Abstract
Background
Limited data are available for the diagnostic value, and for the diagnostic sensitivity and specificity of joint detection of serum lactate dehydrogenase (sLDH)/pleural fluid adenosine deaminase (pADA) and pleural fluid carcinoembryonic antigen (pCEA) in malignant pleural effusion (MPE).
Methods
We collected 987 pleural effusion specimens (of which 318 were malignant pleural effusion, 374 were tubercular pleural effusion, and 295 were parapneumonic effusion specimens) from the First Affiliated Hospital of Wenzhou Medical University from July 2012 to March 2016. The pADA, sLDH, pleural fluid LDH (pLDH), serum C‐reactive protein (sCRP), pleural fluid protein, pCEA, white blood cell (WBC), and red blood cell (RBC) were analyzed, and the clinical data of each group were collected for statistical analysis.
Results
The level of sLDH/pADA, pCEA, and RBC from the MPE group was markedly higher than the tuberculosis pleural effusion (TB) group (Mann‐Whitney U=28422.000, 9278.000, 30518, P=.000, .000, .000) and the parapneumonic pleural fluid group (Mann‐Whitney U=5972.500, 7113.000, 36750.500, P=.000, .000, .000). The receiver operating characteristic curve ROC showed that the area under the ROC curve (AUC) (=0.924, 0.841) of pCEA and sLDH/pADA (cutoff=4.9, 10.6) were significantly higher than other markers for the diagnosis of MPE. Thus, joint detection of pCEA and sLDH/pADA suggested that the sensitivity, specificity, and AUC was 0.94, 81.70, and 94.32 at the cutoff 0.16 and diagnostic performance was higher than pCEA or sLDH/pADA.
Conclusion
Joint detection of sLDH/pADA and pCEA can be used as a good indicator for the identification of benign and MPE with higher sensitivity and specificity than pCEA or sLDH/pADA.
Keywords: malignant pleural effusion, pleural fluid carcinoembryonic antigen, serum lactate dehydrogenase/pleural fluid adenosine deaminase
1. Introduction
A pleural effusion is an abnormal collection of fluid resulting from medical conditions such as tuberculosis, cancer, and pneumonia, etc. A cytology test is used for diagnosis of malignant pleural effusion (MPE), but the positive rate of the test varies from 11% to 78%.1 Thoracoscopy can greatly increase the diagnostic sensitivity (90%)2; however, it results in injury and other related complications, thus limiting its clinical application. Nowadays, conventional test markers of pleural fluid, including serum lactate dehydrogenase (sLDH), pleural fluid LDH (pLDH), pleural fluid adenosine deaminase (pADA), pleural effusion protein, pleural effusion white cell count(WBC), pleural effusion red cell count(RBC), have low sensitivity and specificity for diagnosis of MPE.
Efforts have been made to find better markers to improve the detection rate of MPE. Akash Verma3 found that sLDH/pADA was significantly higher in cancer patients presenting with exudative pleural effusion. The cut‐off level for sLDH/pADA ratio of ≥20 is highly predictive of malignancy in patients with exudative pleural effusion with high sensitivity and specificity.
Tumor markers are substances secreted from cancer cells or by other cells in host in response to cancer during tumor occurrence, development, invasion, and metastasis. These substances are then released into the blood or pleural effusion, and their concentrations in pleural effusion could be much higher than that of serum.4 Carcinoembryonic antigen (CEA) is one of the most common tumor markers, and pleural effusion CEA (pCEA) is a good marker for MPE.5, 6
However, limited data are available for the diagnostic value, and for the diagnostic sensitivity and specificity of joint detection of sLDH/pADA and pCEA in MPE. In this study, we aimed to determine the clinical role and laboratory diagnostic value of joint detection of sLDH/pADA and pCEA to provide a better and early diagnosis of MPE, so that early treatment can be initiated and better prognosis can be achieved.
