Abstract
Background
Copeptin, also termed C‐terminal pre‐pro‐vasopressin or CTproAVP, mirrors endogenous vasopressin (anti‐diuretic hormone, ADH) activity and might thereby serve as a biomarker reflecting the biological stress level. We therefore hypothesized that copeptin plasma concentrations are associated with disease severity in critically ill patients and could predict mortality.
Methods
We analyzed plasma copeptin levels in a prospective, single‐center, observational study comprising 218 critically ill patients at admission to the medical intensive care unit (ICU). Mortality was assessed during a 2‐year observational follow‐up period.
Results
Copeptin plasma levels were significantly elevated in critically ill patients (n = 218) at ICU admission, as compared with 66 healthy controls. Neither sepsis as the cause of critical illness nor pre‐existing metabolic disorders (type 2 diabetes, obesity) were found to influence copeptin levels. On the contrary, plasma copeptin was closely associated with disease severity (eg APACHE‐II score) and correlated with biomarkers of inflammation, renal failure, metabolism, vascular tone, and tissue perfusion. Elevated copeptin levels at ICU admission predicted short‐term and long‐term mortality.
Conclusions
Copeptin plasma concentrations are significantly elevated in critically ill patients, correlate with disease severity and predict ICU and long‐term outcome. Thus, copeptin could be a promising tool for prognostication and management of critically ill patients.
Keywords: anti‐diuretic hormone, arginine vasopressin, C‐terminal pre‐pro‐vasopressin, ICU, organ failure, prognosis, sepsis, vasopressin
1. INTRODUCTION
Critical illness is a disease condition comprising a remarkable heterogeneity of underlying insults, such as shock, infections, metabolic derangements, burns, trauma, or severe blood loss. Despite these broad range of different initial causes, the body's response to such threats is relatively uniform, and severity of critical illness is determined by the degree of systemic inflammation and subsequent hemodynamic changes, the extent of biological stress, the resultant organ failure(s) and, ultimately, death.1 Circulating mediators of such core pathways could serve as prognostic biomarkers in critical care medicine.2
The activation of neuroendocrine pathways, in particular the hypothalamic‐pituitary‐adrenal axis (eg corticotropin‐releasing hormone [CRH]—adrenocorticotropic hormone [ACTH]—cortisol) and vasopressin release, is a characteristic response to biological stress.3 Vasopressin, also known as arginine vasopressin (AVP) or anti‐diuretic hormone (ADH), is secreted from the pituitary gland in response to hypovolemia, hypoxia, acidosis, and changes in plasma osmolality.4, 5 Vasopressin is co‐released with neurophysin II and the C‐terminal part of the precursor pre‐pro‐vasopressin, termed CTproAVP or copeptin. In contrast to vasopressin, which has a short half‐life in blood, is bound to a great extent to platelets and is biochemically instable, copeptin is a stable protein in the circulation and reliably mirrors biologically functional vasopressin in both healthy and acutely ill patients.5, 6, 7, 8
Due to its biochemical properties as a potential biomarker, copeptin has been investigated in different acute and chronic diseases. In line with the biological functions of vasopressin, such as water reabsorption, regulating osmolality, vasoconstriction, and central nervous effects, copeptin levels were found elevated in patients with diabetes insipidus, metabolic diseases, chronic kidney diseases, and cardiovascular disorders.4, 9, 10 In cohorts of patients with acute illnesses, copeptin levels were increased, especially in patients with sepsis, shock, heart failure, and respiratory distress.6, 10, 11, 12, 13 Some of these studies suggested that elevated copeptin plasma concentrations might indicate an increased short‐term mortality risk.6, 11, 12, 13 The favourable biochemical properties of copeptin, its implication in pathways of biological stress and the reported association with short‐term outcome in acute illness prompted us to investigate plasma copeptin concentrations in a large, prospectively enrolled cohort of critically ill medical patients.
