Abstract
Background
The homeostasis of essential trace elements such as selenium and manganese may be altered in patients with severe diseases of various etiologies (trauma brain injuries, tumors, leukemias, lymphomas, neurological diseases).
Methods
Concentration of manganese and selenium were determined in cerebrospinal fluid by electrothermal atomic absorption spectrometry in 50 hospitalized children with various clinical ethiologies including oncological, neurological, and brain related diseases.
Results
The concentrations of manganese in cerebrospinal fluid of children were 0.97±0.67 μg/L. The concentrations of selenium were 13.3±3.5 μg/L. The concentrations were similar as published in adults. The values did not correlated with the age, gender and severity of the disease.
Conclusion
We evaluated values of selenium and manganese in cerebrospinal fluid of seriously diseased children.
Keywords: cerebrospinal fluid, children, electrothermal atomic absorption spectroscopy, manganese, selenium, values
1. Introduction
The cerebrospinal fluid (CSF) has the important potential to reveal malfunction in many diseases. The CSF contains small molecules, salt, ions peptides and proteins that play critical roles in many physiological processes. Trace elements plays important role in metabolic, synthetic and biochemical activities in living cells. They are an integral parts of enzymes and protein structures. Previously published data show that in different pathological processes, alterations have been reported in composition of trace elements in CSF.1, 2, 3, 4 The studies are mainly focused on investigation in adults, data for children are scarce.5 Aim of the presented study was to estimate the reference values of selected trace elements (manganese and selenium) in critically ill children.
2. Materials and Methods
2.1. Patient selection and sample collection
Fifty children with severe diseases of various etiologies including oncological, neurological, and brain related diseases were enrolled into the study (Table 1). A CSF specimen was extracted by means of lumbar puncture through the third or fourth lumbar invertebral space. All samples were collected in the plastic metal free polyethylene tubes, centrifuged at 3000 g at 4°C for 10 minutes and then were immediately deep frozen and stored at −80°C for further analysis. Samples containing blood were excluded from the further investigation. Samples from patients were obtained in accordance with the Helsinki Declaration of 1964, as revised in 2000. CSF samples for Se and Mn were obtained as regular samples for routine testing in these patients, no extra sampling was needed. Parents signed informed consent with analysis due to the fact that there was no extra collection of CSF sample. Ethical board of the University Hospital Motol approved the study.
Table 1.
Subjects characteristics
| Patients | Oncological diseases | Neurological diseases | Brain related diseases | |
|---|---|---|---|---|
| N | 50 | 19 | 11 | 20 |
| M/F | 28/22 | 13/6 | 4/7 | 11/9 |
| Mean age (range) years | 5.1 (0.3‐17) | 5.5 (0.3‐17) | 7.6 (1‐13) | 4.4 (0.5‐16) |
| Se (mean±SD) μg/L | 13.3±3.5 | 13.8±3.9 | 9.8±6.3 | 12.5±3.5 |
| Mn (mean±SD) μg/L | 0.97±0.67 | 1.11±0.66 | 0.74±0.51 | 0.88±0.78 |
| Total Protein (mean with range) mg/L | 510 (64‐3198) | 378 (104‐3198) | 563 (168‐2610) | 615 (64‐1943) |
2.2. Methods
An electrothermal atomic absorption spectrometry (AAS) with Zeeman correction on Varian SpectrAA220Z spectrometer (Varian, Mulgrave, Australia) was used for determination of the selenium and manganese in cerebrospinal fluid. The following wavelengths were used, Se: 196 nm, Mn: 279.5 nm. The limit of detection (3SD of blank) for Se was 2.9 μg/L, limit of quantification (10 SD of blank) was 8.7 μg/L. For Mn, the limit of detection (3 SD of blank) was 0.26 μg/L, limit of quantification (10 SD of blank) was 0.87 μg/L. High grade chemicals evaluated for AAS trace element analysis (acquired from Fluka Analytika, Germany) were used for the preparation of Se and Mn working standards. ClinChek Serum Controls, Level 1 and 2 were used for QC evaluation. The measured values for controls were as follows: ClinChek, Level 1: Se=68.12 μg/L (control range 50.6‐76 μg/L), Mn=31.06 μg/L (control range 22.7‐34.1 μg/L), Level 2: Se=103 μg/L (control range 82.4‐124 μg/L), Mn=30.96 (control range 26.2‐34.2 μg/L).