2. Materials and Methods
Pleural effusion specimens were prospectively collected from 987 patients from the First Affiliated Hospital of Wenzhou Medical College, China, from July 2012 to March 2016. There were 318 MPE patients diagnosed using histology, including 292 lung cancer, 13 breast cancer, 4 stomach cancer, 3 colorectal cancer, 2 malignant lymphoma, 2 esophageal cancer, and 2 cervical cancer patients. The MPE sample consisted of 165 men and 153 women, aged between 22 to 89 years (64.4±12.3). There were 374 tuberculosis pleural effusion (TB) patients consisting of 268 men and 106 women, aged from 21 to 90 years (63.1±20.1), and 295 parapneumonic pleural effusion patients consisting of 229 men and 66 women, aged from 13 to 93 years (61.6±5.1). Statistical analysis showed no significant differences in age and gender composition among three groups (P>.05). These cases were confirmed by X‐ray, chest CT, tuberculosis infected T cells spot test (TSPOT‐TB), bacteriology, pathology, pleural biopsy, fiberoptic bronchoscopy, pleural fluid cytology, and so on. This study was approved by the Institutional Ethics Review Board of the First Affiliated Hospital of Wenzhou Medical University, and all patients provided written informed consent to this study.
Peripheral venous blood (2 mL) was collected from patients, centrifuged at 1509.3 g for 20 minutes, and separated serum was stored at −20°C in a refrigerator. Pleural fluid (10 mL) was collected from patients by routine thoracentesis, centrifuged (4°C, 603.72 g, 10 minutes) after routine testing, and then the separated supernatant was refrigerated at −20°C.
Carcinoembryonic antigen (CEA) was detected using the chemiluminescence method on a DXI800 luminescence analyzer (Beckman, Brea, CA, USA). CRP was detected using the immunoturbidimetry method on an IMMAGE 800 analyzer (Beckman). Protein, LDH, and ADA were determined by a Hitachi 7600 biochemist analyzer (Hitachi, Tokyo, Japan). WBC and RBC levels were detected by a Niu Bao's counting board.
The Shapiro‐Wilk test was used to evaluate the distribution. Differences in variables among groups were tested using the Kruskal‐Wallis test for nonnormal distribution. A comparison between the two groups was performed using the Mann‐Whitney U test or t test. Differences in rate among different groups were tested using the Chi‐square test. A value of P<.05 was considered to be statistically significant. The receiver operating characteristic curve (ROC) was analyzed, and the area under the ROC curve (AUC) was used to evaluate the ability. All statistical analyses were performed with SPSS 18.0 (Statistical Package for the Social Sciences Corporation, Chicago, IL, USA) and MedCalc (MedCalc Software, Ostend, Belgium).
3. Results
The Kruskal‐Wallis test showed that sLDH, pADA, sCRP, pCEA, sLDH/pADA, pLDH/sLDH, protein, WBC, and RBC levels were different (P=.000, .000, .000, .000, .000, .001, .000, .000, and .000) in three groups of pleural effusion, while pLDH was not statistically different (P=.132). The results were further analyzed using the Mann‐Whitney U test and the following analyses were obtained: the sCRP level in the parapneumonic pleural fluid group was significantly higher than the TB group (Mann‐Whitney U=39702.500, P=.000) and the MPE group (Mann‐Whitney U=19370.000, P=.000); the sLDH was higher in the TB group (Mann‐Whitney U=41451.000, P=.000) while it showed no clear difference in the MPE group (Mann‐Whitney U=45389.000, P=.489); pLDH/sLDH in the parapneumonic pleural fluid group showed no significant difference when compared with the TB group (Mann‐Whitney U=52534.000, P=.289) and the MPE group (Mann‐Whitney U=45270.500, P=.456). The pADA, protein, and WBC in the TB group were higher than the parapneumonic pleural fluid group (Mann‐Whitney U=19239.000, 24971.500, 38730.500, P=.000, .000, .000) and the MPE group (Mann‐Whitney U=5478.500, 33587.500, 41893.500, P=.000, .000, .000). The level of sLDH/pADA, pCEA, and RBC in the MPE group was markedly higher than the TB group (Mann‐Whitney U=28422.000, 9278.000, 30518, P=.000, .000, .000) and the parapneumonic pleural fluid group (Mann‐Whitney U=5972.500, 7113.000, 36750.500, P=.000, .000, .000) (see Figure 1 and Table 1).
Figure 1.
