Abstract
Background
Being able to detect the presence of autoantibodies to interferon (IFN)‐γ in serum is essential for evaluating patients with suspected adult‐onset immunodeficiency (AOID) with unusual intracellular infections. Most reported patients with AOID have been Asian, although the exact prevalence of this illness is unknown. To date, no standard assay exists to detect autoantibodies to IFN‐γ. An easy‐to‐use, low‐cost assay that can be performed in any laboratory would be a valuable tool for clinical management of AOID, as well as better reveal its prevalence.
Methods
Our experimental study exploited a dot enzyme‐linked immunosorbent assay (Dot‐ELISA) strip to detect autoantibodies to IFN‐γ. Sera from 66 HIV‐negative patients having autoantibodies to IFN‐γ as determined by indirect ELISA were tested.
Results
Dot enzyme‐linked immunosorbent assay was sensitive (100%) and specific (94.5%), with a positive predictive value of 97.6% and a negative predictive value of 100%.
Conclusion
This simple method provides prompt qualitative results that can be read visually and used in facilities with limited testing capabilities.
Keywords: adult‐onset immunodeficiency, autoantibody, dot enzyme‐linked immunosorbent assay, interferon gamma, screening
1. INTRODUCTION
Adult‐onset immunodeficiency (AOID) is an immunodeficiency syndrome associated with intracellular infections, especially nontuberculous Mycobacteria (NTM), along with other opportunistic microorganisms.1, 2, 3 Many reports have described an association of the syndrome with the presence of autoantibodies to interferon (IFN)‐γ. Most patients that have been diagnosed with AOID have been of Asian extraction and were negative to human immunodeficiency virus (HIV).1, 4
No standard assay exists to measure autoantibodies to IFN‐γ, either qualitatively or quantitatively. Most commonly, sera from reported patients have been tested for the existence of autoantibodies to IFN‐γ by immunological assay, that is, enzyme‐linked immunosorbent assay (ELISA),1, 5 antigen capture assay.6, 7 Most reports of AOID associated with the presence of autoantibodies to IFN‐γ, including ours, were from teaching hospitals, where patients might be referred from smaller healthcare centers.1, 4, 8, 9 As assays for autoantibodies to IFN‐γ in general hospitals may not be readily available, the number of cases of AOID may be substantially underestimated. In this study, we described a simple, user‐friendly, in‐house dot enzyme‐linked immunosorbent assay (Dot‐ELISA) for detecting autoantibodies to IFN‐γ.
2. MATERIAL AND METHODS
Sera were collected from 66 HIV‐negative patients having a history or being infected with proven opportunistic infections at Chiang Mai University Hospital, Chiang Mai, Thailand, as described previously.1 All patients were positive for autoantibodies to IFN‐γ, as determined by indirect ELISA. Control sera were collected from 30 healthy individuals who gave negative results to autoantibodies to IFN‐γ. The ethics committees of the Research Institute for Health Sciences and the Faculty of Medicine, Chiang Mai University approved this study. All participants were enrolled into the study with written consent. All procedures were performed in accordance with the ethical standards and with the 1964 Helsinki Declaration.
Nitrocellulose membrane (0.45 μm pores; Bio‐Rad Laboratories, USA) was prepared as strips of 0.4 × 2.5 cm. All antigens and antibodies were titrated for appropriate concentrations before use. Discrete dots of 1 μL of 20 μg/mL of IFN‐γ (R&D, Minneapolis, USA), human serum albumin (Sigma‐Aldrich, MO, USA), and the F(ab′)2 fragment donkey anti‐human IgG (H + L) (Jackson ImmunoResearch, West Grove, PA, USA) were deposited on the center line of each strip. The gap between dots was 7 mm. (Figure 1A). The strips were dried at ambient temperature for at least 24 hours.
Figure 1.
