Abstract
Background
Coronary artery disease (CAD) is the leading cause of death worldwide and remains a major health problem, providing the rationale for identification of molecular markers for detection of individuals at high risk of developing CAD. Tumor necrosis factor‐α (TNF‐α) plays a crucial role in the pathogenesis of CAD. We have therefore explored the association of TNF‐α 308 (G/A) gene polymorphism in 903 individuals with/without CAD.
Methods
TNF‐α 308 gene polymorphism was analyzed in 903 subjects of whom 222 were healthy controls. Among the 681 patients who were investigated angiographically, 468 had ≧50% stenosis and 213 patients had <50% stenosis. Biochemical profiles (eg, triglycerides, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, fasting blood glucose, and CRP) were evaluated. Associations between TNF‐α genotypes with biochemical and anthropometric characteristics were determined.
Results
The frequencies of TNF‐α‐AA or AG genotypes were significantly lower in patients classified as CAD patients with ≥ or <50% obstruction in at least one coronary artery, compared to the control group. We observed that CAD patients with ≥50% stenosis and with AA genotype were associated with higher risk of CAD with OR of 3.56 (95%CI: 1.02‐12.41; P=.046) using multivariate analysis. Moreover, we found that TNF‐α‐308‐AA genotype was associated with blood pressure and CRP level in CAD patients, compared to the wild type‐genotype.
Conclusion
Our data showed an association of TNF‐α‐308G/A polymorphism with CAD patients with ≥50% obstruction, supporting the need for further investigations on the role of TNF‐α‐308G/A polymorphism with hypertension.
Keywords: coronary artery disease, genotype, tumor necrosis factor‐α
1. Introduction
Cardiovascular disease, including coronary artery disease (CAD), is the leading cause of death worldwide.1, 2 CAD is a chronic inflammatory process which is mediated, in part, by pro‐inflammatory cytokines, such as tumor necrosis factor‐α (TNF‐α). It has been shown that TNF‐α plays an essential role in the pathogenesis of CAD3, 4, 5 as well as in different processes that may contribute to CAD risk, such as insulin resistance,6 lipid metabolism disorders, and systemic lupus erythematosus (SLE).7 The human TNF‐α gene is located near the major histocompatibility complex (MHC) class III region on chromosome 6p21.3.8, 9, 10 Several functional promoter polymorphisms have been identified in this gene.11, 12, 13, 14, 15
There is emerging evidence that of an association of TNF‐α‐308 polymorphism, a G/A transition at ‐308‐bp position, which may alter promoter activity, with TNF‐α expression in vitro, as well as plasma concentrations.16 An increased serum concentration of TNF‐α is an independent predictor of cardiovascular disease 17 and is a marker for recurrent coronary events following a previous acute myocardial infarction.18 This polymorphism is associated with several conditions,19, 20, 21 including infection, asthma,20 autoimmune disease,21, 22, and immune‐mediated diseases, such as rheumatoid arthritis.22 The TNF‐α‐308 polymorphism is also considered to play a key pathogenic role in CAD.23
The relationship between the TNF‐α 308G/A gene polymorphism (rs1800629) has not been studied in Iranian population with CAD. The aim of our study was to evaluate the association of the TNF‐α 308G/A polymorphism with the presence of CAD and its association with other important clinical variables in an Iranian population consists of 903 subjects.
2. Materials and Methods
2.1. Population
In the present cross‐sectional study, 903 subjects, including 681 patients with clinically diagnosed CAD who had angiographically defined disease, and 222 controls, without evidence of CAD, all from Mashhad city, in northeastern Iran, were recruited. Patients who had a history of stroke, myocardial infarction, and diabetes mellitus were excluded from this study. Indications for coronary angiography were stable or unstable angina, acute MI, recurrence of symptoms after restoring reperfusion, valvular heart disease, patients undergoing non‐cardiac surgery (preoperative), congestive heart failure (CHF), congenital heart disorders, aortic dissection, hypertrophic cardiomyopathy, arteritis, and chest trauma, based on ACC/AHA guidelines for coronary angiography (report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines). According to the results of angiography, the subjects were divided into two groups. Patients whose angiography results showed ≥50% obstruction in at least one coronary artery, who were assigned to the obstructive coronary disease group (n=468). Patients who had less than 50% obstruction in their coronary arteries were assigned to the non‐obstructive coronary disease group (n=213). Healthy subjects were those whom referred for their annual medical checkup or pre‐employment examinations. They were used as the reference (control) group as they gave no history of cardiac symptoms and their electrocardiogram (ECG) had no abnormalities. The study was performed according to the declaration of Helsinki. Informed consent was obtained from all subjects using protocols approved by the Ethics Committee of the MUMS.
