Abstract
Objective
The aim of this study was to compare serum anti‐phenolic glycolipid‐1 IgA, IgG, and IgM levels in leprosy patients and controls.
Method
Analysis of anti‐PGL‐1 IgA, IgG, or IgM in serum samples from multibacillary (MB, n=32) and paucibacillary (PB, n=22) leprosy patients, and in non‐endemic controls (n=17), using an indirect enzyme‐linked immunosorbent assay.
Results
A strong correlation between serum IgM and IgA isotypes was found (r=.745, P<.0001) in MB patients. A moderate correlation was found in all analyses in PB patients. A moderate agreement was found between anti‐PGL1 IgA and IgM tests. Based on the ROC curves, the cut‐off values were selected and the parameters of validation were calculated. Considering the clinical forms altogether, the diagnostic sensitivities were 50.0% for IgA, 22.2% for IgG, and 74.1% for IgM. The positive (VPP) and negative (VPN) predictive values were estimated for each isotype. For IgA, the VPP and VPN were, respectively, 100.0% (87.0%‐100.0%; 95% confidence interval) and 38.7% (24.4%‐54.5%); for IgG, 100% (87.0%‐100.0%) and 28.8% (17.8%‐42.1%), respectively; and for IgM, 95.2% (83.8%‐99.4%) and 51.7% (32.5%‐70.6%), respectively.
Conclusion
Despite the limiting factors, anti‐PGL1 IgA correlates to IgM levels and it could be considered as a possible laboratorial tool to be also used, for instance, in serological follow‐up studies.
Keywords: IgA, IgG, IgM, isotypes, Mycobacterium leprae, native PGL1, serum antibodies
1. INTRODUCTION
Leprosy is a chronic infectious disease which is transmitted through droplets from an index case presenting the multibacillary form of the disease to persons living in the same house or nearby1; nonetheless, it cannot be ruled out that skin lesions rich in viable bacilli might be potential sources of transmission.2 Brazil comprises the group of 14 countries responsible for 95% of new cases of leprosy in the world.3 The overall detection rate of cases in Brazil was 14.06 cases per 100 000 inhabitants in 2015.4 The North and Midwest regions presented the highest incidence of leprosy, that is, 29.59 and 36.69 new cases per 100 000 inhabitants, respectively, in that same year.4 The Northeastern Region showed an overall detection rate of 22.71 cases per 100 000 inhabitants.4 In Alagoas (which is situated in the Northeastern Region), the Secretariat of Health reported that Rio Largo and Santana do Ipanema presented an overall detection rate of 19.82 and 39.79 cases per 100 000 inhabitants, respectively, in 2015.4 Another important parameter in the epidemiology of leprosy concerns to the case detection rate among children under 15 years of age, once it is probably related to recent infection and active focus of transmission in the community.5 The Northern, Midwestern, and Northeastern regions also presented the highest frequencies of leprosy cases among children under 15 years of age, that is, 9.62, 7.89, and 7.46 cases per 100 000 individuals.4 The diagnosis of leprosy is essentially based on clinical and epidemiological studies. Considering the mode of transmission, contacts from non‐treated multibacillary leprosy patients, are more prone to develop the disease than the general population.6, 7 One of the major challenges in leprosy is the disease diagnosis which is based mostly on the professional skills in searching for skin lesions and peripheral nerve alteration. Skin smears are neither often positive nor the serology. Although serology is not used in routine, it has been widely explored in research studies since the discovery of the phenolic glycolipid antigen 1 (PGL 1), a molecule specific of Mycobacterium leprae.8, 9 Enzyme‐linked immunosorbent assays (ELISA) for the detection of anti‐PGL 1 IgM antibodies in serum have shown high diagnostic sensitivity mostly for the multibacillary form of the disease.10, 11 Based on the fact that phenolic glycolipid antigen is a T‐independent antigen, the IgM isotype against PGL‐1 is the only isotype most frequently searched in serum samples from patients and contacts.12, 13 Although the test for anti‐PGL1 serum IgG presents a high diagnostic specificity,14 it lacks diagnostic sensitivity.15 Very few reports were found concerning serum anti‐PGL1 IgA isotype.16, 17 According to these authors, the subclass antibody anti‐PGL1 IgA1 was correlated with the bacterial index in leprosy.