2. MATERIALS & METHODS
2.1. Study design and patient characteristics
Critically ill patients were included at admission to the medical intensive care unit (ICU) at the University Hospital Aachen, Germany. Patients, who were admitted for post‐interventional observational stay or underwent an elective procedure, were excluded.14 The local ethics committee approved our study in accordance to the ethical standards laid down in the Declaration of Helsinki (reference number EK 150/06). Informed consent was obtained from each participant or their spouse. The patients were categorized as sepsis and non‐sepsis according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3),15 and were treated following the current guidelines for treatment of sepsis (Surviving Sepsis Campaign).16 As a healthy control group, we analyzed blood donors with normal blood counts, normal values of liver enzymes and a negative serology for viral hepatitis and HIV.17
In order to determine long‐term outcome, we contacted the patients, their relatives and/or the general practitioner in approximately 6‐months intervals after discharge from the hospital for 2 years.17
2.2. Measurements of copeptin and other experimental markers
Blood samples were collected at the time of admission (before specific therapeutic measures), centrifuged, and serum and plasma samples were stored at −80°C. Copeptin was measured from EDTA plasma using a commercially available fluorescent immunoassay (BRAHMS GmbH/ThermoFischer Scientific, Henningsdorf, Germany) following the manufacturer's protocol.
The adipocytokines resistin, adiponectin, leptin, and retinol‐binding protein 4 (RBP4) have been quantified from serum of the same patients, as previously described.18, 19, 20, 21
2.3. Statistical analysis
All copeptin plasma concentrations are presented as median and range. The Mann‐Whitney U test was used to test differences between two groups, correlations were tested according to the Spearman's rank correlation method. All values, including outside values as well as far out values, were included. P‐values less than .05 were considered as statistically significant.
The prognostic value of copeptin on the outcome was evaluated by Cox regression models. Survival curves were generated by Kaplan‐Meier analyses with a copeptin cut‐off level calculated via the Youden‐Index.22 All analyses were performed with IBM SPSS Statistics (SPSS; Chicago, Illinois).
3. RESULTS
3.1. Copeptin plasma levels are significantly elevated in critically ill patients as compared with healthy controls, independent of sepsis
Copeptin plasma levels were significantly elevated in a large cohort of 218 critically ill medical patients (median 46.4 pmol/L, range 4.8‐791.4, Table 1) at admission to the ICU, as compared with 66 healthy controls (median 4.7 pmol/L, range 4.6‐8.5, P < .001; Figure 1A). Within the cohort of ICU patients, copeptin levels did not differ between patients with sepsis (n = 145, median copeptin 45.9 pmol/L, range 4.8‐791.4) and patients without sepsis (n = 73, median 46.8 pmol/L, range 4.8‐496.7; Figure 1B). Typical sites of infection in sepsis were pneumonia, abdominal, and urogenital tract, while non‐sepsis causes of critical illness included, among others, cardiopulmonary diseases, acute pancreatitis, and decompensated liver cirrhosis (not shown). Notably, plasma copeptin concentrations were associated with disease severity, as expressed by the correlations between copeptin and ICU scores, such as the Acute Physiology And Chronic Health Evaluation‐II (APACHE‐II) and the Simplified Acute Physiology Score 2 (SAPS2) (Table 2). Patients with a high APACHE‐II score (above 10) had significantly higher copeptin levels than patients with an APACHE‐II score below or equal to 10 (Figure 1C).
Table 1.
Baseline patient characteristics and copeptin plasma measurements
| Parameter | All patients |
|---|---|
| Number | 218 |
| Sex (male/female) | 133/85 |
| Age median (range) [y] | 64 (18‐90) |
| APACHE‐II score median (range) | 18 (2‐43) |
| ICU days median (range) | 7 (1‐137) |
| Death during ICU n(%) | 48 (22%) |
| Death during follow‐up (total) n(%) | 87 (40%) |
| Mechanical ventilation n(%) | 145 (67%) |
| Pre‐existing diabetes n(%) | 66 (30%) |
| BMI median (range) [m²/kg] | 26 (15.3‐86.5) |
| WBC median (range) [×10³/μL] | 13.0 (0‐208) |
| CRP median (range) [mg/dL] | 100.5 (0.2‐230) |
| Procalcitonin median (range) [μg/L] | 0.7 (0‐207.5) |
| Creatinine median (range) [mg/dL] | 1.3 (0.1‐15) |
| INR median (range) | 1.16 (0‐13) |
| Copeptin median (range) [pmol/L] | 46.4 (4.8‐791.4) |
For quantitative variables, median and range (in parenthesis) are given.