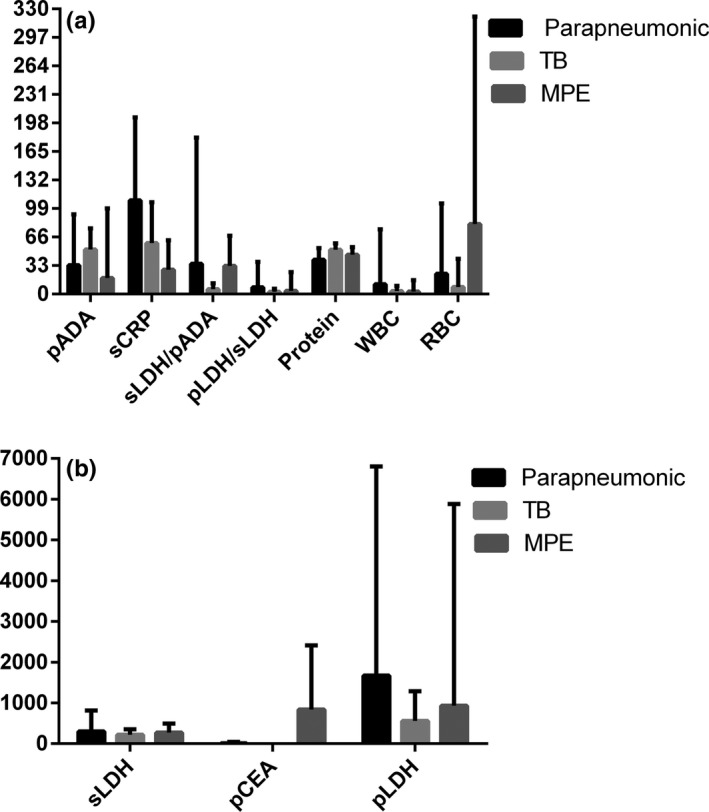
Comparison of the parameters in pleural effusion from the three groups. (A) The red blood cell and serum lactate dehydrogenase/pleural fluid adenosine deaminase levels in the malignant pleural effusion (MPE) group were obviously higher than those in the parapneumonic group and the TB group. (B) The pleural fluid CEA level in MPE was markedly higher than those in the parapneumonic group and the TB group
Table 1.
Comparison of the parameters in pleural effusion in the three groups
| Parapneumonic | TB | MPE | Kruskal‐Wallis test | P | |
|---|---|---|---|---|---|
| sLDH (U/L) | 219 (98‐6064) | 195 (114‐1871) | 215 (100‐2705) | 36.835 | .000 |
| pADA (U/L) | 18 (1‐464) | 50 (2‐185) | 10 (2‐1242) | 491.867 | .000 |
| sCRP (mg/L) | 87.3 (1.0‐467.0) | 53.5 (1.0‐261.0) | 14.1 (1.0‐211.0) | 196.832 | .000 |
| pCEA (ng/mL) | 1.6 (0.2‐489.2) | 0.9 (0.2‐34.9) | 148.3 (0.2‐11386.8) | 480.862 | .000 |
| pLDH (U/L) | 435.0 (1.0‐43761) | 390.0 (2.0‐8929) | 333.0 (77.0‐86301) | 4.047 | .132 |
| sLDH/pADA | 11.5 (0.3‐2395.5) | 4.0 (0.8‐79.0) | 23.0 (0.3‐399.8) | 477.376 | .000 |
| pLDH/sLDH | 1.8 (0.0‐383.7) | 2.0 (0.1‐40.1) | 1.5 (0.2‐385.3) | 13.764 | .001 |
| Protein(g/l) | 42.4 (5.0‐71.2) | 52.0 (13.0‐81.2) | 45.6 (20.0‐78.1) | 181.68 | .000 |
| WBC (103/μL) | 1.15 (0.02‐88.00) | 2.33 (0.01‐88.00) | 1.44 (0.06‐220.80) | 38.481 | .000 |
| RBC (103/μL) | 2.88 (0.00‐700.00) | 2.64 (0.03‐393.60) | 6.60 (0.05‐1856.00) | 59.087 | .000 |
Median (min‐max) in the parameters in the table.
The ROC analysis found that the AUC (=0.924, 0.841) of pCEA and sLDH/pADA (cutoff=4.9,10.6) were significantly higher than sLDH (0.546), pADA (0.144), sCRP (0.244), pLDH (0.450), pLDH/sLDH (0.430), protein (0.415), WBC (0.439), or RBC (0.562), and sensitivity and specificity of pCEA and sLDH/pADA for diagnosis of MPE were 83.06% and 94.62%, and 94.03% and 72.65% showing better diagnostic efficiency. We found that the AUC, sensitivity, and specificity for combined detection of pCEA and sLDH/pADA were 0.94, 81.70, and 94.32 at the cutoff 0.16, and diagnostic performance was significantly higher than pCEA (z statistic=3.381, P=.0007) and sLDH/pADA (z statistic=7.295, P<.0001) (see Figure 2 and Table 2).