The importance of IgA for mucosal host immunity is well established18, 19, 20; however, knowledge about its protective role in systemic circulation is still doubtful.21 Nonetheless, in the last few years there has been increasing evidence of its usefulness for diagnosis of infectious diseases, such as leptospirosis,22 toxoplasmosis,23 and dengue.24, 25, 26 The IgA isotype presents an advantage in comparison to the IgM and IgG isotypes because it appears early in infection and disappears much faster than them.26 Thus, the purpose of the present work was to compare serum anti‐PGL1 IgA, IgG and IgM levels in leprosy patients and controls.
2. MATERIALS AND METHODS
2.1. Controls and leprosy patients
The leprosy patients (n=54) were divided according to the WHO operational classification,27 that is, up to five skin lesions (paucibacillary form) and more than five skin lesions (multibacillary form). Blood samples were collected from 32 multibacillary (MB) patients (14 females and 17 males, age range of 10‐80 years old; median 46 years old) and 22 paucibacillary (PB) patients (15 females and 7 males, age range of 15‐69 years old; median 44 years old) living in the cities of Santana do Ipanema and Rio Largo, Alagoas state, Brazil, or in the cities of Crato and Maracanaú, Ceará State, Brazil.
The patients were selected according to the registration at the National Notifiable Diseases System (SINAN) by the Municipal Health Secretariat. The patients were invited to go to the municipal health centers where the project was explained, and, after informed consent, a questionnaire was applied.
Seventeen individuals (who reported no contact with leprosy patients) living in São Paulo city which presents a low incidence of leprosy (1.47 cases per 100 000 inhabitants),4 were also included in the project for serological evaluation (control group ‐ CT group).
The study was approved by the Ethics Committee of Universidade Federal do Ceará, and each participant or his/her guardian was asked to sign a written informed consent.
2.2. Samples
Five milliliters of venous blood were collected into clot activator tubes containing gel separator. After 30 minutes, the samples were centrifuged at 1500× g for 10 minutes, and the serum was separated and stored at −20°C.
2.3. Serum antibody titers
Serological analyses for anti‐PGL1 antibodies were performed according to the procedure described elsewhere,28 with some modifications. Ninety‐six well flat‐bottom microplates (code 3590, Costar, USA) were coated with 5 mg/L of native PGL‐1 in absolute ethyl alcohol (50 μL per well), kindly donated by the Bei Resources/ATCC (Manassas, USA). The plates were left uncovered for 2 hour at 37°C. After, they were incubated with 1% inactivated fetal bovine serum (FBS; LGC Bio, Brazil) in phosphate‐buffered saline (PBS, pH 7.4) for 2 hour at 37°C in a humid chamber. After five washes with PBS‐0.05% FBS, serum samples previously diluted (1:200 for IgG and IgM and 1:50 for IgA) in PBS‐1% FBS were added to the plates (50 μL per well, in duplicate). The plates were then incubated for 2 hour at 37°C. After washing, peroxidase‐labeled anti‐IgG (A0170, Sigma, USA) anti‐IgM (SAB3701404, Sigma, USA) or anti‐IgA (A0295, Sigma, USA), previously diluted to 1:2500, 1:9000, 1:1000 (respectively) was added to the plates and incubated for 1.5 hour at 37°C. After washing, the plates were incubated for 30 minutes with the substrate solution (100 μL per well) which contained 0.4 mg orthophenylenediamine/mL of 0.01 mol L−1 citrate‐phosphate buffer, pH 5.0 and 0.01% H2O2 final concentration. The reaction was interrupted by adding 25 μL 2.5 N sulfuric acid. The analysis was performed at 492 nm using an ELISA plate reader (ASYS Expert Plus, Biochrom, UK). Fifty serum samples from blood donors were pooled and used as cut‐off sample in all assays. Negative and positive controls were included in each assay. The cut‐off sample and controls were run in quadruplicate.
The results were expressed in indices according to the following formula: optical density (OD) mean of the test sample (minus blank) divided by the OD mean of the normal human serum pool (minus blank).28 Blood donors were seronegative donors to HIV, Chagas, hepatitis B and C, HTLV, syphilis and that did not have leprosy at the time of sample collection.
