) or the expression (thalassemias) of the different hemoglobin fractions. Almost 7% of the world population carries a mutation in globulin genes, of which most remain asymptomatic.1 However, some of these mutations can cause clinically relevant anemia.
In the blood, hemoglobin circulates in the form of three physiological fractions that differ in their globin chain composition: the major fraction HbA0 (α2β2), the minor fraction HbA2 (α2δ2) and a small fraction HbF (α2γ2). Thalassemia syndromes are characterized by an altered synthesis ratio between the different globin chains that constitute these physiological hemoglobin fractions. α‐ and β‐thalassemias have clinical significance as the affected α‐ and β‐globin chains are the components of the main hemoglobin fraction HbA0. α‐thalassemia combines microcytic hypochromic anemia with low or normal levels of HbA2, while β‐thalassemia combines microcytic hypochromic anemia with high levels of HbA2 (heterozygous) or high levels of HbF (homozygous). Hemoglobin variants are characterized by an alteration of the structure of one of the hemoglobin fractions. This alteration results in different physicochemical properties of the affected fraction and can be detected by the presence of an abnormal, unphysiological hemoglobin fraction. Analytical screening for hemoglobinopathies therefore requires the separation of hemoglobin in its different physiological fractions with accurate measurement of HbA2 and HbF for thalassemia diagnosis, and sensitive detection of abnormal hemoglobin fractions for the diagnosis of hemoglobin variants.
Modern automated devices used to quantify the glycated form of Hb (HbA1c) in diabetes diagnosis and follow‐up are able to separate and quantitate the different hemoglobin fractions. This separation is achieved by electrophoresis or by high performance liquid chromatography (HPLC). Automated analyzers either measure HbA1c and screen for hemoglobinopathies in a single mode, or in two different dedicated modes on the same analyzer.
In the past decade, several studies evaluated and compared the performance of hemoglobin separation devices for hemoglobinopathy diagnosis.2, 3, 4, 5, 6 In these studies, hemoglobinopathy analysis was executed in the dedicated Hb‐pathy mode of the analyzers. However, the presence of a previously unknown hemoglobinopathy can be a coincidental finding in patients suspected of having diabetes during routine analysis in the HbA1c mode of the analyzer. Moreover, hemoglobin variants can interfere with HbA1c measurements, stressing the importance of their proper detection. Therefore, the evaluation of both Hb‐pathy mode and HbA1c mode of the analyzers is of clinical relevance.
As newer versions of some hemoglobin separation devices were recently introduced, it was the aim of the present study to compare four commercially available analyzers in their ability to detect hemoglobinopathies, both in the hemoglobinopathy and in the HbA1c mode, if applicable.
2. Materials and Methods
2.1. Samples
58 patient samples were tested: 32 samples containing a hemoglobin variant (9 HbA/S, 4 HbS/S, 9 HbA/E, 5 HbA/C, 2 HbA/D‐Punjab, 1 HbA/Lepore, 1 HbA/J‐Baltimore, 1 HbA/Muravera), 16 samples containing an aberrant value of HbA2 or HbF (of which 1 α‐thalassemia and 6 β‐thalassemia samples, all confirmed by molecular analysis) and 10 normal samples. Blood was collected in EDTA tubes. Normal samples were analyzed on fresh whole blood. All other samples were centrifuged (6 minutes at 1590 g) and red blood cell fractions were washed three times with NaCl 0.9% and stored at −80°C prior to analysis. All samples underwent only one thaw cycle before measurement. Furthermore, dedicated normal and abnormal quality controls for the specific mode of the specific analyzers were used for imprecision testing.