APACHE, acute physiology and chronic health evaluation; BMI, body mass index; CRP, C‐reactive protein; ICU, intensive care unit; INR, international normalized ration; WBC, white blood cell.
Figure 1.
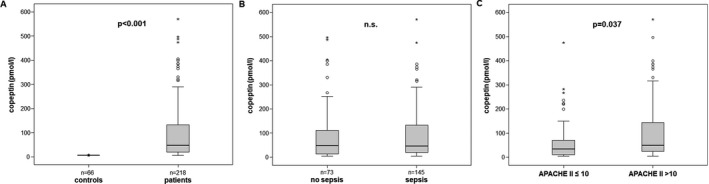
Copeptin levels in critically ill patients. A, Copeptin plasma concentrations are significantly elevated in critically ill patients compared with healthy controls. B, Copeptin levels do not differ between ICU patients with or without sepsis. C, High disease severity, as defined by an APACHE II score above 10, is associated with elevated plasma copeptin. P‐values (U‐test) are given in the figure
Table 2.
Correlations with copeptin plasma concentrations at ICU admission (Spearman rank correlation test, only significant results are shown)
| ICU patients | ||
|---|---|---|
| Parameters | r | P |
| Disease severity | ||
| APACHE II score | .218 | .002 |
| SAPS2 score | .450 | <.001 |
| Inflammation | ||
| Procalcitonin | .164 | .039 |
| suPAR | .200 | .018 |
| Interleukin‐10 | .276 | .004 |
| Renal function | ||
| Creatinine | .631 | <.001 |
| GFR (creatinine) | −.664 | <.001 |
| Cystatin C | .473 | <.001 |
| GFR (cystatin C) | −.518 | <.001 |
| Urea | .566 | <.001 |
| Uric acid | .320 | <.001 |
| Vascular tone & perfusion | ||
| CT‐proET‐1 | .419 | <.001 |
| ADMA | .250 | .001 |
| SDMA | .538 | <.001 |
| NT‐proCNP | .575 | <.001 |
| NT‐proBNP | .492 | <.001 |
| Lactate | .157 | .021 |
| pH | −.301 | <.001 |
| Metabolism | ||
| Blood glucose | .241 | <.001 |
| Resistin | .286 | .031 |
| RBP4 | .485 | <.001 |
| Visfatin | .482 | <.001 |
ADMA, asymmetric dimethylarginine; APACHE, acute physiology and chronic health evaluation; CT‐proET‐1, C‐terminal proendothelin‐1; GFR, glomerular filtration rate; NT‐proBNP, amino‐terminal pro‐B‐type natriuretic peptide; NT‐proCNP, amino‐terminal pro‐C‐type natriuretic peptide; RBP4, retinol binding protein 4; SAPS, Simplified Acute Physiology Score; SDMA, symmetric dimethylarginine; suPAR, soluble urokinase plasminogen activator receptor.
3.2. Diabetes or obesity did not impact copeptin levels at admission to the ICU
Copeptin has been associated with glucose abnormalities, insulin resistance, type 2 diabetes, and the metabolic syndrome.9, 10 We therefore assessed whether these comorbidities impacted copeptin levels at ICU admission. Interestingly, patients with or without concomitant type 2 diabetes did not differ regarding their copeptin levels, although diabetic patients showed a tendency towards higher copeptin plasma concentrations (median 54.6 pmol/L in diabetics vs. median 44.3 pmol/L in non‐diabetics, not significant; Figure 2A). However, we observed significant correlations (Table 2) between copeptin and blood glucose as well as retinol‐binding protein 4 (RBP4), a marker of insulin resistance.20
Figure 2.