2.4. Statistical analysis
The data were analyzed using non‐parametric tests once the data did not follow a Gaussian distribution (Kolgomorov‐Smirnov test). The Kruskall‐Wallis and the Dunn′s tests were used to compare values among three or more unpaired groups. Paired data were analyzed by the Spearman correlation test. The analyses were performed using the GraphPad Prism version 5.0 and the GraphPad Instat version 3.01 programs. The level of statistical significance was considered to be equal to or less than .05. The Spearman coefficients were also used to assess the degree of correlation between the tests. The values were interpreted as follows29: r=.10 to .30 (weak); r=.40 to .6 (moderate); r=.70 to 1 (strong). Diagnostic sensitivity and specificity, positive and negative predictive values, and the receiver operating characteristic curves were obtained by the GraphPad Prism version 5.0. The Kappa index (k) was applied to evaluate the degree of agreement between the results obtained. Kappa values can be interpreted as follows: 0, no agreement; 0 to 0.19, low concordance; 0.20 to 0.39, fair agreement; 0.40 to 0.59, moderate; 0.60 to 0.79 substantial, 0.80‐1.00, almost perfect agreement.30
3. RESULTS
3.1. Serum anti‐PGL1 antibodies in controls and leprosy patients
The patients with the multibacillary form of the disease (MB) showed much higher anti‐PGL1 IgM titers than controls (P<.0001). Similar observations were done for the IgG isotype (P<.05). For the anti‐PGL1 IgA isotype, the PB and MB patients presented higher levels than the controls (P<.05 and P<.01), respectively (Figure 1).
Figure 1.
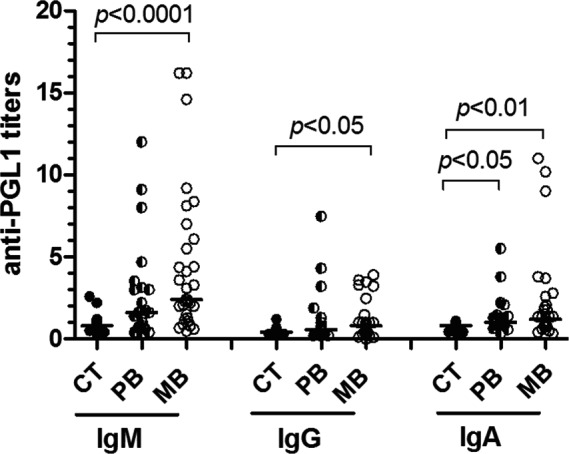
Titers of serum anti‐PGL1 IgM, IgG, and IgA in non‐endemic controls (CT, n=17), and in paucibacillary (PB, n=22) and multibacillary (MB, n=32) leprosy patients
3.2. Correlation between serum anti‐PGL1 antibody titers
A weak correlation was found between serum IgG and IgA isotypes among the MB patients (Spearman correlation, r=.363; P=.04, Figure 2A); a moderate correlation was found between serum IgM and IgG isotypes (r=.479, P=.0055, Figure 2B), and a strong correlation was found between serum IgM and IgA isotypes (r=.745, P<.0001, Figure 2C). In respect to the PB patients (n=22), a moderate correlation was found in all analyses, that is, between serum IgG and IgA isotypes (r=.509; P=.015, Figure 3A), between serum IgM and IgG isotypes (r=.645, P=.0012, Figure 3B), and between serum IgM and IgA isotypes (r=.595, P=.0035, Figure 3C).
Figure 2.
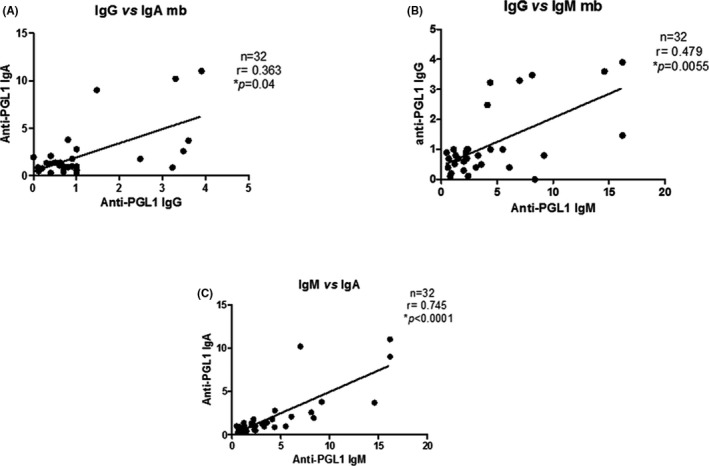
Correlation between serum anti‐PGL1 IgG, IgA, and IgM in multibacillary leprosy patients
Figure 3.