Figure 2.
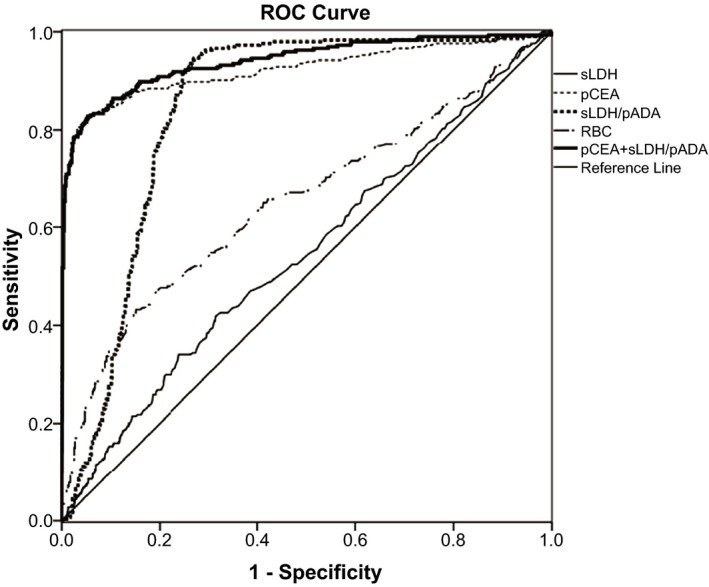
Receiver operating characteristic curve of some parameters for malignant pleural effusion (MPE). At the cutoff=0.16, the sensitivity and specificity of pCEA+sLDH/pADA were 81.70% and 94.32% for the diagnosis of MPE, and the AUC (0.940) was the highest among all markers tested
Table 2.
The AUC, cutoff value, sensitivity, and specificity of parameters for the diagnosis of malignant pleural effusion
| AUC | 95% confidence Interval | P | Cutoff | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|
| sLDH (U/L) | 0.546 | 0.505‐0.586 | .026 | 230 | 42.45 | 68.01 |
| pADA (U/L) | 0.144 | 0.118‐0.169 | .000 | 486 | 0.800 | 100 |
| sCRP (mg/L) | 0.244 | 0.212‐0.276 | .000 | 39.3 | 77.99 | 63.08 |
| pCEA (ng/mL) | 0.924 | 0.900‐0.947 | .000 | 4.9 | 83.06 | 94.62 |
| pLDH (U/L) | 0.450 | 0.411‐0.489 | .015 | 339 | 51.57 | 58.89 |
| sLDH/pADA | 0.841 | 0.815‐0.868 | .000 | 10.6 | 94.03 | 72.65 |
| pLDH/sLDH | 0.430 | 0.392‐0.468 | .001 | 2.15 | 70.44 | 45.14 |
| Protein (g/L) | 0.415 | 0.377‐0.453 | .000 | 48 | 63.8 | 53.7 |
| WBC (103/μL) | 0.439 | 0.401‐0.476 | .003 | 3.00 | 80.26 | 34.22 |
| RBC (103/μL) | 0.652 | 0.612‐0.693 | .000 | 10.56 | 43.09 | 84.93 |
| pCEA+sLDH/pADA | 0.940 | 0.921‐0.959 | .000 | 0.16 | 81.70 | 94.32 |
4. Discussion
The main causes of pleural effusion in China are tuberculosis and cancers. The prognosis of three types of pleural effusion is different, so differential diagnosis is crucial to determine the type of pleural effusion. But the gold standard methods, such as cytology and histopathology, for sensitivity of diagnosis are not high, so the etiological diagnosis of benign and malignant pleural effusion is a common clinical problem. Clinically, it is necessary to find some indicators to be screened for MPE, in order to improve the detection rate of MPE.