2.2. Analyzers
Four analyzers were compared: D‐100 (Bio‐Rad Laboratories, Hercules, CA, USA), HA 8180T (Menarini, Florence, Italy), HLC‐723G8 (Tosoh Bioscience, Tokyo, Japan) and Capillarys 2 Flex Piercing (Sebia, Lisses, France). These analyzers separate hemoglobin fractions either by high performance liquid chromatography (HPLC; D‐100, HA 8180, HLC‐723G8) or by capillary electrophoresis (CE; Capillarys 2 Flex Piercing; Table 1). All analyzers are able to quantify HbA1c. In order to fully separate different hemoglobin fractions, some manufacturers developed an extra hemoglobinopathy (Hb‐pathy) mode, which is the case for HLC‐723G8 and Capillarys 2. Table 1 summarizes the main characteristics of the four analyzers.
Table 1.
Characteristics of the four analyzers and their specific mode
| D‐100 | HA 8180T | HLC‐723G8 | Capillarys | |||
|---|---|---|---|---|---|---|
| HbA1c mode | Hb‐pathy mode | HbA1c mode | Hb‐pathy mode | |||
| Measurement principle | HPLC | HPLC | HPLC | HPLC | CE | CE |
| Throughput (tests/h) | 80 | 17 | 37 | 10 | 38 | 38 |
| HbF quantification | Yes | Yes | Yes | Yes | Yes | Yes |
| HbA2 quantification | No | Yes | No | Yes | Yes | Yes |
| Hb variant detection | Yes | Yes | Yes | Yes | Yes | Yes |
| Hb variant identification | Yes (S/C/E/D) | Yes (S/C/E/D) | Yes (S/C/D)a , b | Yes (S/C/D)a | No | Yes (S/C/E/D) |
| Hb variant quantification | Yes | Yes | No | Yes | Yes | Yes |
HbE co‐eluates with HbA2 which is indicated by an error flag.
Variants are not identified as D, S and C, but as HV0, HV1 and HV2 respectively.
2.2.1. D‐100
The D‐100 was released in Europe in 2015 as the successor of the Variant II and Variant II Turbo. Although this HPLC analyzer detects, identifies and quantifies several variants and HbF, its initial purpose is to quantify HbA1c for diabetes diagnosis and follow‐up. In contrast to HbA1c, values for HbF and for variants are not calibrated. A dedicated Hb‐pathy mode for the D‐100 is still under development. Therefore, until the dedicated Hb‐pathy mode for the D‐100 is available, Bio‐Rad Laboratories propose the Variant II HPLC analyzer for hemoglobinopathy screening, but this analyzer was not tested in the current study. HbA2 is not measured on the D‐100 (see Table 1).
2.2.2. HA 8180T
The HA 8180T was released in Europe in 2015 as the successor of the HA 8160. This HPLC analyzer is able to detect, identify and quantify hemoglobin variants and measure HbA1c, HbA0, HbF and HbA2 in a single mode. In addition to the HA 8180T, Menarini also distributes the HA 8180V which operates in either a fast mode for HbA1c quantification or a variant mode for HbA1c, HbF and variant quantification. The HA 8180V, however, was not tested in the current study. One notable improvement of the HA 8180T compared to the former HA 8160 is the ability to identify HbS and HbC fraction in a separate window.
2.2.3. HLC‐723G8
HLC‐723G8 is a HPLC analyzer that operates in two modes and was released in Europe in 2007. One mode is dedicated to measurement of HbA1c and detection of Hb variants and is called “variant mode”. The other modus is dedicated to the measurement of HbA2 and the identification of the most common Hb variants and is called “beta thalassemia mode”. In order to avoid confusion, the “variant mode” on HLC‐723G8 was named “HbA1c mode” and the “beta thalassemia mode” was named “Hb‐pathy mode” throughout the manuscript.
2.2.4. Capillarys 2 Flex Piercing
The Capillarys 2 Flex Piercing was released in Europe in 2011 as the successor of the Capillarys 2. This CE analyzer is equipped with eight parallel capillaries enabling multiple simultaneous analyses. One notable improvement compared to the former Capillarys 2, is the availability of a cap piercing function, thereby avoiding the necessity to open the tubes manually prior to analysis. Moreover, Capillarys 2 Flex Piercing has a mixing system consisting of seven sample inversions, avoiding manual mixing.