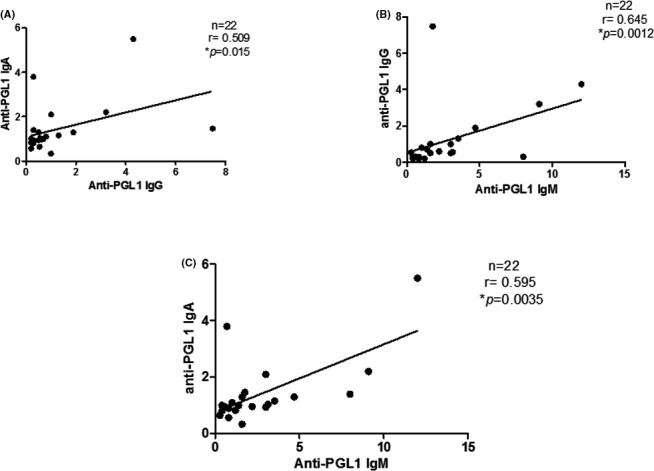
Correlation between serum anti‐PGL1 IgG, IgA and IgM in paucibacillary leprosy patients
3.3. Receiver operating characteristic (ROC) curves, diagnostic sensitivities and specificities, negative and positive predictive values
Data from patients and controls were used to build the ROC curves, as represented in Figures 4A‐C. Based on the ROC curves, it was possible to select the cut‐off values, that is, 1.1 for IgA, 1.2 for IgM and IgG tests. After selection of the cut‐off points, the following parameters were calculated: The diagnostic sensitivities for serum anti‐PGL1 IgA, IgG, and IgM in PB patients were 40.9%, 22.7%, and 59.1%, respectively, and 53.1%, 21.9%, and 81.3% for the same isotypes in MB patients (Table 1). The diagnostic specificities were 100.0% for the IgA and IgG isotypes, and 88.2% for the IgM isotype. Considering the clinical forms altogether, the diagnostic sensitivities were 50.0% for IgA, 22.2% for IgG and 74.1% for IgM. The positive (VPP) and negative (VPN) predictive values were estimated for each isotype. For IgA, the VPP and VPN were, respectively, 100.0% (87.0%‐100.0%; 95% confidence interval) and 38.7% (24.4%‐54.5%); for IgG, 100% (87.0%‐100.0%) and 28.8% (17.8%‐42.1%), respectively; and for IgM, 95.2% (83.8%‐99.4%) and 51.7% (32.5%‐70.6%), respectively.
Figure 4.
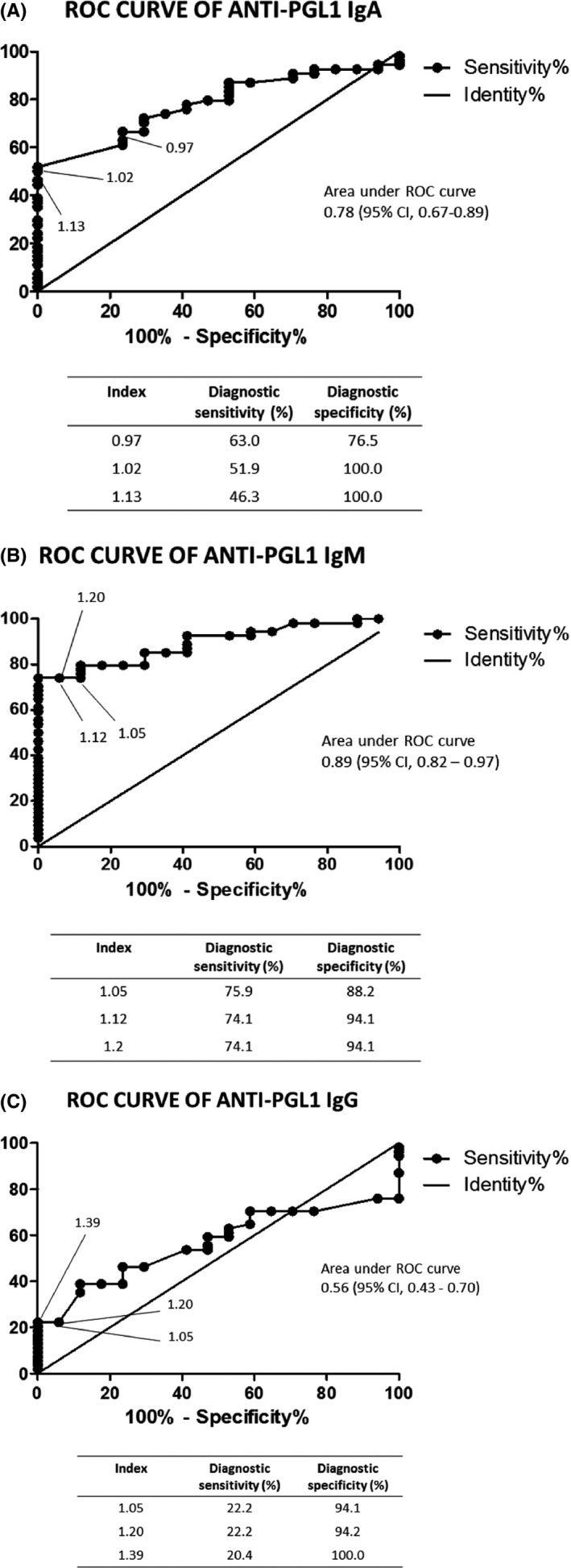
(A) Receiver operating characteristic curve for anti‐PGL1 IgA. Fifty‐four serum samples from leprosy patients were tested. (B) Receiver operating characteristic curve for anti‐PGL1 IgM. Fifty‐four serum samples from leprosy patients were tested.(C) Receiver operating characteristic curve for anti‐PGL1 IgG. Fifty‐four serum samples from leprosy patients were tested
Table 1.
