Abstract
Background
We found that it is not necessary to simultaneously detect both creatinine (CREA) and urea until the concentration of CREA is lower than the certain level. To reduce urea testing, we suggest measuring urea only when CREA or estimated glomerular filtration rate (eGFR) exceeds a predetermined limit.
Materials and methods
CREA and urea data were analyzed consisting of almost all of people age above 65 years old check‐up (n=95441) in Shuyang countryside, and inpatients (n=101631), outpatients (n=18474) and Routine Health Check‐up (n=20509) in Shuyang People's Hospital. The proportions of elevated urea were derived. The data used in this study was generated from people more than 13 years old in both outpatients and inpatients.
Results
When the limits for initiating urea testing were used at 85 μmol/L CREA and 120 mL/min/1.73 m2 eGFR, the percentage of unnecessary urea test are 94.5% and 64.7% (elderly health check‐up), 67.9% and 84.5% (outpatients), 88.5% and 73.2% (inpatients), 92.2% and 81.7% (routine health check‐up). The missing rate of urea are 1%, 2.5%, 4.6% and 9.2%, 0.1%, 0.4%, 0.9% and 1.8%, 0.4%, 0.8%, 1.4%, and 2.5%, 0.05%, 0.1%, 1.1%, and 0.8% of ureas exceeding 9.28 mmol/L and 8.3 mmol/L in above each group, respectively. If the CREA≤85 μmol/L or eGFR≥90 mL/min/1.73 m2, there is 97.5% urea <10.1 mmol/L, the proportion of elevated urea missed is 2.5%.
Conclusions
We suggest that the initiating urea testing should be based on the upper limit of Reference Intervals serum CREA of females or a 120 mL/min/1.73 m2 eGFR limit. Conservatively, the urea testing would be reduced by 65% at least.
Keywords: creatinine, estimated glomerular filtration rate, kidney function tests, urea
1. Introduction
Creatinine (CREA) and urea or BUN are commonly ordered in clinical application for assessing the progression of kidney diseases. As we all know, when the functions of the kidney are decreased, circulating blood urea and CREA will be increased, and the blood urea and CREA will drop down while the kidney functions recovered. The Blood urea and CREA are positively correlated. An elevated CREA will usually be accompanied by an elevated urea. However, blood CREA and urea are not identical in the evaluation of kidney functions. CREA is believed a more specific indicator of kidney disease than urea.1 In American clinical laboratory, the groups of BMP (basic metabolic panel)2 and CMP (comprehensive metabolic panel)3, 4 recommend both tests of urea and CREA to patients. Thus, most clinical laboratories in the world follow these criteria that both CREA and urea are ordered for patients with kidney disease. However, according to statistics of large amounts of data, we conclude that it is not necessary to simultaneously detect urea and CREA.
The present study aims to reduce urea tests that are ordered in tandem with CREA or eGFR (CREA‐based estimating equations for estimated glomerular filtration rate), which is used for the CKD‐EPI1 exceeds a predetermined limit.1, 5, 6 Through the retrospective analysis of paired CREA and urea data, we determined the proportions of elevated urea that would not be measured based on the CREA or eGFR limit.
2. Materials and Methods
2.1. Materials
We analyzed 3 years of paired urea and CREA test results obtained from a single, large outpatients and inpatients in Shuyang People's Hospitals and routine health check‐up (routine), and almost the health check‐up for elderly, only once for everyone between April 26, 2012, and December 31, 2015 in Shuyang countryside. The people of the <13 years of age of outpatients and inpatients were deleted, and the same of name and years old were removed too. Table 1 shows the characteristics of the participants.
Table 1.