2.2. Anthropometric measurements
Anthropometric parameters including weight, height, BMI, waist circumference, hip circumference, and waist/hip ratio as well as systolic and diastolic blood pressures were measured as previously described.24
2.3. Lipid profile, fasting glucose, and hsCRP measurements
Total serum cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), and triglycerides (TG), and fasting blood glucose (FBG) concentrations were determined by standard enzymatic techniques, while serum C‐reactive protein (CRP) levels were measured using a polyethylene glycol‐enhanced immunoturbidimetry method, as described previously.24
2.4. Genotyping
Peripheral blood samples were obtained from the individuals, and genomic DNA was extracted using a Biogene DNA extraction kit according to the manufacturer's protocol (Biogene, Iran). A polymerase chain reaction‐amplification refractory mutation system (ARMS‐PCR) was performed to determine the TNF‐α gene polymorphism at position ‐308 G/A. The two forward and reverse primers used for amplification of TNF‐α gene were as follows: Forward primers: 5′‐ ACC CAA ACA CAG GCC TCA GGA CTC AAC A‐3 and 5′‐ TGG AGG CAA TAG CTT TTG AGG GGC AGG A ‐3′; reverse primers: 5′‐ AGT TGG GGA CAC GCA AGC ATG AAG GAT A ‐3′and 5′‐ TAG GAC CCT GGA GGC TAG ACC CCG TAC C ‐3′. The reaction was performed in total volume of 20 μL, using 2 μL Buffer, 1.6 μL dNTPs, 2 μL MgCl2, 1 μL for each forward and reverse outer primers, 3 μL for each forward and reverse inner primers, 0.2 μL Taq Polymerase, 20ng DNA, and distilled water. The samples were amplified using the following thermal profile: 94°C for 4 minutes followed by 30 cycles of denaturation at 94°C for 40 seconds, annealing at 62°C for 30 seconds, DNA extension at 72°C for 35 seconds with final extension at 72°C for 5 minutes. PCR system Veriti 96 well thermocycler (Applied Biosystems, USA) was used for amplification. PCR products were then separated by 2% agarose gels for 45 minutes at 80 V, and stained with ethidium bromide.
2.5. DNA sequencing
DNAs were amplified as described above, sequenced using Sanger sequencing and then analyzed with DNA Analyzer (Sequetech, USA) to confirm the genotypes obtained by ARMS‐PCR.
2.6. Statistical analysis
Data were analyzed using SPSS‐11.5 software (SPSS Inc., IL, USA). The normality of distribution was assessed using the Kolmogorov‐Smirnov test. Data were expressed as mean±SD (standard deviation) for normally distributed variables or as median±IQR for not normally distributed variables. For normally distributed variables, the student t test was used to compare the baseline demographics between the groups. Mann‐Whitney U test, Chi‐square test, and/or Fisher exact test was used for not normally distributed variables. Logistic regression was used to determine the odds ratios for association of the polymorphism and CAD. A two‐sided P‐value <.05 was considered statistically significant.
3. Results
3.1. Characteristics of the population
The clinical features and baseline characteristics of the subjects are summarized in Table 1. Not surprisingly, the levels of TG, FBG, CRP, SBP, and DBP were significantly higher in the CAD patients with ≥50% obstruction in at least one coronary artery, compared to the CAD patients with <50% obstruction. Moreover, CAD patients with ≥50% obstruction also had markedly higher levels of TG, HDL‐C, CRP and FBG, compared to the other group (Table 1).
Table 1.