For Hb separation, Capillarys operates in two modes. One mode is dedicated to HbA1c measurement, but HbF and HbA2 are also measured. Variants can be detected and quantified, but are not identified. The other mode is specially developed to separate different Hb variants, in order to correctly identify and quantify them. As with HLC‐723G8, these modes were named “HbA1c mode” and “Hb‐pathy mode” respectively throughout the manuscript. Variability between the eight capillaries was assessed prior to this study and proved to be lower than 3% for both the HbA1c and the Hb‐pathy mode (data not shown).
Separation plots of the different analyzers are exemplified in Figure 1, demonstrating the results for a heterozygous HbS sample.
Figure 1.
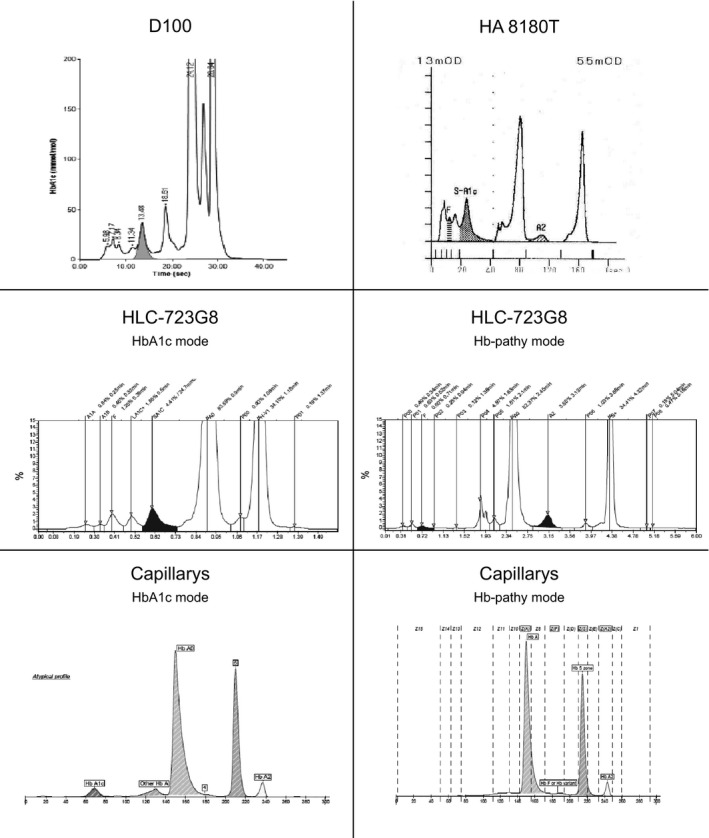
Example of HbA/S on the different analyzers
2.3. Analytical performances
2.3.1. Imprecision
Imprecision was measured by analyzing the dedicated normal and abnormal quality controls for the specific mode of the specific analyzer on 10 different days. As the Capillarys does not report a value for HbF on quality control materials, two blood samples with respectively a low and a high value for HbF were analyzed twice daily over 5 days to asses imprecision of this parameter in the Hb‐pathy mode of this analyzer.
2.3.2. Correlation of HbF and HbA2
Correlation of HbF and HbA2 measurements with the reference comparator was tested on the 26 samples without a Hb variant, since Hb variants can interfere with HbA2 values on some analyzers. The mean values of HbF and HbA2 on the different analyzers, measured in the Hb‐pathy mode, were used as reference comparator.
2.3.3. Sensitivity for β‐thalassemia
A high HbA2 value (i.e. >3.5%) is used in the detection of β‐thalassemia minor. In order to evaluate the sensitivity of the different analyzers in detecting β‐thalassemia minor, HbA2 of six heterozygote β‐thalassemia samples was measured to verify whether values exceeded the cut‐off level for each sample.
2.3.4. Sensitivity for α‐thalassemia
In contrast to β‐thalassemia, α‐thalassemia has no specific hallmark on HPLC or CE analysis as normal levels of HbA2 do not exclude its presence. Therefore, analytical sensitivity for α‐thalassemia could not be assessed. When microcytic normochromic anemia cannot be explained by iron deficiency in a patient with a normal hemoglobin separation pattern, molecular analysis for α‐thalassemia detection is required.