The characteristics of the participants
| Institution | n | Age (y) | Urea (mmol/L) | CREA (μmol/L) | eGFR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5% | 50.0% | 97.5% | 2.5% | 50.0% | 97.5% | 2.5% | 50.0% | 97.5% | 2.5% | 50.0% | 97.5% | |||
| Elderly | Male | 39 185 | 65 | 70 | 85 | 3.84 | 6.50 | 11.0 | 46.0 | 66.0 | 103 | ‐ | ‐ | ‐ |
| Female | 56 256 | 65 | 70 | 85 | 3.60 | 6.00 | 10.3 | 39.0 | 53.0 | 85.0 | ‐ | ‐ | ‐ | |
| Total | 95441 | 65 | 70 | 85 | 3.67 | 6.20 | 10.7 | 40.0 | 59.0 | 96.0 | 54.4 | 86.2 | 110 | |
| Outpatient | Male | 9839 | 15 | 48 | 83 | 2.60 | 5.20 | 25.1 | 42.0 | 73.0 | 857 | ‐ | ‐ | ‐ |
| Female | 8635 | 18 | 48 | 79 | 2.36 | 4.80 | 23.8 | 35.8 | 54.2 | 661 | ‐ | ‐ | ‐ | |
| Total | 18 474 | 15 | 48 | 81 | 2.43 | 5.00 | 24.3 | 37.0 | 64.0 | 760 | 24.6 | 94.7 | 154 | |
| Inpatient | Male | 48 667 | 16 | 53 | 87 | 1.80 | 4.50 | 13.5 | 21.0 | 64.9 | 178 | ‐ | ‐ | ‐ |
| Female | 52 964 | 15 | 44 | 82 | 1.80 | 4.10 | 10.2 | 23.1 | 49.1 | 105 | ‐ | ‐ | ‐ | |
| Total | 101 631 | 16 | 48 | 85 | 1.80 | 4.30 | 11.8 | 22.0 | 56.0 | 144 | 34.1 | 105 | 189 | |
| Routine | Male | 12 716 | 25 | 39 | 65 | 3.00 | 5.00 | 8.20 | 51.0 | 72.0 | 98.0 | ‐ | ‐ | ‐ |
| Female | 7793 | 22 | 31 | 65 | 2.50 | 4.30 | 7.30 | 39.8 | 54.0 | 77.0 | ‐ | ‐ | ‐ | |
| Total | 20 509 | 23 | 35 | 65 | 2.70 | 4.70 | 7.90 | 41.5 | 63.0 | 93.6 | 85.1 | 114 | 142 | |
“‐” need not. eGFR (mL/min/1.73 m2) was calculated with using CKD‐EPI.5
2.2. Methods
We computed the reduction in urea testing and the proportions of elevated urea that would be missed when the CREA or eGFR limit was varied from 53 to 300 μmol/L or 20 to 120 mL/min/1.73 m2. Two limits which were used to define an elevated urea are 9.28 mmol/L (=26 mg/dL)7 and the upper limit of Reference Intervals of urea for our lab was 8.3 mmol/L.
All things considered with the upper limit of Reference Intervals (or normal results) of CREA for our lab were 104 μmol/L (male) and 84 μmol/L (female) and the Reference Intervals (or normal results) of elderly of the local region.8 We tabulated the proportions of elevated and nonelevated urea that would not be done using a cut‐off of 85 and 100.9 mmol/L for CREA and a cutoff of 90 and 120 mL/min/1.73 m2 for eGFR.
The urea (liquid, UV‐GLDH Method) and CREA (Sarcosine Oxidase‐PAP Method) values were measured by the TBA2000FR automatic biochemical analyzer (Toshiba Co., Ltd., Japan) were adjusted after being the installer. The quality of results for urea and CREA were validated by regular internal quality control (IQC) procedures and participation in an External Quality Assessment Scheme (EQAS), eGFR (mL/min/1.73 m2) use for CKD‐EPI.5
| Sex | Serum creatinine (mg/dL) | eGFR (mL/min/1.73 m2) |
|---|---|---|
| Female | ≤0.7 | 151×(0.993)Age×(Scr/0.7)−0.328 |
| Female | >0.7 | 151×(0.993)Age×(Scr/0.7)−1.210 |
| Male | ≤0.9 | 149×(0.993)Age×(Scr/0.7)−0.415 |
| Male | >0.9 | 149×(0.993)Age×(Scr/0.7)−1.210 |
1 mg/dL (CREA)=88.42 μmol/L.
2.3. Statistical analysis
All data were from the laboratory information system, the statistical analyses were performed using EXCEL (Armonk, NY, USA) and SPSS 17.0 (IBM, Beijing, china).
3. Results
In total, 2 360 055 pairs of CREA and urea tests between October 4, 2011, and December 31, 2015 in Shuyang countryside were drawn and analyzed, including 18 474 pairs at Outpatient, 101 631 pairs at Shuyang People's Hospital (hospital), 20 509 of Routine Health Check‐up (routine), and 95 441 of elderly health check‐up (elderly; Table 1).
The levels of urea in different CREA and eGFR (Table 2), it Shows the 97.5% of urea is <10.1 mmol/L, the proportion of elevated urea missed is 2.5%.
Table 2.