Additionally, total protein was investigated in all CSF samples to check the blood brain barrier integrity using commercially available turbidimetric assay on Integra 400 analyzer (Roche, Mannheim, Germany).
2.3. Statistical analysis of the results
The D′Agostino Pearson omnibus normality test was used to determine the data distribution.
Correlation of Mn and Se values with age was calculated by using ether by Pearson or by Spearman correlation coefficient. The differences between groups were investigated either by the unpaired t‐test in case of normal data distribution or by the non parametric Mann–Whitney U‐test. A value of P<.05 was considered as significant. Multiple linear regression analysis was applied to assess the association between CSF selenium and manganese values (dependent variables) and other variables such as the age, gender, barrier integrity and severity of the disease. Statistical Software GraphPad Prism version 6.01 (San Diego, DA, USA) and MedCalc version 13.0.1 (Oostende, Belgium) were used for data evaluation.
3. Results
We evaluated CSF concentrations of Mn and Se in critically ill children. The obtained values were as follows: Se: 13.3±3.5 μg/L and Mn 0.97±0.67 μg/L.
Distribution of Se and Mn values in CSF in children is summarized in Figure 1.
Figure 1.
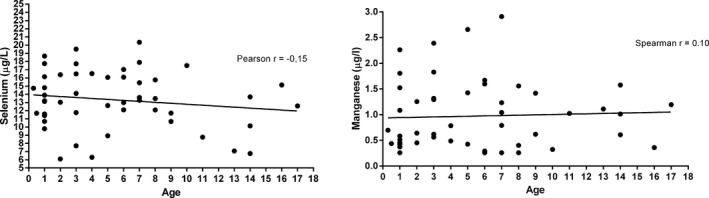
Distribution of Se and Mn values in CSF
4. Discussion
Concentrations of Mn and Se in CSF correlated well with the results published in other studies.2, 3, 6, 7, 8 The relationship of altered trace elements concentrations particularly with various neurological diseases including Parkinson disease, Alzheimer disease, progressive central and peripheral demyelinating disease1, 4, 9 and oncological diseases 10 was referred. Investigation of plasma trace elements including Selenium and Manganese are important in patients on long‐term parenteral nutrition and suffering from chronic illnesses.
We were interested in possible changes in CSF concentrations in studied groups comparing with published reference intervals.
Currently, reference values for Se in cerebrospinal fluid of children with neurological diseases were published,5 nevertheless, presented study was first study focused on investigation of Mn and Se in CSF of children with severe diseases of various etiologies.
It was shown that concentrations of Mn and Se are related to the applied analytical method.5, 8, 11
Typically, determination methods applied were atomic absorption spectrometry (AAS), inductively coupled plasma atomic emission spectrometry (ICP‐AES) and ICP‐ mass spectrometry (ICP‐MS). For AAS, the published reference values for Mn in CSF (expressed as mean±SD) ranged between 0.97±0.34 μg/L to 6±1.3 μg/L,8, 11 for Se in CSF the values (expressed as mean±SD) ranged between 13.5±8.2 μg/L to 22.7±2.1 μg/L.8, 11 We evaluated values of Mn and Se in CSF of critically ill children. The concentrations were as follows: Se: 13.3±3.5 μg/L and Mn 0.97±0.67 μg/L. The presented results showed that the values of Se and Mn in CSF of critically ill children were similar with published values in adults.
Previously published data showed that the concentration of elements in cerebrospinal fluid can be related to the age.7, 9 Our results showed, that the correlations of manganese and selenium in CSF of critically ill children with the age were weak (Se: Pearson r=−.15, P=.28, Mn: Spearman r=.10, P=.48), as is shown in Figure 1 and thus, the values were not significantly related to age.
It is well known, that the concentrations of trace elements in CSF are strongly affected by the various clinical states including infection, inflammatory, tumors, injuries, blood occurrence in the spinal fluid which alter blood brain barrier integrity. Concentrations of total protein in CSF elevated above the age specific upper reference limit characterized possible alteration of the barrier, although the concentrations of total protein in the reference range are associated with non‐altered barrier. We assessed concentration of total protein in CSF to investigate the possible role of barrier leakage for the final Se and Mn concentration. We found that the concentrations of Se and Mn in CSF in patients with various clinical states were similar (Se: P=.51, Mn: P=.54), and thus the concentrations of Mn and Se in CSF were not significantly related to the barrier integrity.