Demographic, anthropometric, and biochemical characteristics of the population
| Variables | CAD patients with ≥50% obstruction (n=468) | CAD patients with <50% obstruction (n=213) | Control (n=222) | P‐value | ||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | ||||
| Age, (y) | 57±10 | 52±10 | 51±10 | <.001 | <.001 | <.001 |
| Sex, male (%) | 281 (63.0) | 64 (30.0) | 101 (45.4) | <.001 | <.001 | <.001 |
| BMI (kg/m2) | 27±5 | 26±5 | 26±4 | .1 | >.05 | >.05 |
| Weight(kg) | 70±13 | 67±14 | 70±12 | .05 | .05 | .5 |
| WC (cm) | 93±12 | 92±12 | 92±12 | .3 | >.05 | >.05 |
| Height(cm) | 159±19 | 158±14 | 144±49 | <.001 | .8 | <.001 |
| TC(mg/dL) | 169±45 | 170±45 | 194±37 | <.001 | .5 | <.001 |
| TG(mg/dL) | 142 (59) | 132 (54) | 118 (97) | <.001 | <.001 | .001 |
| HDL‐C(mg/dL) | 40±13 | 43±11 | 41±8 | .03 | .02 | .9 |
| LDL‐C(mg/dL) | 98±34 | 99±36 | 118±36 | <.001 | .9 | <.001 |
| FBG(mg/dL) | 131±60 | 115±49 | 87±27 | <.001 | .001 | <.001 |
| HC(cm) | 99±9 | 98±8 | 101±8 | .03 | .3 | .2 |
| SBP(mmHg) | 139±25 | 135±24 | 123±18 | <.001 | .2 | <.001 |
| DBP(mmHg) | 83±11 | 82±11 | 76±10 | <.001 | .2 | <.001 |
| MCI (%) | 230 (49.1) | 103 (48.3) | ‐ | <.001 | ‐ | ‐ |
| hsCRP (mg/dL) | 5.5 (2.1‐6.4) | 4.3 (1.7‐4.9) | 1.7 (1.1‐3.5) | .199 | .025 | .718 |
Values are expressed as mean±SD or median range. Comparisons were made by the χ2 test (for categorical data), one‐way ANOVA and Kruskal‐Wallis test (for numerical data). Also, the post hoc test was used for comparison between groups. P1: comparison between CAD patients with ≥50% obstruction and CAD patients with <50% obstruction group. p2: comparison between CAD patients with ≥50% obstruction and control group. P3: comparison between CAD patients with <50% obstruction and control group. BMI: body mass index; TC: total cholesterol; WC: Waist circumference, HC: Hip circumference, TG: triglycerides; HDL‐C: high‐density lipoprotein cholesterol; LDL‐C: low‐density lipoprotein cholesterol; FBG: fasting blood glucose; SBP: systolic blood pressure; DBP: diastolic blood pressure; MCI: History of myocardial infarction.
3.2. Association of TNF‐α ‐308G/A polymorphism with baseline characteristics of the groups
In order to explore whether there was an association between TNF‐α ‐308G/A polymorphism and clinical and baseline characteristics of CAD patients and control groups, we performed genotyping as described earlier (Figure 1). Genotyping was successfully performed, and no discrepancies were found in the samples analyzed in duplicate. As shown in Table 2, the TNF‐α‐AA genotype had a frequency of 13.2%, whereas the AG and GG genotypes were found in 33.8% and 53% of the CAD patients with ≥50% obstruction, respectively, while these frequencies in the other CAD group were 14.9% (AA), 39.6% (AG), and 45.5% (GG). There was no significant difference in genotype frequencies between the patient and control groups (Table 2).
Figure 1.