The levels of urea in different levels of creatinine and eGFR
| Institution | Urea/mmol/L (CREA≤85 μmol/L) | Urea/mmol/L (CREA≤100.9 μmol/L) | Urea/mmol/L (eGFR≥120 mL/min/1.73 m2) | Urea/mmol/L (eGFR≥90 mL/min/1.73 m2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | 2.5% | 50% | 97.5% | n (%) | 2.5% | 50% | 97.5% | n (%) | 2.5% | 50% | 97.5% | n (%) | 2.5% | 50% | 97.5% | |
| Elderly | 90 167 (94.5) | 3.60 | 6.12 | 10.1 | 93 684 (98.2) | 3.70 | 6.20 | 10.3 | 61 774 (64.7) | 3.50 | 5.90 | 9.60 | 89 210 (93.5) | 3.60 | 6.10 | 10.1 |
| Outpatient | 15 644 (84.7) | 2.50 | 4.70 | 8.10 | 16 907 (90.2) | 2.50 | 4.80 | 8.50 | 12 550 (67.9) | 2.40 | 4.60 | 7.80 | 16 312 (88.3) | 2.50 | 4.80 | 8.20 |
| Inpatient | 89 993 (88.5) | 2.10 | 4.40 | 8.50 | 95 371 (93.8) | 2.10 | 4.40 | 8.90 | 74 421 (73.2) | 2.00 | 4.20 | 8.00 | 91 875 (90.4) | 2.10 | 4.40 | 8.60 |
| Routine | 18 915 (92.2) | 2.70 | 4.70 | 7.60 | 20 102 (98.1) | 2.70 | 4.70 | 7.80 | 16 748 (81.7) | 2.70 | 4.60 | 7.50 | 20 174 (98.5) | 2.70 | 4.70 | 7.80 |
eGFR (mL/min/1.73 m2) was calculated with using CKD‐EPI.5
The results of setting a CREA limit of 85 μmol/L, 100.9 μmol/L, 120 mL/min/1.73 m2, and 90 mL/min/1.73 m2 as a condition for a further urea test. A larger proportion of urea was eliminated in all participants (Tables 3 and 4).
Table 3.
Effectiveness of setting 85 and 100.9 μmol/L as the CREA limit for initiating urea testing
| Institution | Total urea not done, no. (%) | CREA≤85 μmol/L | Total urea not done, no. (%) | CREA≤100.9 μmol/L | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Elevated urea missed, no. (%) | Nonelevated urea tests eliminated, no. (%) | Elevated urea missed, no. (%) | Nonelevated urea tests eliminated, no. (%) | |||||||
| >8.30 mmol/L | >9.28 mmol/L | <8.30 mmol/L | <9.28 mmol/L | >8.30 mmol/L | >9.28 mmol/L | <8.30 mmol/L | <9.28 mmol/L | |||
| Elderly | 90 167 (94.5) | 8752 (9.2) | 2386 (2.5) | 81 415 (85.3) | 87 781 (92.0) | 93 684 (98.2) | 9883 (10.4) | 2863 (3.0) | 83 801 (87.8) | 90 821 (95.2) |
| Outpatient | 15 644 (84.7) | 339 (1.8) | 74 (0.4) | 15 305 (82.9) | 15 570 (84.3) | 16 907 (91.5) | 483 (2.6) | 129 (0.7) | 16 424 (88.9) | 16 778 (89.8) |
| Inpatient | 89 993 (88.5) | 2516 (2.5) | 813 (0.8) | 87 477 (86.0) | 89 180 (87.7) | 95 371 (93.8) | 3478 (3.4) | 1220 (1.2) | 91 893 (90.4) | 94 151 (92.6) |
| Routine | 18 915 (92.2) | 174 (0.8) | 21 (0.1) | 18 741 (91.4) | 18 894 (92.1) | 20 102 (98.1) | 228 (1.1) | 21 (0.1) | 19 874 (97.0) | 20 081 (98.0) |
eGFR (mL/min/1.73 m2) was calculated with using CKD‐EPI.5
Table 4.