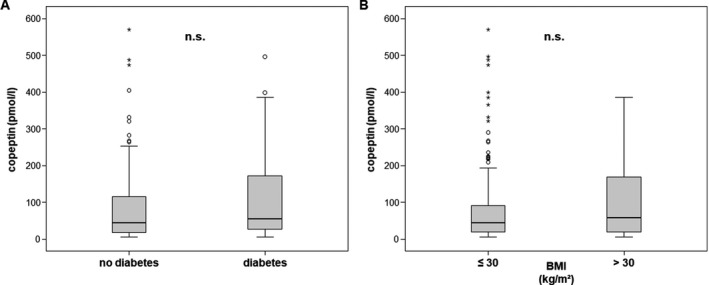
Impact of metabolic comorbidities on copeptin levels. Copeptin plasma concentrations did not differ between ICU patients with or without pre‐existing type 2 diabetes (A) or obesity, as defined by a body‐mass index (BMI) above 30 kg/m² (B)
A similar observation was found regarding obesity. Patients with pre‐existing obesity, defined as a body mass index above 30 kg/m², showed a slight, but insignificant trend towards higher copeptin levels at ICU admission (median 57.5 pmol/L vs median 43.7 pmol/L in non‐obese patients, not significant). However, copeptin plasma concentrations correlated with the adipose tissue related factors (“adipocytokines”) resistin and visfatin (Table 2).
3.3. Copeptin levels are correlated with biomarkers of renal failure and metabolic disturbances in critically ill patients
As copeptin mirrors endogenous vasopressin levels, and thereby its hemodynamic and osmoregulatory functions,23 we hypothesized that copeptin plasma concentrations might be associated with biomarkers of organ function, tissue perfusion and metabolism. In fact, copeptin was closely correlated with biomarkers reflecting renal function, inflammation, vascular tone, and tissue perfusion (Table 2). The association between copeptin levels and renal failure is well established.4 While copeptin concentrations were closely correlated with creatinine, cystatin C or urea, patients that developed subsequent renal failure and required renal replacement therapy at the ICU had significantly elevated circulating copeptin levels at ICU admission already (median copeptin 33.9 pmol/L in patients without vs median 91.2 pmol/L in patients with subsequent renal replacement therapy, P < .001).
Regarding vasculature and tissue perfusion, copeptin levels correlated with C‐terminal proEndothelin‐1 (CT‐proET‐1), the circulating precursor protein of the vasoconstrictor Endothelin 1,22 asymmetric and symmetric dimethylarginine, two regulators of the endothelial nitric oxide pathways,14, 24 and N‐terminal pro‐C‐type natriuretic peptide (NT‐proCNP, Table 2), a paracrine molecule synthesized in the vasculature with vasorelaxant effects.25
3.4. High copeptin plasma concentrations at ICU admission are associated with adverse prognosis
By categorizing our critically ill patients into patients that survived ICU treatment (170/218, 78%) and patients that died during critical illness (48/218, 22%), we found significantly elevated copeptin plasma levels at the time‐point of ICU admission in the patients that subsequently passed away at the ICU (median copeptin 39.6 pmol/L in ICU survivors vs. median 75.8 pmol/L in ICU deaths, P = .007; Figure 3A). We next calculated the optimal cut‐off value of copeptin for predicting survival using the Youden index.26 In fact, patients with copeptin levels above 65 pmol/L showed significantly decreased survival rates (P = .009), as displayed by Kaplan‐Meier curve analysis (Figure 3B).
Figure 3.