Diagnostic sensitivity and specificity, negative and positive predictive values of anti‐PGL1 IgA, IgM, and IgG tests in leprosy patients with the multibacillary (n=32) and paucibacillary (n=22) forms and in non‐endemic controls (n=17). The cut‐off values were 1.1 for IgA and 1.2 for IgG and IgM isotypes
| Anti‐PGL1 | Diagnostic Sensitivity (%) (95% CI) | Diagnostic sensitivity (%) | Diagnostic Specificity (%) | Negative predictive value (%) | Positive predictive value (%) | |
|---|---|---|---|---|---|---|
| Multibacillary form | Paucibacillary form | Multibacillary + Paucibacillary forms | ||||
| IgA | 53.1 (34.7‐70.9) | 40.9 (20.7‐63.6) | 50.0 (36.0‐64.0) | 100.0 (81.0‐100.0) | 38.7 (24.4‐54.5) | 100.0 (87.0‐100.0) |
| IgG | 21.9 (9.3‐40.0) | 22.7 (7.8‐45.4) | 22.2 (12.0‐35.6) | 100.0 (81.0‐100.0) | 28.8 (17.8‐42.1) | 100.0 (87.0‐100.0) |
| IgM | 81.3 (63.6‐92.8) | 59.1 (36.4‐79.3) | 74.1 (60.4‐85.0) | 88.2 (63.6‐98.5) | 51.7 (32.5‐70.6) | 95.2 (83.8‐99.4) |
CI, Confidence interval.
3.4. Assessment of the degree of agreement between serum anti‐PGL1 IgM and anti‐PGL1 IgA tests
Serum samples from the leprosy patients (n=54) were tested for agreement between anti‐PGL1 IgA and IgM tests. The Table 2 shows the frequency of positive and negative titers for both isotypes. The index Kappa was 0.48, calculated according to described elsewhere,30 and presented a moderate agreement.
Table 2.
Assessment of the degree of agreement between serum anti‐PGL1 IgM and anti‐PGL1 IgA tests, according to the Kappa index 30
| Serum anti‐PGL1 IgA | Serum anti‐PGL1 IgM | |
|---|---|---|
| Positive titers n | Negative titers n | |
| Positive titers | 25 | 2 |
| Negative titers | 12 | 15 |
Kappa index=0.48 (moderate).
4. DISCUSSION
The high number of new cases of leprosy reported every year in Brazil reveals that the control of the disease is far from being achieved. Poor effective surveillance system, very few laboratory diagnostic tools, skin smears done without quality assurance,31 professionals not skilled in making early diagnosis 5 are some of the factors that contribute to the late diagnosis of leprosy and consequently poor control of the disease and its transmission. On this aspect, the huge challenge is to reduce transmission and to be able to identify those people who are prone to develop the disease. For this reason, search for biomarkers is absolutely primordial.32
Few considerations must be done about the technique. We have demonstrated recently 14 that 2 of 17 people who reported not to have contact with leprosy patients presented positive serum anti‐PGL1 IgM antibodies. In that work, we have found a high frequency of positive anti‐PGL1 IgM among the negative anti‐PGL1 IgG samples. The question that has arisen at that time was in respect to the real meaning of seropositivity of anti‐PGL 1 IgM antibodies. False‐positive results are described in the literature in various circumstances. For example, rheumatoid factors may be produced in low amounts in infectious diseases, such as toxoplasmosis,33 malaria,34 dengue,35 which lead to non‐specific IgM detection. Some authors have described the existence of cross‐reacting idiotypes, that is, specific antibodies that use the same antigenic site to interact with an unrelated epitope.36
In respect to the PGL‐1 antigen, as a glycophenolic molecule, it is at risk of being affected when a long period of incubation is performed. For this reason, the adsorption incubation step was reduced to 2 hours at 37o C.