Effectiveness of setting 90 and 120 mL/min/1.73 m2 as the eGFR limit for initiating urea testing
| Institution | Total urea not done, no. (%) | eGFR≥120 mL/min/1.73 m2 | Total urea not done, no. (%) | eGFR≥90 mL/min/1.73 m2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Elevated urea missed, no. (%) | Nonelevated urea tests eliminated, no. (%) | Elevated urea missed, no. (%) | Nonelevated urea tests eliminated, no. (%) | |||||||
| >8.30 mmol/L | >9.28 mmol/L | <8.30 mmol/L | <9.28 mmol/L | >8.30 mmol/L | >9.28 mmol/L | <8.30 mmol/L | <9.28 mmol/L | |||
| Elderly | 61 774 (64.7) | 4396 (4.6) | 954 (1.0) | 91 045 (60.1) | 60 820 (63.7) | 89 237 (93.5) | 8464 (8.9) | 2291 (2.4) | 80 773 (84.6) | 86 919 (91.1) |
| Outpatient | 12 550 (67.9) | 163 (0.9) | 19 (0.1) | 12 387 (67.0) | 12 631 (67.8) | 16 311 (88.3) | 350 (1.9) | 74 (0.4) | 15 961 (86.4) | 16 238 (87.9) |
| Inpatient | 74 421 (73.2) | 1394 (1.4) | 407 (0.4) | 73 027 (71.8) | 74 014 (72.8) | 91 875 (90.4) | 2734 (2.7) | 915 (0.9) | 89 141 (87.7) | 90 960 (89.5) |
| Routine | 16 748 (81.7) | 228 (1.1) | 10 (0.05) | 16 520 (80.6) | 16 738 (81.6) | 20 174 (98.4) | 117 (0.6) | 20 (0.1) | 20 057 (97.8) | 16 728 (98.3) |
eGFR (mL/min/1.73 m2) was calculated with using CKD‐EPI.5
The proportion of elevated urea tests that are ordered (either exceeding 85 μmol/L and 100.9 μmol/L, 120 mL/min/1.73 m2, and 90 mL/min/1.73 m2) and the percentage of urea tests not done. Benefits of such conditional analysis are greater, with more reductions in urea testing and fewer “positive” urea tests missed (Figures 1 and 2).
Figure 1.
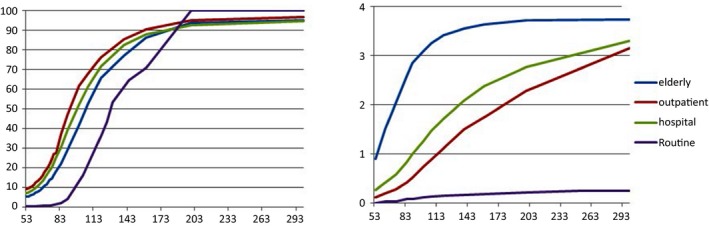
The line represents the proportion of urea not done as a result of the creaeatinine (CREA, μmol/L) time limit (left fig). The line demonstrate the proportion of missed urea exceeding 9.28 mmol/L (right fig)
Figure 2.
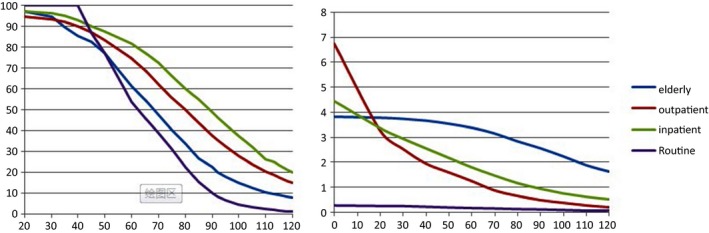
The line represents the proportion of urea not done as a result of the estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) time limit (left fig). The line demonstrates the proportion of missed urea exceeding 9.28 mmol/L (right fig)
When the eGFR≥90 mL/min/1.73 m2, the max values of CREA were 95.4, 111.0, 114.6, and 90 μmol/L for the elderly, outpatient, hospital and routine, respectively. When eGFR≥120 mL/min/1.73 m2, the max value were 74.2, 92.2, 97.1, and 109 μmol/L in above groups.
4. Discussion
Urea is produced by liver protein decomposition and excreted through the kidney, so that the blood urea concentration is relatively constant. When renal function is damaged, the decreased renal excretion function can be reflected with a high concentration of blood urea. On the other hand, urea concentration is also easily affected by excessive protein breakdown (catabolism), which could be resulted from high protein diet9 or gastrointestinal bleeding.10
However, the pairs of urea and CREA tests are ordered all of the chemistry labs in China and United States. First, less urea ordered is the most obvious savings doing (or buying) the urea test. The second savings is that urea reagents are relatively inexpensive, typically costing approximately CAD 4‐6 yuan RMB per urea analysis. If the Outpatient employed a cutoff of 85 μmol/L for CREA or 120 mL/min/1.73 m2 for eGFR, the urea testing would be reduced by 68% and 85%, and the urea testing of the elderly check‐up would be reduced more than 90%. In fact, the CREA is not 100% predictive of the urea level, and some “positive” urea results will be missed.