Recent studies considered neurological patients as control group.5, 8, 11 Our study consisted group of severely ill children of various severe etiologies including neurological, oncological, and brain related diseases (see Table 1). This heterogeneity will affect the evaluation of the values. For this, we evaluated comparison of Mn and Se concentrations in CSF of patients with neurological diseases, oncological diseases and brain related diseases and we did not found significant differences (Se: P=.07, Mn: P=.08). This showed that the severity of the disease did not significantly affect the concentrations and thus, we can evaluated Mn and Se levels from whole group. Previously performed findings were confirmed by the multiple regression analysis showing that Se and Mn in CSF were not related to the age, gender, and severity of the disease.
Presented study has some limitations. First, the results need to be verified on the large number of samples. Second, the analytical methodology (GFAAS) and QC samples used in this study are routinely developed for investigation of Mn and Se in blood, Third, review data of the reference values in adults used for comparison mentioned an uncompleted data about the QC and should be considered with caution.
We evaluated values of selenium and manganese in cerebrospinal fluid of severely ill children. We did not find any correlation with the age, gender, severity of the disease. The results need to be verified on the large number of the samples.
Acknowledgment
We would like to express our thanks to Mrs. Romana Pospíšilová and Ms. Lucie Jiraková for their excellent technical assistance.
Franěk T, Kotaška K, and Průša R. Manganese and selenium concentrations in cerebrospinal fluid of seriously ill children. J Clin Lab Anal. 2016;31:e22122 10.1002/jcla.22122
References
- 1. Gerhardsson L, Lundh T, Minthon L, Londos E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;25:508–515. [DOI] [PubMed] [Google Scholar]
- 2. Alimonti A, Bocca B, Pino A, Ruggieri F, Forte G, Sancesario G. Elemental profile of cerebrospinal fluid in patients with Parkinson's disease. J Trace Elem Med Biol. 2007;21:234–241. [DOI] [PubMed] [Google Scholar]
- 3. Forte G, Bocca B, Senofonte O, et al. Trace and major elements in whole blood, serum, cerebrospinal fluid and urine of patients with Parkinson's disease. J Neural Transm (Vienna). 2004;111:1031–1040. [DOI] [PubMed] [Google Scholar]
- 4. Gellein K, Skogholt JH, Aaseth J, et al. Trace elements in cerebrospinal fluid and blood from patients with a rare progressive central and peripheral demyelinating disease. J Neurol Sci. 2008;266:70–78. [DOI] [PubMed] [Google Scholar]
- 5. Tondo M, Moreno J, Casado M, et al. Selenium concentration in cerebrospinal fluid samples from a paediatric population. Neurochem Res. 2010;35:1290–1293. [DOI] [PubMed] [Google Scholar]
- 6. Qureshi GA, Qureshi AA, Memon SA, Parvez SH. Impact of selenium, iron, copper and zinc in on/off Parkinson's patients on L‐dopa therapy. J Neural Transm. 2006;Suppl:229–236. [DOI] [PubMed] [Google Scholar]
- 7. Aguilar MV, Jimenez‐Jimenez FJ, Molina JA, et al. Cerebrospinal fluid selenium and chromium levels in patients with Parkinson's disease. J Neural Transm (Vienna). 1998;105:1245–1251. [DOI] [PubMed] [Google Scholar]
- 8. Speziali M, Di Casa M, editors. Minor and Trace Elements in Cerebrospinal Fluid of Parkinson's Patients – Suggestions After a Critical Review of the Analytical Data. Rijeka: InTech; 2011:149–164. [Google Scholar]
- 9. Gazzaniga GC, Ferraro B, Camerlingo M, Casto L, Viscardi M, Mamoli A. A case control study of CSF copper, iron and manganese in Parkinson disease. Ital J Neurol Sci. 1992;13:239–243. [DOI] [PubMed] [Google Scholar]
- 10. El‐Yazigi A, Al‐Saleh I, Al‐Mefty O. Concentrations of Ag, Al, Au, Bi, Cd, Cu, Pb, Sb, and Se in cerebrospinal fluid of patients with cerebral neoplasms. Clin Chem. 1984;30:1358–1360. [PubMed] [Google Scholar]
- 11. Michalke B, Nischwitz V. Review on metal speciation analysis in cerebrospinal fluid‐current methods and results: a review. Anal Chim Acta. 2010;682:23–36. [DOI] [PubMed] [Google Scholar]