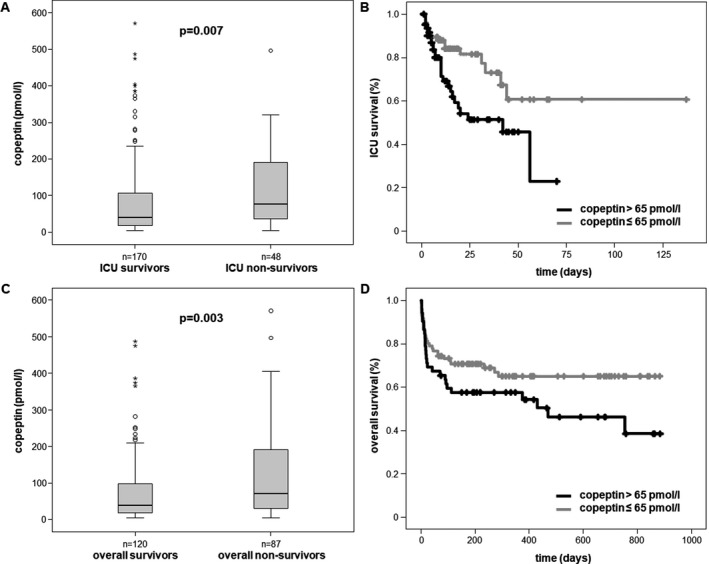
Copeptin is a prognostic biomarker for mortality in critically ill patients. A, Patients that died during the course of ICU treatment are characterized by significantly higher plasma copeptin concentrations already at ICU admission. B, Using a calculated cut‐off of 65 pmol/L, high copeptin levels are associated with decreased ICU survival, as depicted by Kaplan‐Meier survival curve analysis. C, Patients that died either at the ICU or during the observation follow‐up period of approximately two years have higher copeptin levels at ICU admission compared to overall survivors. D, Elevated copeptin concentrations (>65 pmol/L) at ICU admission indicate overall mortality during long‐term follow‐up, as depicted by Kaplan‐Meier survival curve analysis
In 207 out of our 218 patients, follow‐up data on long‐term survival for up to 3 years were available. Strikingly, copeptin levels obtained at ICU admission were significantly elevated in the 87 patients that died overall (median copeptin 38.1 pmol/L in survivors vs 70.3 pmol/L in deaths, P = .003; Figure 3C). Also for the overall survival rate, patients with copeptin plasma levels above 65 pmol/L showed an increased mortality by Kaplan‐Meier curve analysis (Figure 3D).
4. DISCUSSION
We conducted a large observational study, in which we prospectively enrolled 218 critically ill patients from our medical ICU, to determine the value of copeptin as a biomarker in the intensive care setting. In line with previous reports of patients with acute illnesses,6, 10, 11, 12, 13 critically ill patients had significantly higher circulating copeptin levels as compared to healthy controls. However, the disease entity (eg sepsis) or metabolic comorbidities that would affect copeptin levels in the chronic setting 4, 9 did not determine copeptin concentrations in critical illness. On the contrary, copeptin levels closely correlated with disease severity as well as biomarkers of renal failure, liver failure, altered metabolism, and tissue perfusion. As a consequence, high copeptin plasma concentrations at ICU admission reliably predicted short‐term and long‐term mortality in our cohort.
In contrast to our study, a large observational single‐center study from Beijing that included 461 patients admitted to the Emergency Department reported that copeptin levels might serve as an early diagnostic indicator of sepsis.13 Importantly, this study also noted an association between copeptin and disease severity as well as short‐term mortality in the Emergency Department setting.13 Our study that focused on critically ill patients at the ICU revealed that not the presence of sepsis, but the severity of critical illness as reflected by organ failure(s) and hemodynamic alterations was the main determinant for elevated copeptin. Possibly, sepsis is simply a characteristic condition, in which neuroendocrine pathway activation and subsequent vasopressin release reflect the stress level of critically ill patients. Of note, in the ICU setting, two smaller observational studies reported a stepwise increase in circulating copeptin levels from sepsis to severe sepsis to septic shock,12, 27 which particularly mirrors the extent of cardiovascular instability.