2.3.5. Sensitivity for Hb variants
The 32 samples of patients with a known Hb variant were analyzed on the different analyzers and the detection and identification of the different types of variants was studied. Because of insufficient sample volume, not all samples were measured on all modes of the different analyzers. As a consequence, the number of analyzed samples was 28 for the HbA1c mode on the HLC‐723G8, 25 for the HbA1c mode on the Capillarys and 30 for the D‐100 (Table 5).
Table 5.
Detection of Hb variants in known samples
| Genotype group | # | D‐100 | HA 8180T | HLC‐723G8 | Capillarys | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c mode | Hb‐pathy mode | HbA1c mode | Hb‐pathy mode | ||||||||||
| Detection | ID | Detection | ID | Detection | ID | Detection | ID | Detection | ID | Detection | ID | ||
| HbA/S | 9 | 9/9 | S | 9/9 | S | 9/9 | NA | 9/9 | S | 7/7 | NA | 9/9 | S |
| HbS/S | 4 | 4/4 | S | 4/4 | S | 4/4 | NA | 4/4 | S | 3/3 | NA | 2/4 | S |
| 2/4 | “zone 2” a | ||||||||||||
| HbA/E | 9 | 9/9 | E | 9/9 | E | 9/9 | NA | 8/9 | A2 | 8/8 | NA | 9/9 | E |
| 1/9 | “P06” | ||||||||||||
| HbA/C | 5 | 4/4 | C | 5/5 | C | 3/3 | NA | 5/5 | C | 3/3 | NA | 4/5 | C |
| 1/5 | NI | ||||||||||||
| HbA/D‐Punjab | 2 | 1/1 | D | 2/2 | D | 1/1 | NA | 2/2 | D | 2/2 | NA | 2/2 | D |
| HbA/Lepore | 1 | 1/1 | “unknown” | 0/1 | A0 | 1/1 | A2 | 1/1 | D | ||||
| HbA/J‐Baltimore | 1 | 1/1 | “unknown” | 1/1 | P5 | 0/1 | A0 | 1/1 | “P07” | 1/1 | NA | 1/1 | “Z12 zone” |
| HbA/Muravera | 1 | 1/1 | “unknown” | 1/1 | E | 0/1 | A0 | 1/1 | A2 | 1/1 | NA | 1/1 | D |
ID, “identification”; NA, “not applicable”; NI, “not identified”.
Underlined+bold: not detected; Italic+bold: incorrect identification of Hb C, D, E or S (aberrant fraction detected, but incorrectly identified).
Correct identification after mixture with normal blood sample.
2.4. Statistics
Statistical analysis was performed using Microsoft Excel 2013 (Microsoft Corp., Redmond, WA, USA) and MEDCALC version 12.6 (MedCalc Software bvba, Mariakerke, Belgium). Imprecision was calculated using the coefficient of variation (CV). Correlation between methods was measured by Passing‐Bablok regression to determine proportional and constant bias via, respectively, slopes and intercepts of the regression lines of pairwise assay comparisons.
3. Results
3.1. Imprecision
Imprecision was <10% for both HbF and HbA2 in all modes of all analyzers (Table 2). The International Committee for Standardization in Haematology (ICSH) recommends a maximum imprecision of 2% for HbA2 measurements.7 Only the HA 8180T obtained such low imprecision on both measured samples. For HbA1c mode on Capillarys, imprecision of HbF could not be measured because both normal and abnormal quality control material of the manufacturer does not contain a detectable level of HbF. For both D‐100 and HbA1c mode of HLC‐723G8, imprecision of HbA2 was not measured because both analyzers do not report HbA2.
Table 2.