Under certain circumstances, the ratio of CREA to urea is very helpful to determine the cause of the changed urine. Thus, it is necessary to detect urea and CREA simultaneously for congestive heart failure11, 12 or dehydration or gastrointestinal bleeding10 or malnutrition, etc.13 Moreover, the estimated glomerular filtration rate (eGFR) is another important value in evaluating renal function because it is adjusted for age and gender so that the influence of UREA on age and gender is minimized. This may prompt with eGFR boundary value to reduce urea test more scientific.
To the best of our knowledge, this study is the first report about the Limiting the Testing of urea. There are two advantages in our study. First, we had selected a large number research objects, including inpatients and outpatients, routine healthy check‐up and almost all elderly healthy check‐up in Shuyang county. Second, we obtained the limit of CREA and eGRF for initiating urea testing by statistical analysis of mass data. Of course, this study had some limitations: (i) This study was confined in Shuyang County, Jiangsu Province, China and the results might not be useful for other areas; (ii) Reagents used in this study were from China, and detection methods of urea and CREA are both for enzymatic method, and others method of which is out of our study; (iii) The abnormal result of the line was potentially due to the fact that the order of urea and CREA is pertinence to the disease of outpatients, and the order regarded as routine check for inpatients.
In summary, the initiating urea testing should be based on the upper limit of Reference Intervals serum CREA of females or a 120 mL/min/1.73 m2 eGFR limit. If performed accordingly, the urea testing can be reduced at least 65%.
Ethics Approval
This study was approved by the ethics committee of the Shuyang People's Hospital.
Acknowledgments
We would like to thank all the subjects and all the staff members of the Laboratory Department, Shuyang People's Hospital for their participation in this project. We also thank Dr. Baojin Ding for his initial review and constructive comments on our manuscript.
Zhang G‐M, Guo X‐X, Zhang G‐M. Limiting the testing of urea: Urea along with every plasma creatinine test?. J Clin Lab Anal. 2017;31:e22103 10.1002/jcla.22103
All authors are co‐first authors, and contributed equally to this work.
Contributor Information
Xu‐Xiao Guo, Email: guoxuxiao180@163.com.
Guo‐Ming Zhang, Email: zly52120@163.com.
References
- 1. Traynor J, Mactier R, Geddes CC, et al. How to measure renal function in clinical practice. BMJ. 2006;333:733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. https://www.nlm.nih.gov/medlineplus/ency/article/003462.htm. Accessed October 10, 2016.
- 3. https://labtestsonline.org/understanding/analytes/bun/tab/test. Accessed October 10, 2016.
- 4. https://www.nlm.nih.gov/medlineplus/ency/article/003468.htm. Accessed October 10, 2016.
- 5. Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levey AS, Eckfeldt JH. Using glomerular filtration rate estimating equations: clinical and laboratory considerations. Clin Chem. 2015;61:1226–1229. [DOI] [PubMed] [Google Scholar]
- 7. http://www.westgard.com/decision.htm. Accessed October 10, 2016.
- 8. Hang GM, Xia YJ, Guo XX, et al. Reference intervals for total bilirubin, ALT, AST and creatinine in healthy Chinese elderly. Med Sci Monit. 2014;20:1778–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luke RG. Uremia and the BUN. N Engl J Med. 1981;305:1213–1215. [DOI] [PubMed] [Google Scholar]
- 10. Dunstan EJ. Urea/creatinine ratios, age, and gastrointestinal bleeding. Lancet. 1986;1:1327. [DOI] [PubMed] [Google Scholar]
- 11. Brisco MA, Coca SG, Chen J, et al. Blood urea nitrogen/creatinine ratio identifies a high‐risk but potentially reversible form of renal dysfunction in patients with decompensated heart failure. Circ Heart Fail. 2013;6:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiba N. Blood urea Nitrogen/CREAeatinine ratio in acute heart failure patients. Circ J. 2015;79:1446–1447. [DOI] [PubMed] [Google Scholar]
- 13. Marshall S. Urea‐creatinine ratio in obstructive uropathy and renal hypertension. JAMA. 1964;190:719–720. [DOI] [PubMed] [Google Scholar]