Another observation was related to the blocking step. Some samples, especially those which contain high titers of antibodies, may present non‐specific reactivity to bovine serum albumin (BSA). For managing such circumstances, various concentrations of BSA in blocking solution were tested, but inhibition of nonspecific binding could not be completely achieved. Fetal bovine serum (FBS) showed more confident results with lower background reaction, besides the lower price.
The cut‐off value for each isotype was selected based on the ROC curves. Unfortunately, it was not possible to obtain very high diagnostic sensitivities.37 We speculate that for leprosy surveys, it is more important that the test presents a good specificity in spite of its sensitivity. Our suggestion is based on the fact that leprosy contacts with abnormal levels of anti‐PGL1 shall be surveyed. For this reason, it is rather necessary that the biomarker bear a good specificity in order that it will provide a good positive predictive value. On the other hand, to compensate for the low negative predictive value it is useful to use two or more serological parameters.
As it has been shown in previous studies, lipid antigen may elicit T‐dependent humoral immune responses in case the bacteria are ingested by phagocytes.38 As the presence of anti‐PGL1 IgG has been demonstrated in leprosy patients28 and in contacts,14 we have decided in the present work to evaluate anti‐PGL1 IgA titers.
IgA is being considered as an alternative or complementary biomarker in the diagnosis of some pathologies. For instance, it is considered to be more sensitive than the IgM test for the diagnosis of congenital toxoplasmosis.39 IgA is also considered to be a good candidate for the diagnosis of acute dengue by the fact it remains shorter time in circulation than IgM does.26 Finally, in the decade of 1990, it has been suggested that IgA could be used in early stages of the leprosy disease and in subclinical infection.16, 17
Our current data demonstrated a better performance of the IgA than the IgG isotype. IgG presented a moderate correlation with IgM (r=.479, P=.0055) and a very low diagnostic sensitivity even in MB patients. On the other hand, IgA showed a strong correlation with IgM (r=.745, P<.0001). Its diagnosis sensitivity (53.1%) was much higher than that of IgG (21.9%), but still far from that of IgM (81.3%) in MB patients.
The moderate Kappa index obtained between IgM and IgA probably indicates how complex the immunological mechanisms are in leprosy disease. The complexity is not only restricted to the dichotomy cellular/humoral immune response but also in the humoral immune repertoire. The PGL‐1 molecule probably behaves as a “pure” T‐independent antigen in various patients, inducing solely the IgM isotype; nonetheless, in other patients, it behaves as a “partial” T‐dependent antigen, also inducing IgG and/or IgA.
By one side, a question remains if all the individuals who show anti‐PGL1 IgM titers can be considered at risk of developing leprosy disease once the frequency of positivity is high among household contacts.14 On the other hand, is it reasonable to expect that a contact who presents positive IgA is really at high risk of developing the disease. For this reason, despite the mentioned limiting factors, we strongly recommend that IgA be considered as a biomarker adjuvant to IgM to be used in serological and clinical follow‐up studies of household leprosy contacts in municipalities with high endemicity of the disease.
ACKNOWLEDGMENTS
We gratefully acknowledge the kindly assistance provided by the health professionals, particularly, Ana Lúcia Carneiro Leal and Gilvânia França Vilela, and to the League of Neglected Diseases with emphasis on leprosy Professora Noraci Pedrosa, Maceió, Alagoas, Brazil. This research was financially supported by the MCTI/CNPq/MS‐SCTIE (process 403461/2012‐0).
de Macedo AC, Guimarães JA, Rodrigues RO, et al. Serum anti‐phenolic glycolipid—1 IgA correlates to IgM isotype in leprosy patients: a possible candidate for seroepidemiological surveys?. J Clin Lab Anal. 2018;32:e22276 10.1002/jcla.22276
REFERENCES
- 1. Huang CL. The transmission of leprosy in man. Int J Lepr Other Mycobact Dis. 1980;48(3):309‐318. [PubMed] [Google Scholar]
- 2. Job CK, Jayakumar J, Kearney M, Gillis TP. Transmission of leprosy: a study of skin and nasal secretions of household contacts of leprosy patients using PCR. Am J Trop Med Hyg. 2008;78:518‐521. [PubMed] [Google Scholar]
- 3. World Health Organization . Weekly Epidemiol. Record, v. 91, n. 35, p. 405‐420, 2016. Disponível em: http://www.who.int/wer. Accessed on March 4th, 2017.