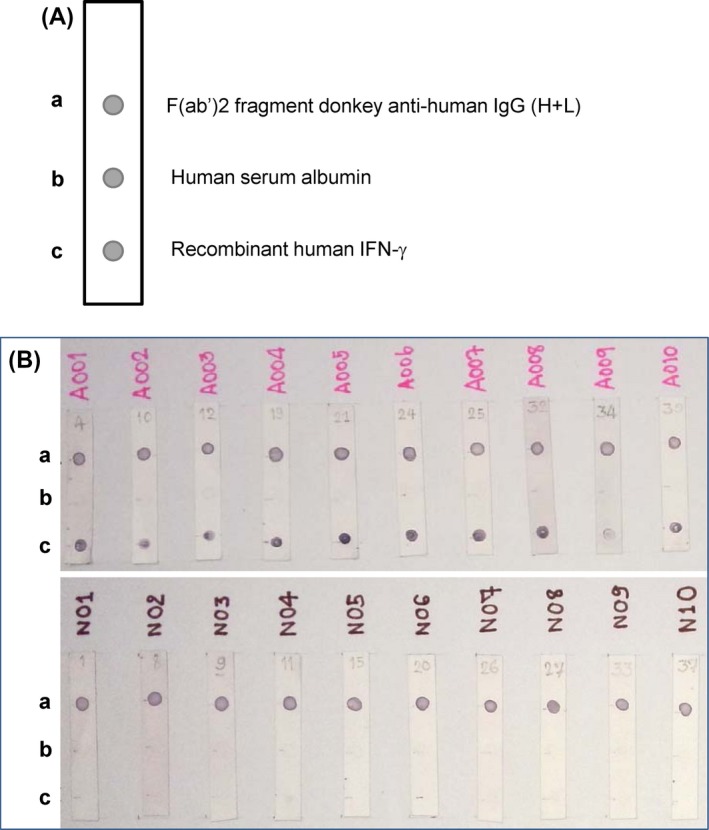
A, Schematic illustration of a dot enzyme‐linked immunosorbent assay (Dot‐ELISA) strip. a: positive control spot with donkey anti‐human IgG, b: negative control spot with human serum albumin, and c: test spot with recombinant human interferon (IFN)‐γ. B, Examples of Dot‐ELISA results obtained with sera from samples that gave positive (upper panel) and negative results (lower panel) to autoantibodies to IFN‐γ determined by ELISA. a, b, and c represent the positions of donkey anti‐human IgG, human serum albumin, and recombinant human IFN‐γ, respectively
The experiment was designed as single blind with 3 repetitions. Individual strips were put into 1.5‐mL microcentrifuge tubes, and the strips were soaked with 10% skimmed milk in PBS for 60 minutes. After discarding the solution, 1 mL of individual serum diluted 1:100 was added into each tube and incubated for 60 minutes. The strips were washed 5 times with 0.05% Tween‐20 in PBS, and 1 mL of 1:1000 diluted Biotin‐SP‐conjugated AffiniPure F(ab′)2 fragment donkey anti‐human IgG (Jackson ImmunoResearch) was added to each tube and incubated for 60 minutes. After washing, the strips were incubated in 1:1000 diluted alkaline phosphatase‐conjugated streptavidin (Vector Laboratories, Burlingame, CA, USA) for 60 minutes. All strips were transferred into a reservoir containing BCIP/NBT substrate (Vector Laboratories) and stirred for 15 minutes. The reaction was stopped by immersing the strips in distilled water; the strips were then left to dry. The results were read 15‐20 minutes after stopping the reaction.
3. RESULTS
This study used human serum albumin and donkey anti‐human IgG as negative and positive controls, respectively. A positive result was identified when visible dark‐purple dots appeared at the positions of anti‐human IgG and recombinant IFNγ; a negative result was read when a dot appeared only at the position of anti‐human IgG (Figure 1B). No dot was observed at the position of human serum albumin.
The results from 3 repetitions are shown in Table 1. The average sensitivity and specificity of Dot‐ELISA compared with indirect ELISA were 100% and 94.45%, respectively. Positive predictive value (PPV), which indicates the probability that the disease is present when the test is positive, and negative predictive value (NPV), which indicates the probability that the disease is not present when the test is negative, were 97.56% and 100%, respectively.
Table 1.
Number of samples tested by Dot‐ELISA, sensitivity, specificity, and positive and negative predictive values of Dot‐ELISA in detecting autoantibody to IFN‐γ
| Patients (N = 66) | Healthy controls (N = 30) | |||||||
|---|---|---|---|---|---|---|---|---|
| Dot‐ELISA assay | Positive | Negative | Positive | Negative | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| 1st Run | 66 | 0 | 3 | 27 | 100.00 | 90.00 | 95.65 | 100.00 |
| 2nd Run | 66 | 0 | 1 | 29 | 100.00 | 96.67 | 98.51 | 100.00 |
| 3rd Run | 66 | 0 | 1 | 29 | 100.00 | 96.67 | 98.51 | 100.00 |
Dot‐ELISA, dot enzyme‐linked immunosorbent assay; IFN, interferon; NPV, negative predictive valve; PPV, positive predictive value.
4. DISCUSSION
Thus far, in‐house assays, mostly ELISA‐based techniques, have been used to detect autoantibodies to IFN‐γ. In our setting, sera from suspected cases were sent to the laboratory sporadically, testing few samples by ELISA was quite costly, given that serial dilutions of positive control serum must be included. The Dot‐ELISA technique developed here allows prompt screening of each suspected case at lower cost.
The strips can be prepared ahead of time in a large batch and stored for later use; the strips have an excellent shelf life, with the same results obtained from strips prepared over a year before (data not shown). As the result can be read visually and no special equipment is needed, the strips could be distributed to public health centers with suspected AOID cases or from where cases were referred. This may increase the chances of accurately diagnosing AOID, improving clinical management and better revealing the scale of the problem.
Our Dot‐ELISA assay has some limitation when compared with standard ELISA. The assay provides qualitative results, therefore the relative concentrations of autoantibodies to IFN‐γ could not be determined. Dot‐ELISA strip also reported some false positives. Several factors are known to contribute to false positivity in immunoassays, such as changes in pH and ionic strength of the reaction mixture or spilling over of reagents that interfere with the color development.10 Other factors causing false positive reaction in immunoassays include rheumatoid factors,11, 12 heterophilic antibodies,13 serum macroglobulins from patients with systemic lupus erythematosus,14 and other proteins.10 Evaluating the factors causing nonspecific binding would improve the effectiveness of these strips. Nevertheless, our strips have demonstrated good sensitivity and specificity, with high PPV and NPV, indicating their potential for rapid detection of autoantibodies to IFN‐γ.