The close association between copeptin and renal failure in ICU patients is not surprising, given the important role of vasopressin in water and sodium resorption, vascular tone of renal vessels and kidney perfusion.5 In our critically ill patients, however, there was also a strong correlation between copeptin and biomarkers of endothelial dysfunction and systemic regulators of vascular tone such as CT‐pro‐ET1, ADMA, SDMA or NT‐proCNP.14, 22, 24, 25 These data indicate that copeptin contributes to hemodynamic alterations resulting in tissue hypoperfusion. This assumption is corroborated by the correlations between copeptin and lactate as well as systemic acidosis in our study.
From our correlative analysis, it is impossible to dissect whether high copeptin in critical illness is cause or consequence of biological stress, vascular dysregulation and tissue hypoperfusion. Biological stress activates key neuroendocrine pathways,3 namely the hypothalamic‐pituitary‐adrenal axis (eg CRH—ACTH—cortisol) as well as vasopressin release, which is derived from the same precursor protein as copeptin. Hypotension further stimulates vasopressin secretion in order to counteract shock by volume retention and vasoconstriction.4 Nonetheless, the activation of this pathway, as reflected by copeptin, is closely related to the patients’ prognosis at and beyond the ICU. In agreement with prior studies in patients with acute illnesses that noted an association with short‐term mortality,6, 11, 12, 13 our study confirmed the value of copeptin levels as a prognostic biomarker for the ICU mortality. More strikingly, copeptin plasma concentrations at the admission to the ICU even predicted the long‐term, overall mortality of critically ill patients in our study. The long‐term outcome of critically ill patients after discharge from the ICU depends on manifold clinical and biological factors, including age, comorbidity, and length of stay at the ICU.28 The association between copeptin plasma levels and overall mortality suggests that copeptin could be a sensitive biomarker for the accurate assessment of the initial disease severity, supporting that the copeptin‐related pathologies as biological stress and hypoperfusion are major determinants of the severity of critical illness. Future studies should prospectively evaluate, whether the implementation of copeptin measurements into multifactorial risk models could improve prognostication and thereby management of critically ill patients.
Koch A, Yagmur E, Hoss A, et al. Clinical relevance of copeptin plasma levels as a biomarker of disease severity and mortality in critically ill patients. J Clin Lab Anal. 2018;32:e22614 10.1002/jcla.22614
Funding information
This work was supported by the German Research Foundation DFG; Ta434/5‐1 and SFB/TRR57 and the Interdisciplinary Center for Clinical Research (IZKF) Aachen.
REFERENCES
- 1. Martin‐Loeches I, Wunderink RG, Nanchal R, et al. Determinants of time to death in hospital in critically ill patients around the world. Intensive Care Med. 2016;42:1454‐1460. [DOI] [PubMed] [Google Scholar]
- 2. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873‐904. [DOI] [PubMed] [Google Scholar]
- 4. Morgenthaler NG. Copeptin: a biomarker of cardiovascular and renal function. Congest Heart Fail. 2010;16(Suppl 1):S37‐S44. [DOI] [PubMed] [Google Scholar]
- 5. Bolignano D, Cabassi A, Fiaccadori E, et al. Copeptin (CTproAVP), a new tool for understanding the role of vasopressin in pathophysiology. Clin Chem Lab Med. 2014;52:1447‐1456. [DOI] [PubMed] [Google Scholar]
- 6. Jochberger S, Morgenthaler NG, Mayr VD, et al. Copeptin and arginine vasopressin concentrations in critically ill patients. J Clin Endocrinol Metab. 2006;91:4381‐4386. [DOI] [PubMed] [Google Scholar]
- 7. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112‐119. [DOI] [PubMed] [Google Scholar]
- 8. Szinnai G, Morgenthaler NG, Berneis K, et al. Changes in plasma copeptin, the c‐terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab. 2007;92:3973‐3978. [DOI] [PubMed] [Google Scholar]