- 4. Brasil. Ministério da Saúde. Sala de apoio à gestão estratégica, SAGE. Hanseníase. Indicadores de morbidade. Available from: http://sage.saude.gov.br/#. Accessed on March 4th, 2017.
- 5. Barreto JG, Guimarães LS, Frade MAC, et al. High rates of undiagnosed leprosy and subclinical infection amongst school children in the Amazon Region. Mem Inst Oswaldo Cruz. 2012;107:60‐67. [DOI] [PubMed] [Google Scholar]
- 6. van Beers SM, de Wit MY, Klatser PR. The epidemiology of Mycobacterium leprae: recent insight. FEMS Microbiol Lett. 1996;136:221‐230. [DOI] [PubMed] [Google Scholar]
- 7. Douglas JT, Cellona RV, Fajardo TT Jr, et al. Prospective study of serological conversion as a risk factor for development of leprosy among household contacts. Clin Diag Lab Immunol. 2004;11:897‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brennan PJ, Barrow WW. Evidence for species‐specific lipid antigens in Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1980;48:382‐387. [PubMed] [Google Scholar]
- 9. Spencer JS, Brennan PJ. The role of Mycobacterium leprae phenolic glycolipid I (PGL‐I) in serodiagnosis and in the pathogenesis of leprosy. Lepr Rev. 2011;82:344‐57. [PubMed] [Google Scholar]
- 10. Kampirapap K, Singtharn N. Anti‐PGL‐1 antibody levels in Thai leprosy patients. Southeast Asian J Trop Med Public Health. 1996;27:728‐33. [PubMed] [Google Scholar]
- 11. Zenha EM, Ferreira MA, Foss NT. Use of anti‐PGL‐1 antibodies to monitor therapy regimes in leprosy patients. Braz J Med Biol Res. 2009;42:968‐972. [DOI] [PubMed] [Google Scholar]
- 12. Barreto JG, Guimarães Lde S, Leão MR, et al. Anti‐PGL‐I seroepidemiology in leprosy cases: household contacts and school children from a hyperendemic municipality of the Brazilian Amazon. Lepr Rev. 2011;82:358‐370. [PubMed] [Google Scholar]
- 13. Lobato J, Costa MP, Reis Ede M, et al. Comparison of three immunological tests for leprosy diagnosis and detection of subclinical infection. Lepr Rev. 2011;82:389‐401. [PubMed] [Google Scholar]
- 14. Brito e Cabral P, Júnior JE, .de Macedo AC , et al. Anti‐PGL1 salivary IgA/IgM, serum IgG/IgM, and nasal Mycobacterium leprae DNA in individuals with household contact with leprosy. Int J Infect Dis. 2013;17:e1005‐10. [DOI] [PubMed] [Google Scholar]
- 15. Nagao‐Dias AT, Yokobatake‐Souza ER, Oliveira MF, et al. Títulos de anticorpos anti‐PGL1 de Mycobacterium leprae em pacientes com as diversas formas clínicas da hanseníase em diferentes períodos de tratamento In: Lessa APG, Costa JI, Silva LA. et al. eds. Série Pesquisa para o SUS Ceará. Fortaleza: Secretaria da Saúde do Estado do Ceará, Inc. 2; 2013:13‐17. [Google Scholar]
- 16. Schwerer B, Chujor CS, Bernheimer H, et al. IgA antibodies against phenolic glycolipid I from Mycobacterium leprae in serum of leprosy patients and contacts: subclass distribution and relation to disease activity. Clin Immunol Immunopathol. 1989;53:202‐211. [DOI] [PubMed] [Google Scholar]
- 17. Chujor CS, Bernheimer H, Levis WR, et al. Serum IgA1 and IgM antibodies against Mycobacterium leprae‐derived phenolic glycolipid‐I: a comparative study in leprosy patients and their contacts. Int J Lepr Other Mycobact Dis. 1991;59:441‐449. [PubMed] [Google Scholar]
- 18. Lamm ME. The IgA mucosal immune system. Am J Kidney Dis. 1988;12:384‐387. [DOI] [PubMed] [Google Scholar]
- 19. Woof JM. Immunoglobulin A: Molecular Mechanisms of Function and Role in Immune Defence In: Nimmerjahn F, ed. Molecular and Cellular Mechanisms of Antibody Activity. New York: Springer‐Verlag, Inc.; 2013:31‐60. [Google Scholar]
- 20. Mantis NJ, Rol N, Corthésy B. Secretory IgA's Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 2011;4:603‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blutt SE, Miller AD, Salmon SL, et al. IgA is important for clearance and critical for protection from rotavirus infection. Mucosal Immunol. 2012;5:712‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silva MV, Camargo ED. Teste imunoenzimático (ELISA) para detecção de anticorpos circulantes da classe IgA na leptospirose humana. Rev Inst Med Trop Sao Paulo. 1992;34:239‐242. [DOI] [PubMed] [Google Scholar]