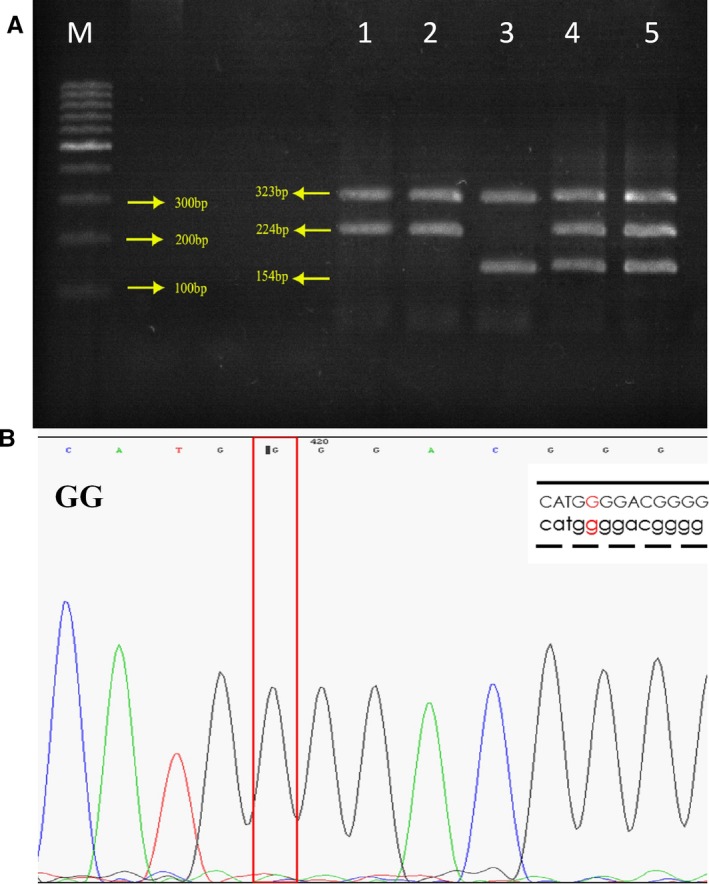
(A‐B) ARMS‐PCR and sequence of the amplified fragment in TNF‐α gene polymorphism. The genotype was labeled on corresponding sequences, and the sites which were marked were the SNP of TNF‐α gene electrophoresed on 2% agarose, stained with ethidium bromide. Left to right: M, DNA marker (100bp), Lanes 1 and 2: GG homozygous (323bp and 224 bp), Lane 3: homozygote AA (323bp and 154 bp) and Lanes 4 and 5: AG heterozygote (323 bp, 224 bp, and 154 bp) for TNF‐α genotype
Table 2.
Genotype distribution and allele frequency of TNF‐α polymorphism in all the subjects
| Genotype | CAD patients with ≥50% obstruction, % | CAD patients with <50% obstruction, % | Control n=222, % | Significance |
|---|---|---|---|---|
| Genotype distribution | ||||
| (GG) | 53.0 (248) | 60.6 (129) | 45.5 (101) | χ²=10.48; df=4; P=.03 |
| (AG) | 33.8 (158) | 26.8 (57) | 39.6 (88) | |
| (AA) | 13.2 (62) | 12.7 (27) | 14.9 (33) | |
| HWE | P<.05 | P<.05 | P=.17 | |
| Allele frequency | ||||
| A | 30.1 (282) | 26.1 (111) | 34.7 (154) | χ²=7.689; df=2; P=0.021 |
| G | 69.9 (654) | 73.9 (315) | 65.3 (290) | |
Comparisons were made by the χ2 test.
Furthermore, we explored the association of the genotypes with biochemical and anthropometric characteristics of the populations. This analysis showed that the TNF‐α‐308G/A polymorphism was related to an altered systolic blood pressure and CRP level in CAD patients (Table 3). Individuals with the 308G/A polymorphism‐AA genotype or those who carried the A allele of the 308G/A polymorphism were likely to have CAD (Table 4). In particular, AA genotype in CAD patients with ≥50% obstruction was associated with higher risk of CAD with OR of 3.56 (95%CI: 1.02‐12.41; P‐value=.046) (Table 4) using multivariate analysis.
Table 3.
Association of TNF‐α polymorphism with biochemical and anthrometric characteristics of subjects
| Groups | Genotype | Height | Weight | BMI | WC | HC | TC | TG | HDL | LDL | FBG | SBP | DBP | hsCRP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | GG | 161±9 | 69±12 | 25±4 | 91±12 | 99±7 | 192±37 | 135.3±70.6 | 41±8 | 115±38 | 84±16 | 121±16 | 74±10 | 1.4 (0.8‐2.3) |
| GA | 159±14 | 71±13 | 26±4 | 92±13 | 102±10 | 193±36 | 133.5±76.2 | 41±8 | 120±32 | 92±38 | 125±20 | 77±9 | 1.7 (1.0‐5.0) | |
| AA | 159±9 | 71±9 | 26±2 | 94±10 | 103±7 | 203±41 | 154.2±108.2 | 39±8 | 121±43 | 85±15 | 119±17 | 77±12 | 3.5 (1.3‐10.4) | |
| P | .4 | .2 | .8 | .4 | .02 | .3 | .7 | .2 | .5 | .1 | .2 | .1 | <.001 | |
| CAD patients with <50% obstruction | GG | 157±16 | 68±13 | 27±5 | 93±11 | 99±9 | 171±47 | 133.4±58.9 | 43±11 | 100±38 | 116±49 | 134±23 | 81±11 | 3.9 (1.6‐4.9) |
| GA | 159±9 | 66±14 | 25±5 | 93±13 | 100±9 | 173±44 | 148.6±99.9 | 43±10 | 99±36 | 110±38 | 142±25 | 85±12 | 3.9 (1.5‐4.8) | |
| AA | 156±21 | 69±14 | 27±5 | 94±11 | 100±9 | 160±38 | 126.6±56.9 | 42±9 | 97±25 | 124±69 | 127±24 | 81±12 | 4.8 (3.8‐9.1) | |
| P | .7 | .4 | .3 | .5 | .3 | .4 | .5 | .9 | .9 | .5 | .01 | .09 | .023 | |
| CAD patients with ≥50% obstruction | GG | 160±20 | 70±14 | 27±5 | 93±11 | 92±9 | 172±44 | 151.9±74.1 | 40±9 | 101±36 | 127±60 | 140±24 | 84±10 | 5.3 (1.7‐6.4) |