Analytical imprecision values for HbF and HbA2
| D‐100 | HA 8180T | HLC‐723G8 | Capillarys | |||
|---|---|---|---|---|---|---|
| HbA1c mode | Hb‐pathy mode | HbA1c mode | Hb‐pathy mode | |||
| HbF | ||||||
| Normal | 9.2% (1.0) | 1.7% (1.8) | 5.0% (0.6) | 1.9% (2.5) | NA | 9.0% (0.7) |
| Abnormal | 5.2% (1.1) | 1.2% (5.6) | 4.0% (1.0) | 1.6% (7.2) | NA | 2.2% (9.4) |
| HbA2 | ||||||
| Normal | NA | 1.8% (2.9) | NA | 5.0% (2.3) | 1.7% (2.4) | 3.3% (2.6) |
| Abnormal | NA | 1.3% (5.6) | NA | 1.5% (5.6) | 2.9% (2.3) | 1.2% (7.4) |
NA, not applicable.
Data are reported as CV% (mean).
3.2. Correlation of HbF and HbA2
The correlations between values obtained with individual analyzers and the mean reference comparator value are shown in Table 3, while correlation plots are provided in the Supplementary Information. For all analyzers, both the measurements of HbF and HbA2 showed small but statistically significant proportional and constant biases when compared to the reference comparator. For the D‐100, HA8180T, HLC‐723G8 and the Hb‐pathy mode of the Capillarys, these biases were too small to be of clinical relevance. A high proportional and constant bias was demonstrated for HbF measured in the HbA1c mode of the Capillarys. In this mode, for 20 out of the 26 measured samples, no value for HbF was reported as the result was below the detection limit (<0.5%). For two of the six samples for which a value was reported, the result was significantly higher compared to the Hb‐pathy mode of the Capillarys and as compared to the gold standard (sample 1: 6.4%, 1.9% and 2.2% respectively; sample 2: 8.1%, 2.8% and 2.6% respectively).
Table 3.
Correlation of HbF and HbA2
| D‐100 | HA 8180T | HLC‐723G8 | Capillarys | |||
|---|---|---|---|---|---|---|
| HbA1c mode | Hb‐pathy mode | HbA1c mode | Hb‐pathy mode | |||
| HbF | ||||||
| Slope (95% CI) | 1.4 (1.3 to 1.6) | 1.4 (1.2 to 1.5) | 1.3 (1.2 to 1.4) | 1.3 (1.2 to 1.4) | 7.1 | 1.4 (1.2 to 1.7) |
| Intercept (95% CI) | 0.2 (0.1 to 0.4) | −0.3 (−0.5 to −0.2) | 0.0 (−0.1 to 0.1) | −0.1 (−0.2 to 0.0) | −11.2 | −1.0 (−1.4 to −0.6) |
| Correlation coefficient (R) | .972 | .969 | .821 | .979 | .334 | .179 |
| HbA2 | ||||||
| Slope (95% CI) | NA | 0.8 (0.7 to 0.8) | NA | 1.3 (1.2 to 1.3) | 1.0 (0.9 to 1.0) | 1.0 (1.0 to 1.0) |
| Intercept (95% CI) | NA | 0.7 (0.6 to 0.8) | NA | −0.8 (−0.9 to −0.7) | −0.1 (−0.3 to 0.0) | 0.0 (−0.1 to 0.1) |
| Correlation coefficient (R) | NA | .996 | NA | .997 | .997 | .997 |
NA, not applicable.
italic+bold: 1 not included in 95% CI of Slope and 0 not included in 95% CI of Intercept.
3.3. Sensitivity for β‐thalassemia
The clinical significance of the proportional and constant biases for HbA2 demonstrated in the correlation study was assessed by evaluating the sensitivity for detecting β‐thalassemia minor, using a cut‐off of 3.5% (Table 4). Only the Hb‐pathy mode on Capillarys 2 was able to detect all 6 β‐thalassemia samples. HA 8180T, Hb‐pathy mode on HLC‐723G8 and HbA1c mode on Capillarys detected all samples but one. However, the sample with discrepant results demonstrated a value close to the cut‐off on all devices. Of note, values for HbA2 were consistently lower in het HbA1c mode as compared to the Hb‐pathy mode on the Capillarys.