In conclusion, our Dot‐ELISA strip was a modified ELISA‐based method for more practical detection of autoantibodies to IFN‐γ in each suspected AOID case; it was quick and economical, required no special technique or equipment, and the results could be read visually. As this syndrome has a high mortality rate (from our observation, 32% of patients died at the median time of 25 months after diagnosis 1), this method may be helpful in diagnosing AOID, predicting its prognosis, and interpreting treatment outcomes.
ACKNOWLEDGMENTS
This research was supported by the Faculty of Medicine Research Fund and the National Research University Project under Thailand's Office of the Higher Education Commission, Chiang Mai University, Chiang Mai, Thailand. We are grateful to all participants, to Narumon Tachawong for her technical assistance, to Mr. Andrew Adam for editorial assistance and to the staff of the Department of Internal Medicine of the Chiang Mai University Hospital for recruiting participants.
Rattanathammethee K, Chawansuntati K, Chaiwarith R, Praparattanapan J, Supparatpinyo K, Wipasa J. Dot enzyme‐linked immunosorbent assay strip as a screening tool for detection of autoantibody to interferon gamma in sera of suspected cases of adult‐onset immunodeficiency. J Clin Lab Anal. 2018;32:e22460 10.1002/jcla.22460
REFERENCES
- 1. Wongkulab P, Wipasa J, Chaiwarith R, Supparatpinyo K. Autoantibody to interferon‐gamma associated with adult‐onset immunodeficiency in non‐HIV individuals in Northern Thailand. PLoS One. 2013;8:e76371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang HH, Huang YC, Chen WY, Chiang YY. Subcutaneous Sweet syndrome associated with disseminated nontuberculous mycobacterial infection leading to the diagnosis of adult‐onset immunodeficiency. J Am Acad Dermatol. 2014;71:e20‐e22. [DOI] [PubMed] [Google Scholar]
- 3. Lee WI, Huang JL, Wu TS, et al. Patients with inhibitory and neutralizing auto‐antibodies to interferon‐gamma resemble the sporadic adult‐onset phenotype of Mendelian Susceptibility to Mycobacterial Disease (MSMD) lacking Bacille Calmette‐Guerin (BCG)‐induced diseases. Immunobiology. 2013;218:762‐771. [DOI] [PubMed] [Google Scholar]
- 4. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult‐onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367:725‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madariaga L, Amurrio C, Martin G, et al. Detection of anti‐interferon‐gamma autoantibodies in subjects infected by Mycobacterium tuberculosis . Int J Tuberc Lung Dis. 1998;2:62‐68. [PubMed] [Google Scholar]
- 6. Shima K, Sakagami T, Tanabe Y, et al. Novel assay to detect increased level of neutralizing anti‐interferon gamma autoantibodies in non‐tuberculous mycobacterial patients. J Infect Chemother. 2014;20:52‐56. [DOI] [PubMed] [Google Scholar]
- 7. Nei T, Okabe M, Mikami I, et al. A non‐HIV case with disseminated Mycobacterium kansasii disease associated with strong neutralizing autoantibody to interferon‐gamma. Respir Med Case Rep. 2013;8:10‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kampitak T, Suwanpimolkul G, Browne S, Suankratay C. Anti‐interferon‐gamma autoantibody and opportunistic infections: case series and review of the literature. Infection. 2011;39:65‐71. [DOI] [PubMed] [Google Scholar]
- 9. Pruetpongpun N, Khawcharoenporn T, Damronglerd P, et al. Disseminated talaromyces marneffei and Mycobacterium abscessus in a patient with anti‐interferon‐gamma autoantibodies. Open Forum Infect Dis. 2016;3:ofw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004;25:105‐120. [PMC free article] [PubMed] [Google Scholar]
- 11. Norden AG, Jackson RA, Norden LE, Griffin AJ, Barnes MA, Little JA. Misleading results from immunoassays of serum free thyroxine in the presence of rheumatoid factor. Clin Chem. 1997;43(6 Pt 1):957‐962. [PubMed] [Google Scholar]
- 12. Li YC, Yang F, Ji XY, Fang ZJ, Liu J, Wang Y. False human immunodeficiency virus test results associated with rheumatoid factors in rheumatoid arthritis. Chin Med Sci J. 2014;29:103‐106. [DOI] [PubMed] [Google Scholar]
- 13. Ward G, McKinnon L, Badrick T, Hickman PE. Heterophilic antibodies remain a problem for the immunoassay laboratory. Am J Clin Pathol. 1997;108:417‐421. [DOI] [PubMed] [Google Scholar]
- 14. Engler HD, Shea YR. Effect of potential interference factors on performance of enzyme immunoassay and latex agglutination assay for cryptococcal antigen. J Clin Microbiol. 1994;32:2307‐2308. [DOI] [PMC free article] [PubMed] [Google Scholar]