| GA | 158±20 | 70±12 | 27±5 | 93±13 | 100±10 | 165±43 | 144.6±75.1 | 42±19 | 95±31 | 136±64 | 134±25 | 81±10 | 5.8 (2.3‐6.5) | |
| AA | 160±11 | 69±13 | 27±5 | 93±12 | 100±9 | 171±55 | 171.7±113.2 | 39±10 | 94±29 | 134±53 | 143±28 | 80±13 | 5.4 (2.3‐6.8) | |
| P | .7 | .8 | .8 | .9 | .4 | .3 | .3 | .2 | .1 | .3 | .03 | .002 | .125 |
BMI, body mass index; WC, Waist circumference, HC, Hip circumference, TC, total cholesterol; TG, triglycerides; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Values are expressed as mean±SD or median and interquartile range. Comparisons were made by the χ2 test (for categorical data), one‐way ANOVA and Kruskal‐Wallis test (for numerical data). Also, the post hoc and Mann‐whitney test were used for comparison between groups.
P‐value <.05 was considered significant.
Table 4.
Association between TNF‐α polymorphism and CAD
| Genotypes | Univariate analysis OR (95% CI) | P | Multivariate analysis OR (95%CI) | P a | |
|---|---|---|---|---|---|
| CAD patients with<50% obstruction | GG | 1 | ‐ | 1 | ‐ |
| GA | 0.507 (0.33‐0.77) | .002 | 0.96 (0.43‐2.17) | .968 | |
| AA | 0.64 (0.36‐1.13) | .127 | 2.35 (0.64‐8.56) | .193 | |
| CAD patients with ≥50% obstruction | GG | 1 | ‐ | 1 | ‐ |
| GA | 0.73 (0.51‐1.03) | .078 | 1.66 (0.81‐3.40) | .163 | |
| AA | 0.76 (0.47‐1.23) | .276 | 3.56 (1.02‐12.41) | .046 |
OR, Odds ratio.
After correction for age, sex, smoking, HDL‐C, TG, LDL‐C, FBG, SBP, DBP, and BMI.
4. Discussion
We demonstrated that the AA genotype of TNF‐α 308 gene polymorphism was associated with the presence of ≥50% coronary artery stenosis in patients being investigated by angiography.
TNF‐α alters vascular endothelial cell function, inducing the production and activation of growth factors and adhesion molecules such as intercellular adhesion molecule‐1 (ICAM‐1), vascular cell adhesion molecule‐1 (VCAM‐1), and E‐selectin.25, 26 It has been shown that TNF‐α plays an important role in the pathogenesis of atherosclerosis. It has been reported that patients with CAD have circulating mononuclear leukocytes that have an increased potential to secrete TNF.27
There is growing evidence of an association between the TNF‐α SNP and cardiovascular disease.21 However, the data are inconsistent.16, 27, 28 Ghazouani et al.16 have reported that the TNF‐α 308 (G/A) SNP was not associated with CAD in Tunisian patients. A further study also showed the lack of association of TNF‐α 308G/A polymorphism and risk of coronary artery disease and myocardial infarction.27 Koch et al.29 evaluated the association between this genetic polymorphism and coronary artery disease in a Caucasian population finding no association with CAD. Padovani and colleagues investigated two genetic polymorphisms in the TNF‐α gene (‐308 G→A and LT‐alpha +252 A→G) as risk factors for CAD by determining its prevalence in 148 survivors of myocardial infarction (MI) with angiographically proven severe CAD.30 They were unable to find a significant association between the TNF‐α polymorphisms and MI.27 This lack of association can be explained at least in part by life style, severity of the disease, small sample size, and ethnic diversity.