Table 4.
Measurement of HbA2 in known heterozygote β‐thalassemia samples
| D‐100 | HA 8180T | HLC‐723G8 | Capillarys | |||
|---|---|---|---|---|---|---|
| HbA1c mode | Hb‐pathy mode | HbA1c mode | Hb‐pathy mode | |||
| Samples | ||||||
| 1 | NA | 4.3 | NA | 4.9 | 4.2 | 4.5 |
| 2 | NA | 3.4 | NA | 3.4 | 3.2 | 3.6 |
| 3 | NA | 5.3 | NA | 6.6 | 5.4 | 5.8 |
| 4 | NA | 4.4 | NA | 5.2 | 4.4 | 4.7 |
| 5 | NA | 5.2 | NA | 6.5 | 5.5 | 5.7 |
| 6 | NA | 4.7 | NA | 5.6 | 4.8 | 5.2 |
| Total | NA | 5/6 | NA | 5/6 | 5/6 | 6/6 |
NA, not applicable.
italic+bold: lower than 3.5%.
3.4. Sensitivity for Hb variants
Table 5 summarizes the results for the 32 samples with known Hb variant. The D‐100 detected all variants as an aberrant peak, and all Hb C, D, E and S were correctly identified. The HA 8180T detected all variants but one (Hb Lepore) as an aberrant peak, and all Hb C, D, E and S were correctly identified. The Hb Lepore was missed as this variant co‐elutes with HbA0 on this device. Two rare variants (Hb Baltimore and Hb Muravera) were missed on the HbA1c‐mode of the HLC‐723G8, but all Hb C, D, E and S were detected. The Hb‐pathy mode of the same device detected all variants and all Hb C, D and S were correctly identified. HbE, Hb Lepore and Hb Muravera co‐eluted with HbA2 in the Hb‐pathy mode of the HLC‐723G8, but their presence could be suspected by the extreme high value for HbA2 (>10%). Moreover, co‐elution of HbE with HbA2 is indicated by an error flag. In the HbA1c‐mode of the Capillarys, all measured variants were detected as an aberrant peak. In this mode, variants are never identified. Likewise, all variants were detected in the Hb‐pathy mode of this device but two HbS out of four homozygous samples and one HbC out of five heterozygous samples did not migrate in the appropriate identification zone. In case of the unidentified homozygous HbS samples, correct identification was obtained after 1/1 mixing with a normal whole blood sample, as recommended by the manufacturer. In case of the unidentified heterozygous HbC sample, none of the identification zones were named by the software for an unknown reason. In such situation the manufacturer recommends to repeat the analysis, which was not done in this study.
4. Discussion
Automated analyzers either measure HbA1c and screen for hemoglobinopathies in a single mode, or in two different dedicated modes on the same analyzer. In the latter case, several manipulations of the analyzer need to be performed when changing between different modes, including switching of reagents and performing quality controls. Longer separation procedures entail a more distinctive pattern of Hb fractions, at the expense of longer turnaround times. When separate modes are present, focus in the HbA1c mode lies on a short turnaround time resulting in a lower separation capacity, while the Hb‐pathy mode focusses on separation capacity inducing longer turnaround times. Devices that work in a single mode do not achieve the short turnaround time of a dedicated HbA1c mode, nor the separation capacity of a dedicated Hb‐pathy mode, but they try to find the ideal compromise between both, thereby avoiding the need to switch between modes.
The present study compares four hemoglobin separation devices in their ability to detect and identify hemoglobinopathies. As two of these analyzers, the HA8180T and the D100, only recently appeared on the market, their analytical performance has not been evaluated before. Both analyzers operate in a single mode. The two other analyzers, the HLC‐723G8 and the Capillarys, operate in two modes. The good analytical performance of the dedicated Hb‐pathy mode of these latter analyzers has been well established before,2, 3, 4, 5, 6 but whether their HbA1c mode is sensitive enough to be used as a screening tool for the presence of hemoglobinopathies remained to be investigated.