- 9. Saleem U, Khaleghi M, Morgenthaler NG, et al. Plasma carboxy‐terminal provasopressin (copeptin): a novel marker of insulin resistance and metabolic syndrome. J Clin Endocrinol Metab. 2009;94:2558‐2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smaradottir MI, Ritsinger V, Gyberg V, Norhammar A, Nasman P, Mellbin LG. Copeptin in patients with acute myocardial infarction and newly detected glucose abnormalities ‐ A marker of increased stress susceptibility? A report from the glucose in acute myocardial infarction cohort. Diab Vasc Dis Res. 2017;14:69‐76. [DOI] [PubMed] [Google Scholar]
- 11. Krychtiuk KA, Honeder MC, Lenz M, et al. Copeptin predicts mortality in critically ill patients. PLoS One. 2017;12:e0170436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seligman R, Papassotiriou J, Morgenthaler NG, Meisner M, Teixeira PJ. Copeptin, a novel prognostic biomarker in ventilator‐associated pneumonia. Crit Care. 2008;12:R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Q, Dong G, Zhao X, Wang M, Li CS. Prognostic significance of hypothalamic‐pituitary‐adrenal axis hormones in early sepsis: a study performed in the emergency department. Intensive Care Med. 2014;40:1499‐1508. [DOI] [PubMed] [Google Scholar]
- 14. Koch A, Weiskirchen R, Kunze J, et al. Elevated asymmetric dimethylarginine levels predict short‐ and long‐term mortality risk in critically ill patients. J Crit Care. 2013;28:947‐953. [DOI] [PubMed] [Google Scholar]
- 15. Buendgens L, Yagmur E, Bruensing J, et al. Growth differentiation factor‐15 is a predictor of mortality in critically ill patients with sepsis. Dis Markers. 2017;2017:5271203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304‐377. [DOI] [PubMed] [Google Scholar]
- 17. Koch A, Voigt S, Kruschinski C, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch A, Gressner OA, Sanson E, Tacke F, Trautwein C. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non‐septic patients. Crit Care. 2009;13:R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koch A, Sanson E, Voigt S, Helm A, Trautwein C, Tacke F. Serum adiponectin upon admission to the intensive care unit may predict mortality in critically ill patients. J Crit Care. 2011;26:166‐174. [DOI] [PubMed] [Google Scholar]
- 20. Koch A, Weiskirchen R, Sanson E, et al. Circulating retinol binding protein 4 in critically ill patients before specific treatment: prognostic impact and correlation with organ function, metabolism and inflammation. Crit Care. 2010;14:R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koch A, Weiskirchen R, Zimmermann HW, Sanson E, Trautwein C, Tacke F. Relevance of serum leptin and leptin‐receptor concentrations in critically ill patients. Mediators Inflamm. 2010;2010:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buendgens L, Yagmur E, Bruensing J, et al. C‐terminal proendothelin‐1 (CT‐proET‐1) is associated with organ failure and predicts mortality in critically ill patients. J Intensive Care. 2017;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katan M, Muller B, Christ‐Crain M. Copeptin: a new and promising diagnostic and prognostic marker. Crit Care. 2008;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koch A, Weiskirchen R, Bruensing J, et al. Regulation and prognostic relevance of symmetric dimethylarginine serum concentrations in critical illness and sepsis. Mediators Inflamm. 2013;2013:413826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koch A, Voigt S, Sanson E, et al. Prognostic value of circulating amino‐terminal pro‐C‐type natriuretic peptide in critically ill patients. Crit Care. 2011;15:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koch A, Weiskirchen R, Ludwig S, et al. Relevance of serum sclerostin concentrations in critically ill patients. J Crit Care. 2017;37:38‐44. [DOI] [PubMed] [Google Scholar]
- 27. Laribi S, Lienart D, Castanares‐Zapatero D, Collienne C, Wittebole X, Laterre PF. CT‐proAVP is not a good predictor of vasopressor need in septic shock. Shock. 2015;44:330‐335. [DOI] [PubMed] [Google Scholar]
- 28. Gayat E, Cariou A, Deye N, et al. Determinants of long‐term outcome in ICU survivors: results from the FROG‐ICU study. Crit Care. 2018;22:8. [DOI] [PMC free article] [PubMed] [Google Scholar]