In the present study, we investigated the association of TNF‐α ‐308G/A polymorphism in a large group of patients with angiographically defined disease. Our data showed that individuals carrying A allele for TNF‐α were at a higher risk of having CAD, which is in line with previous observations. In particular, Kriskovszky and colleagues found that the TNF‐α 308A was related with the low blood pressure in diabetic adolescents.31 Another study showed that TNF‐α 308G was associated with increased risk of hypertension.32 Ito et al.,33 investigated the correlation of TNF‐α with atherogenic risk factors in women. They observed that the serum level of TNF‐α was significant related with obesity, hyperlipidemia and hypertension. These findings are in agreement with our recent data about the role of this marker with hypertension, CAD, and metabolic syndrome.33, 34, 35, 36, 37 Moreover, a recent meta‐analysis revealed that the A allele of TNF‐α G308A gene increases the risk of essential hypertension,38 which is in agreement with our data.
The strength of the present study is that it was carried out in a large number of individuals with or without angiographically defined CAD. The main limitations of this study were the cross‐sectional study design and possible confounding effects of differences in mean age or gender on the outcome in subjects with CAD patients with <50% obstruction vs CAD patients with ≥50% obstruction in at least one coronary artery, although these variables were partially adjusted for in the logistic regression model and multivariate analysis for evaluating the role of this polymorphisms and CAD. Moreover, we cannot exclude the possible ethnic heterogeneity which might be present in Iranian population, supporting further studies in this population.
In conclusion, we demonstrate the possible important role of TNF‐α‐308G/A polymorphism with blood pressure and its association with CAD. Since accumulating data is supporting the role of TNF‐α in CAD, further studies are required to investigate the association of other polymorphisms of TNF‐α with coronary artery disease.
Kazemi E, Jamialahmadi K, Avan A, et al. Association of tumor necrosis factor‐α ‐308 G/A gene polymorphism with coronary artery diseases: An evidence‐based study. J Clin Lab Anal. 2018;32:e22153 10.1002/jcla.22153.
Funding information
This study was supported by a grant from Mashhad University of Medical Sciences, Mashhad, Iran.
Contributor Information
Alireza Pasdar, Email: PasdarA@mums.ac.ir, Email: a.pasdar@abdn.ac.uk.
Majid Ghayour‐Mobarhan, Email: ghayourm@mums.ac.ir.
References
- 1. Banerjee I, Pandey U, Hasan OM, Parihar R, Tripathi V, Ganesh S. Association between inflammatory gene polymorphisms and coronary artery disease in an Indian population. J Thromb Thrombolysis. 2009;27:88–94. [DOI] [PubMed] [Google Scholar]
- 2. Cho HC, Yu G, Lee MY, Kim HS, Shin DH, Kim YN. TNF‐α polymorphisms and coronary artery disease: association study in the Korean population. Cytokine. 2013;62:104–109. [DOI] [PubMed] [Google Scholar]
- 3. Cambien F, Poirier O, Mallet C, Tiret L. Coronary heart disease and genetics an epidemiologist's view. Mol Med Today. 1997;3:197–203. [DOI] [PubMed] [Google Scholar]
- 4. Allen R, Lee E, Roberts D, Park B, Pirmohamed M. Polymorphisms in the TNF‐α and TNF‐receptor genes in patients with coronary artery disease. Eur J Clin Invest. 2001;31:843–851. [DOI] [PubMed] [Google Scholar]
- 5. DąBek J, Świderski R, Głogowska‐Ligus J, Pysz P. Transcriptional activity of tumour necrosis factor α (TNF‐α) in patients with subclinical coronary atherosclerosis—preliminary results. Folia Biol. 2012;58:209–214. [PubMed] [Google Scholar]