4.1. D‐100
The D‐100 shows a low imprecision and a clinically acceptable correlation with other devices for HbF. Although the main objective of the D‐100 is HbA1c analysis and not hemoglobinopathy analysis, thereby operating in a single mode, all tested Hb variants were detected and the most common ones (Hb C, D, E and S) were correctly identified. The major drawback of this analyzer in its present stage is the lack of HbA2 measurement, making it unsuitable to detect β‐thalassemia.
4.2. HA 8180T
HA 8180T operates in a single mode for both HbA1c measurement and hemoglobinopathy detection, making it a versatile and user‐friendly device for routine laboratory use. It measures both HbF and HbA2 and has a low imprecision and acceptable correlation for both parameters. The HA 8180T was the only evaluated device that obtained imprecision levels for HbA2 below 2% on both measured samples, as recommended by the ICSH.7 Based on a cut‐off value for HbA2 of 3.5%, five out of six β‐thalassemia cases were detected. The missed case demonstrated a HbA2 value around the chosen cut‐off. This borderline value for HbA2 was measured in a sample from a 78‐year old female patient and can be explained by the fact that she was a known β‐thalassemia patient and received red blood cell transfusions in the weeks preceding blood sampling, lowering HbA2 levels. Indeed, at diagnosis higher values for HbA2 (4.7%) had been measured in this patient. Good laboratory practice implies that each laboratory should validate a chosen cut‐off or ideally establishes its own reference range. This was not done in the present study as we chose a ‘universal’ cut‐off for all analyzers. A reference range should be determined by analyzing a large number of samples from healthy controls to verify whether the upper cut‐off limit could be lowered to increase sensitivity for β‐thalassemia. This analyzer is able to detect all measured Hb variants, except one. The more seldom Hb variant HbA/Lepore co‐eluted with HbA0 and was therefore not detected. However in case of Hb Lepore, no functional delta chain can be produced by the abnormal chromosome, resulting in an underproduction of HbA2. The concentration of HbA2 is therefore decreased to approximately 50% of normal.8 In this case, concentration of HbA2 was undetectable, which would eventually lead to suspicion of a hemoglobinopathy prompting further molecular analysis.
4.3. HLC‐723G8
HLC‐723G8 operates in two modes, one for HbA1c and one for hemoglobinopathies. Switching between modes implies changing the analytical column and liquid reagents and performing separate quality controls. The HbA1c mode demonstrates a low imprecision and a clinically acceptable correlation for HbF. HbA2 is not quantified in this mode, so β‐thalassemia cannot be detected. Two Hb variants were missed in the HbA1c mode: the rare variants Hb J‐Baltimore and Hb Muravera. In the dedicated Hb‐pathy mode, all variants were correctly detected. This Hb‐pathy mode of HLC‐723G8 has a low imprecision and acceptable correlation for both HbF and HbA2. Users should be aware that HbE is not identified as a separate peak but co‐elutes with HbA2. As for the HA 8180T, one out of six β‐thalassemias was missed because of a value close to the cut‐off.
4.4. Capillarys 2 Flex Piercing
The Capillarys also operates in a HbA1c mode and a Hb‐pathy mode. Switching between modes implies changing liquid reagents and performing separate quality controls. A low imprecision was demonstrated for HbA2 in the HbA1c mode. Correlation of HbF with the gold standard was poor in this mode, although admittedly, the number of samples for which a result was reported was low. In contrast, correlation of HbF was much better in the Hb‐pathy mode. The Capillarys demonstrated the best correlation for HbA2 of all evaluated analyzers, both in the HbA1c and in the Hb‐pathy mode. All variants were detected as a separate peak in both the HbA1c and the Hb‐pathy mode. An advantage of this device is the large number of possible identifications that is given for each aberrant peak in the software, according to its migration zone. The Capillarys software uses normal Hb fractions to mark the different identification zones, and in case of homozygous HbS these Hb fractions are often low or undetectable, preventing a correct identification. In such cases the sample should be repeated after 1/1 mixing with a normal whole blood sample. The Hb fraction of the normal sample will enable a correct identification of the migration zones. This phenomenon was observed with two out of four homozygous HbS samples in this study. In contrast to the other analyzers, the Hb‐pathy mode detected all six β‐thalassemias.