- 23. Takahashi EE, Rossi CL. Use of three immunological techniques for the detection of Toxoplasma sp. IgA antibodies in acute toxoplasmosis. J Clin Pathol. 1994;47:1101‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Talarmin A, Labeau B, Lelarge J. Immunoglobulin A‐specific capture enzyme‐linked immunosorbent assay for diagnosis of dengue fever. J Clin Microbiol. 1998;36:1189‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nawa M, Takasaki T, Ito M, et al. Immunoglobulin A antibody responses in dengue patients: a useful marker for serodiagnosis of dengue virus infection. Clin Diagn Lab Immunol. 2005;12:1235‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Decker S, Vray M, Sistek V, et al. Evaluation of the Diagnostic Accuracy of a New Dengue IgA Capture Assay (Platelia Dengue IgA Capture, Bio‐Rad) for Dengue Infection Detection. PLoS Negl Trop Dis. 2015;9:e0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Diretrizes para vigilância, atenção e eliminação da Hanseníase como problema de saúde pública: manual técnico‐operacional [recurso eletrônico]. Brasília: Ministério da Saúde, 2016. Available from: http://portalarquivos.saude.gov.br/images/pdf/2016/fevereiro/04/diretrizes-eliminacao-hanseniase-4fev16-web.pdf. Accessed on March 7th, 2017 [Google Scholar]
- 28. Nagao‐Dias AT, Almeida TL, Oliveira MF, et al. Salivary anti‐PGL IgM and IgA titers and serum antibody IgG titers and avidities in leprosy patients and their correlation with time of infection and antigen exposure. Braz J Infect Dis. 2007;11:215‐9. [DOI] [PubMed] [Google Scholar]
- 29. Dancey CP, Reidy J, eds. Estatística sem matemática para psicologia: usando SPSS para Windows. Porto Alegre: Artmed Inc.; 2006. [Google Scholar]
- 30. Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85:257‐268. [PubMed] [Google Scholar]
- 31. Waters MFR. To smear or not to smear. Lepr Rev. 2002;73:211‐214. [PubMed] [Google Scholar]
- 32. Cairns W, Smith S, Aerts A. Role of contact tracing and prevention strategies in the interruption of leprosy transmission. Lepr Rev. 2014;85:2‐17. [PubMed] [Google Scholar]
- 33. Naot Y, Desmonts G, Remington JS. IgM enzyme‐linked immunosorbent assay test for the diagnosis of congenital Toxoplasma infection. J Pediatric. 1981;98:32‐36. [DOI] [PubMed] [Google Scholar]
- 34. Iqbal J, Sher A, Rab A. Plasmodium falciparum Histidine‐Rich Protein 2‐Based Immunocapture Diagnostic Assay for Malaria: cross‐Reactivity with Rheumatoid Factors. J Clin Microbiol. 2000;38:1184‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hunsperger EA, Yoksan S, Buchy P, et al. Evaluation of commercially available anti‐dengue virus immunoglobulin M tests. Emerg Infect Dis. 2009;15:436‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karol R, Reichlin M, Nobles RW. Idiotypic cross‐reactivity between antibodies of different specificities. J Exp Med. 1978;148:1488‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96:644‐647. [DOI] [PubMed] [Google Scholar]
- 38. Nagao AT, Friedlander‐Del Nero D, Arslanian C, et al. Elevated levels and different repertoire profile of colostral anti‐LPS antibodies may have a significant role in compensating newborn immunity. Scand J Immunol. 2001;53:602‐609. [DOI] [PubMed] [Google Scholar]
- 39. Center for Disease Control and Prevention (CDC) . DPDx – Laboratory Identification of Parasitic Diseases of Public Health Concern. Atlanta: Division of Parasitic Diseases and Malaria (DPDM); 2017. Available from: http://www.cdc.gov/dpdx/toxoplasmosis/dx.html. Accessed on March 4th, 2017. [Google Scholar]