- 6. Sobti RC, Kler R, Sharma YP, Talwar KK, Singh N. Risk of obesity and type 2 diabetes with tumor necrosis factor‐α 308G/A gene polymorphism in metabolic syndrome and coronary artery disease subjects. Mol Cell Biochem. 2012;360:1–7. [DOI] [PubMed] [Google Scholar]
- 7. Lee YH, Harley JB, Nath SK. Meta‐analysis of TNF‐α promoter −308 A/G polymorphism and SLE susceptibility. Eur J Hum Genet. 2006;14:364–371. [DOI] [PubMed] [Google Scholar]
- 8. Winton HL, Bidwell JL, Armitage WJ. Functional tumor necrosis factor alpha polymorphisms and haplotype analysis in high‐risk corneal transplantation. Transplant Proc. 2014;46:1548–1553. [DOI] [PubMed] [Google Scholar]
- 9. Nedwin GE, Naylor SL, Sakaguchi AY, et al. Human lymphotoxin and tumor necrosis factor genes: structure, homology and chromosomal localization. Nucleic Acids Res. 1985;13:6361–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunham I, Sargent CA, Trowsdale J, Campbell RD. Molecular mapping of the human major histocompatibility complex by pulsed‐field gel electrophoresis. Proc Natl Acad Sci USA. 1987;84:7237–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higuchi T, Seki N, Kamizono S, et al. Polymorphism of the 5‐flanking region of the human tumor necrosis factor (TNF)‐α gene in Japanese. Tissue Antigens. 1998;51:605–612. [DOI] [PubMed] [Google Scholar]
- 12. Abraham LJ, Kroeger KM. Impact of the‐308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene: relevance to disease. J Leukoc Biol. 1999;66:562–566. [DOI] [PubMed] [Google Scholar]
- 13. Skoog T, van't Hooft FM, Kallin B, et al. A common functional polymorphism (C→A substitution at position– 863) in the promoter region of the tumour necrosis factor‐α (TNF‐α) gene associated with reduced circulating levels of TNF‐α. Hum Mol Genet. 1999;8:1443–1449. [DOI] [PubMed] [Google Scholar]
- 14. De Jong BA, Westendorp RGJ, Bakker AM, et al. Polymorphisms in or near tumour necrosis factor (TNF)‐gene do not determine levels of endotoxin‐induced TNF production. Genes Immun. 2002;3:25–29. [DOI] [PubMed] [Google Scholar]
- 15. Soga Y, Nishimura F, Ohyama H, Maeda H, Takashiba S, Murayama Y. Tumor necrosis factor‐alpha gene (TNF‐α) −1031/−863, −857 single‐nucleotide polymorphisms (SNPs) are associated with severe adult periodontitis in Japanese. J Clin Periodontol. 2003;30:524–531. [DOI] [PubMed] [Google Scholar]
- 16. Ghazouani L, Khalifa SBH, Abboud N, et al. –308G> A and–1031T> C tumor necrosis factor gene polymorphisms in Tunisian patients with coronary artery disease. Clin Chem Lab Med. 2009;47:1247–1251. [DOI] [PubMed] [Google Scholar]
- 17. Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events results from the Health ABC Study. Circulation. 2003;108:2317–2322. [DOI] [PubMed] [Google Scholar]
- 18. Ridker PM, Rifai N, Pfeffer M, et al. Elevation of tumor necrosis factor‐α and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. [DOI] [PubMed] [Google Scholar]
- 19. Sri Manjari K, Jyothy A, Shravan Kumar P, et al. A single‐nucleotide polymorphism in tumor necrosis factor‐α (−308 G/A) as a biomarker in chronic pancreatitis. Gene. 2014;539:186–189. [DOI] [PubMed] [Google Scholar]
- 20. Kamali‐Sarvestani E, Ghayomi MA, Nekoee A. Association of TNF‐alpha‐308 G/A and IL‐4‐589 C/T gene promoter polymorphisms with asthma susceptibility in the south of Iran. J Investig Allergol Clin Immunol. 2007;17:361. [PubMed] [Google Scholar]
- 21. Elahi MM, Gilmour A, Matata BM, Mastana SS. A variant of position −308 of the Tumour necrosis factor alpha gene promoter and the risk of coronary heart disease. Heart Lung Circ. 2008;17:14–18. [DOI] [PubMed] [Google Scholar]
- 22. Aranda F, Perés Wingeyer SD, Schneeberger E, et al. The 308 G/A polymorphism in the tumor necrosis factor‐α gene is not associated with development and progression of rheumatoid arthritis in Argentinean patients. Int J Rheum Dis. 2016;19:476–481. [DOI] [PubMed] [Google Scholar]