5. Conclusion
Based on this evaluation of four hemoglobin separation devices, we conclude that HbA2 determination is adequate on all evaluated devices, but this analysis is not yet available on the D‐100, making it inappropriate for β‐thalassemia screening. D‐100, HA8180T, HLC‐723G8 and the Capillarys are all able to detect the most common important Hb variants (Hb C, D, E and S), although not all of these Hb variants were correctly identified. More rare Hb variants can be missed by certain analyzers as they co‐elute with HbA0. The HbA1c mode of the Capillarys correctly detected all measured hemoglobin variants and can therefore be used as a hemoglobinopathy screening device. This was also the case for the most common important Hb variants on the HbA1c mode of the HLC‐723G8. This study stresses the importance for individual laboratories to critically and comprehensively explore the advantages and drawbacks of their hemoglobin separation analyzer and its different modes in the diagnosis of hemoglobinopathies.
Supporting information
Acknowledgments
The authors would like to thank Dr Diane Maisin from the Cliniques Universitaires Saint‐Luc, Brussels, Belgium for kindly providing samples containing Hb variants, and Cedric Vandemergel, Karen Van Maele, Nick Verplancke and Sarah Gils for their dedicated assistance in collecting the samples and performing the analyses.
Degandt S, Coens R, Cauwelier B, Devos H, Langlois M, Emmerechts J. Evaluation of four hemoglobin separation analyzers for hemoglobinopathy diagnosis. J Clin Lab Anal. 2018;32:e22224 10.1002/jcla.22224
References
- 1. Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bull WHO. 2001;79:1‐15. [PMC free article] [PubMed] [Google Scholar]
- 2. Oyaert M, Van Laer C, Claerhout H, et al. Evaluation of the Sebia Minicap Flex Piercing capillary electrophoresis for hemoglobinopathy testing. Int J Lab Hematol. 2015;37:420‐425. [DOI] [PubMed] [Google Scholar]
- 3. Van Delft P, Lenters E, Bakker‐Verweij M, et al. Evaluating five dedicated automatic devices for haemoglobinopathy diagnostics in multi‐ethnic populations. Int J Lab Hematol. 2009;31:484‐495. [DOI] [PubMed] [Google Scholar]
- 4. Sangkitporn S, Sangkitporn SK, Tanjatham S, et al. Multicenter validation of fully automated capillary electrophoresis method for diagnosis of thalassemias and hemoglobinopathies in Thailand. Southeast Asian J Trop Med Public Health. 2011;42:1224‐1232. [PubMed] [Google Scholar]
- 5. Cotton F, Malaviolle X, Vertongen F, Gulbis B. Evaluation of an automated capillary electrophoresis system in the screening for hemoglobinopathies. Clin Lab. 2009;55:217‐221. [PubMed] [Google Scholar]
- 6. Greene DN, Pyle AL, Chang JS, Hoke C, Lorey T. Comparison of Sebia Capillarys Flex capillary electrophoresis with the BioRad Variant II high pressure liquid chromatography in the evaluation of hemoglobinopathies. Clin Chim Acta. 2012;413:1232‐1238. [DOI] [PubMed] [Google Scholar]
- 7. Stephens AD, Colah R, Fucharoen S, et al. ICSH recommendations for assessing automated high‐performance liquid chromatography and capillary electrophoresis equipment for the quantitation of HbA2. Int J Lab Hematol. 2015;37:577‐582. [DOI] [PubMed] [Google Scholar]
- 8. Clarke GM, Higgins TN. Laboratory investigation of hemoglobinopathies and thalassemias: review and update. Clin Chem. 2000;46:1284‐1290. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