Lactate dehydrogenase is widely distributed in various types of cells; when the cells are invaded and destroyed by tumor, LDH is released into the blood, causing a marked increase in plasma LDH level. The level of LDH in plasma acts a diagnostic marker in some types of cancer as described in previous studies.7, 8, 9 and high LDH level was associated with survival of MPE.10 As the diagnosis of LDH is less effective, the need for it to be used in conjunction with other markers is necessary. Currently, the ADA is widely used in the diagnosis of tuberculosis disease, and the ADA level in pleural effusion will not only help in the early diagnosis of tuberculous pleurisy, but also is an important indicator for differentiating tuberculosis pleural effusion and MPE.11 The tumor marker CEA is an important indicator of MPE as reported in previous studies.5, 6
The size of the AUC indicates the accuracy of diagnostic tests. The actual range of AUC is 0.5‐1. It has a lower diagnostic value when AUC is from 0.5 to 0.7 and has the higher diagnostic value when AUC >0.9. Our results found that the AUC (0.924, 0.841) of pCEA and sLDH/pADA (cutoff=4.9, 10.6) were significantly higher than other studies indicators, so the differential diagnosis of benign and malignant pleural effusion has better accuracy, which is consistent with Akash Verma's findings.3 The results of joint detection of pCEA and sLDH/pADA indicated that diagnostic performance was significantly higher than that of pCEA or sLDH/pADA when the cutoff, AUC, sensitivity, and specificity were 0.16, 0.94, 81.70, and 94.32, respectively. Hence, the combined detection of pCEA and sLDH/pADA could improve the diagnostic efficacy. We first reported the diagnostic value of joint detection of pCEA and sLDH/pADA in terms of MPE.
In summary, joint detection of pCEA and sLDH/pADA has a high diagnostic efficacy for differential diagnosis of MPE, and hence, it is an important indicator to determine the nature of pleural effusion, which can help clinicians to determine early effusions for appropriate treatment. Thus, joint detection of pCEA and sLDH/pADA has better diagnostic performance than pCEA or sLDH/pADA. On the other hand, the detection of these conventional markers are simple and noninvasive, and could be used in more reliable routine screening programs.
Zhang F, Hu L, Wang J, Chen J, Chen J, and Wang Y. Clinical value of jointly detecting serum lactate dehydrogenase/pleural fluid adenosine deaminize ratio and pleural fluid carcinoembryonic antigen in the identification of malignant pleural effusion. J Clin Lab Anal. 2017;31:e22106 10.1002/jcla.22106
Contributor Information
Jie Chen, Email: chenjie991300@163.com.
Yumin Wang, Email: wym0577@163.com.
References
- 1. Braunschweig R, Yan P, Guilleret I, et al. Detection of malignant effusions: comparison of a telomerase assay and cytologic examination. Diagn Cytopathol. 2001;24:174–180. [DOI] [PubMed] [Google Scholar]
- 2. Goyal A, Jones MO, Couriel JM, Losty PD. Oesophageal atresia and tracheooesophageal fistula. Arch Dis Child Fetal Neonatal Ed. 2006;91:F381–F384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verma A, Abisheganaden J, Light RW. Identifying malignant pleural effusion by a cancer ratio (serum LDH: pleural fluid ADA ratio). Lung. 2016;194:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trapé J, Molina R, Sant F. Clinical evaluation of the simultaneous determination of tumor markers in fluid and serum and their ratio in the differential diagnosis of serous effusions. Tumor Biol. 2004;25:276–281. [DOI] [PubMed] [Google Scholar]
- 5. Son SM, Han HS, An JY, et al. Diagnostic performance of CD66c in lung adenocarcinoma‐associated malignant pleural effusion: comparison with CEA, CA 19‐9, and CYFRA 21‐1. Pathology. 2015;47:123–129. [DOI] [PubMed] [Google Scholar]
- 6. Gu Y, Zhai K, Shi HZ. Clinical value of tumor markers for determining cause of pleural effusion. Chin Med J (Engl). 2016;129:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ernam D, Atalay F, Hasanoglu HC, et al. Role of biochemical tests in the diagnosis of exudative pleural effusions. Clin Biochem. 2005;38:19–23. [DOI] [PubMed] [Google Scholar]
- 8. Deeba F, Khatun S, Alam MM, et al. Serum LDH and CA‐125: markers for diagnosis of ovarian malignancy. Mymensingh Med J. 2015;24:334–340. [PubMed] [Google Scholar]
- 9. Kayser G, Kassem A, Sienel W, et al. Research Lactate‐Dehydrogenase 5 is overexpressed in non‐small cell lung cancer and correlates with the expression of the transketolase‐like protein. Diagn Pathol. 2010;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bielsa S, Salud A, Martínez M, et al. Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Intern Med. 2008;19:334–339. [DOI] [PubMed] [Google Scholar]
- 11. Koşar F, Yurt S, Arpınar Yiğitbaş B, et al. The comparative value of pleural fluid adenosine deaminase and neopterin levels in diagnostic utility of pleural tuberculosis. Tuberk Toraks. 2015;63:243–249. [DOI] [PubMed] [Google Scholar]