- 23. Vendrell J, Fernandez‐Real JM, Gutierrez C, et al. A polymorphism in the promoter of the tumor necrosis factor‐α gene (−308) is associated with coronary heart disease in type 2 diabetic patients. Atherosclerosis. 2003;167:257–264. [DOI] [PubMed] [Google Scholar]
- 24. Mirhafez SR, Mohebati M, Feiz Disfani M, et al. An imbalance in serum concentrations of inflammatory and anti‐inflammatory cytokines in hypertension. J Am Soc Hypertens. 2014;8:614–623. [DOI] [PubMed] [Google Scholar]
- 25. Yamada Y, Ichihara S, Izawa H, Tanaka M, Yokota M. Genetic risk for coronary artery disease in individuals with or without type 2 diabetes. Mol Genet Metab. 2004;81:282–290. [DOI] [PubMed] [Google Scholar]
- 26. Keso T, Perola M, Laippala P, et al. Polymorphisms within the tumor necrosis factor locus and prevalence of coronary artery disease in middle‐aged men. Atherosclerosis. 2001;154:691–697. [DOI] [PubMed] [Google Scholar]
- 27. Chu H, Yang J, Mi S, et al. Tumor necrosis factor‐alpha G‐308 A polymorphism and risk of coronary heart disease and myocardial infarction: a case–control study and meta‐analysis. J Cardiovasc Dis Res. 2012;3:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Q. Molecular genetics of coronary artery disease. Curr Opin Cardiol. 2005;20:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koch W, Kastrati A, Böttiger C, et al. Interleukin‐10 and tumor necrosis factor gene polymorphisms and risk of coronary artery disease and myocardial infarction. Atherosclerosis. 2001;159:137–144. [DOI] [PubMed] [Google Scholar]
- 30. Padovani JC, Pazin‐Filho A, Simões MV, Marin‐Neto JA, Zago MA, Franco RF. Gene polymorphisms in the TNF locus and the risk of myocardial infarction. Thromb Res. 2000;100:263–269. [DOI] [PubMed] [Google Scholar]
- 31. Krikovszky D, Vásárhelyi B, Tóth‐Heyn P, Körner A, Tulassay T, Madácsy L. Association between G −308A polymorphism of the tumor necrosis factor‐α gene and 24‐hour ambulatory blood pressure values in type 1 diabetic adolescents. Clin Genet. 2002;62:474–477. [DOI] [PubMed] [Google Scholar]
- 32. Yoo C‐S, Hwang W‐J, Hong S‐H, et al. Relationship between iris constitution analysis and TNF‐alpha gene polymorphism in hypertensives. Am J Chin Med. 2007;35:621–629. [DOI] [PubMed] [Google Scholar]
- 33. Ito H, Ohshima A, Tsuzuki M, et al. Association of serum tumour necrosis factor‐α with serum low‐density lipoprotein‐cholesterol and blood pressure in apparently healthy Japanese women. Clin Exp Pharmacol Physiol. 2001;28:188–192. [DOI] [PubMed] [Google Scholar]
- 34. Mirhafez SR, Avan A, Pasdar A, et al. Association of tumor necrosis factor‐α promoter G‐308A gene polymorphism with increased triglyceride level of subjects with metabolic syndrome. Gene. 2015;568:81–84. [DOI] [PubMed] [Google Scholar]
- 35. Mirhafez SR, Zarifian A, Ebrahimi M, et al. Relationship between serum cytokine and growth factor concentrations and coronary artery disease. Clin Biochem. 2015;48:575–580. [DOI] [PubMed] [Google Scholar]
- 36. Mirhafez SR, Pasdar A, Avan A, et al. Cytokine and growth factor profiling in patients with the metabolic syndrome. Br J Nutr. 2015;113:1911–1919. [DOI] [PubMed] [Google Scholar]
- 37. Mirhafez SR, Tajfard M, Avan A, et al. Association between serum cytokine concentrations and the presence of hypertriglyceridemia. Clin Biochem. 2016;49:750–755. [DOI] [PubMed] [Google Scholar]
- 38. Li Y‐Y. Tumor necrosis factor‐alpha G308α gene polymorphism and essential hypertension: a meta‐analysis involving 2244 Participants. PLoS One. 2012;7:e35408. [DOI] [PMC free article] [PubMed] [Google Scholar]


