Abstract
Background
As a result of physiological and metabolic changes during pregnancy, thyroid hormones can be affected significantly throughout entire three trimesters. According to the guidelines published by American Thyroid Association in 2017, it is strongly recommended to establish population‐based trimester‐specific and assay method‐specific reference intervals (RIs) using local population.
Methods
A total of 1209 pregnant women without personal or family history of thyroid disease were recruited from July 2015 to April 2017 at Beijing Obstetrics and Gynecology Hospital. Those initially selected patients were further tested for TSH, FT4 and thyroid peroxidase antibody (aTPO), performed on the chemiluminescent platform Siemens ADVIA Centaur® XP. Only patients tested negative for aTPO were included in reference interval establishment. RIs for both TSH and FT4 were determined as 2.5th percentile to 97.5th percentile on the data distribution.
Results
The TSH and FT4 trimester‐specific RIs were as follows: 0.59‐3.54 mIU/L, 11.8‐18.4 pmol/L (n = 188, 1st trimester); 0.80‐4.46 mIU/L, 11.6‐17.4 pmol/L (n = 133, 2nd trimester); 0.72‐4.19 mIU/L, 9.7‐15.1 pmol/L (n = 157, 3rd trimester). The RIs of TSH and FT4 determined by Hoffmann method for first trimester outpatient pregnant women were 0.33‐3.96 mIU/L (n = 9924) and 11.7‐17.5 pmol/L (n = 10039), respectively.
Conclusion
Trimester‐specific thyroid function tests RIs are distinct from those provided by assay manufacturers. The RIs determined by direct sampling and Hoffmann indirect calculation showed no statistical difference.
Keywords: free thyroxine, Hoffmann, pregnancy, reference interval, thyroid stimulating hormone
1. INTRODUCTION
Profound physiological changes take place in pregnancy and may affect laboratory testing of endocrine systems. Normal pregnancy is associated with dramatic changes in thyroid gland and its function, such as increased iodine renal excretion, increased production of thyroxine binding globulin (TBG) and human chorionic gonadotropin (hCG).1 As a result of increased placental hCG, thyroid hormone secretion is stimulated via direct interaction between hCG and thyroid stimulating hormone (TSH) receptor, leading to suppressed maternal TSH concentration especially in early pregnancy.2 Besides, it is reported that up to 18% of women in pregnancy are positive for thyroid peroxidase antibody (aTPO) or thyroglobulin antibody (aTg),1 which may adversely have significant impact on maternal thyroid function and even on developing fetus. Thyroid autoantibody also leads to increased risk of abnormal thyroid status even in postpartum period. To be adapted to these physiological alterations during pregnancy, thyroid hormone metabolism, iodine uptake and the hypothalamic‐pituitary‐thyroid axis regulation will change accordingly.3, 4
All above factors during pregnancy influence thyroid function tests and make them differ from those of nonpregnant healthy women. More specifically, after conception, the concentrations of both circulating TBG and total thyroxine start to increase from week 7 of gestation and reach peaks around week 16,1 remaining at a high level until delivery. As mentioned above, the stimulating effect of hCG leads to increased thyroid hormones and subsequently decreased TSH. The largest decrease in serum TSH is seen during the first trimester, after which TSH reference intervals (RIs) gradually rise in the second and third trimesters.1 On the contrary, free thyroxine (FT4) serum concentration is highly method‐dependent and shows a significant reduction especially in the third trimester.2, 5, 6 It has been shown that geographic location and ethnicity can have significantly impact on RIs of thyroid function tests. For instance, TSH and FT4 RIs established for Chinese pregnant women in their first trimester are distinct from those reported in Europe and United States, presenting a downward shift in the upper reference range of TSH.7 As recommended in the 2017 guidelines for diagnosis and management of thyroid disease during pregnancy by the American Thyroid Association (ATA), population‐based and trimester‐specific RIs should be established for thyroid function tests.1
RIs are essential for clinical laboratory test interpretation and patient evaluation. Direct RIs determination involves recruiting a minimum of 120 healthy reference subjects. However, health is a relative condition without clear definition and universal standards. Therefore, uncertainty may exist in selecting healthy subjects. Plus, subclinical subjects may be recruited and further undermine the validity of reference group. The difficulty can be further magnified by targeting different age groups of unusual sample types. Therefore, traditional method of establishing clinical test RIs is typically costly and time‐consuming.
Recently, an indirect way of estimating RIs through proper statistical technique and data mining from the laboratory's database began to attract attention. This statistical method was first described by Hoffmann in 1963, to help laboratory professionals to deal with the difficulties encountered in RIs establishment.8 Although the Hoffmann method was put forward more than half century ago and has been widely accepted as an alternative way of RIs determination, only a few publications have actually applied this method in their calculations.9, 10, 11
In this study, trimester‐specific RIs for TSH and FT4 were established, respectively, by recruiting Chinese pregnant women with singleton pregnancy and normal thyroid status. Indirect estimating RIs for pregnant women in first trimester was applied with Hoffmann method. To confirm the validity of Hoffmann method in thyroid function tests during pregnancy, TSH and FT4 RIs were statistically compared between direct measurements and indirect calculations.
2. MATERIALS AND METHODS
2.1. Subjects
According to the recommendation from the 2017 ATA guidelines, patients with optimal iodine intake status were selected based the following exclusion criteria: with a personal or family history of thyroid disease, with a goiter, with more than one fetus or pregnancy complications.1 From July 2015 to April 2017, a total of 1209 pregnant women, between 20 and 40 years old, were recruited at Beijing Obstetrics and Gynecology Hospital for thyroid function tests. Those initially selected patients were further tested for TSH, FT4, and aTPO. Only patients tested negative for aTPO were included in reference interval establishment. After step‐by‐step screening, 732 subjects were excluded due to positive aTPO results; 477 pregnant women were included in RIs establishment for TSH and FT4, including 188 in the first trimester (1‐12 weeks), 132 in the second trimester (13‐28 weeks), and 157 in the third trimester (29‐40 weeks). The study was approved by the Institutional Research Review Board of Beijing Obstetrics and Gynecology Hospital. All participants recruited in the study signed consent forms.
In the RIs study with Hoffmann method, TSH (n = 10053) and FT4 (n = 10051) test results were from pregnant outpatients in their first trimester who visited Department of Obstetrics at Beijing Obstetrics and Gynecology Hospital from January 2016 to December 2016. This part of statistical analysis was determined to be exempt under existing regulations by the Institutional Research Review Board.
2.2. Laboratory methods
About 2 mL serum was collected from each recruited subject after 8‐10 hours fasting and tested for TSH, FT4, and aTPO on the automated chemiluminescent immunoassay platform Siemens ADVIA Centaur® XP.
The limit of detection for serum TSH was 0.001mIU/L. The intra‐assay coefficients of variation (CV) of serum TSH, FT4, and aTPO were 0.79% to 1.44%, 2.56% to 2.68%, and 1.00% to 5.14%, respectively. The inter‐assay CV of serum TSH, FT4, and aTPO were 4.04% to 7.07%, 2.70% to 4.27%, and 3.37% to 3.40%, respectively. The current laboratory reference ranges for all female adults were TSH 0.55 to 4.78 mIU/L, FT4 11.5 to 22.7 pmol/L according to Siemens package inserts ADVIA Centaur TSH3‐Ultra and FT4, respectively.
2.3. Statistical analyses
For trimester‐specific RIs of TSH and FT4 tests, all statistical analyses were performed with Sigmaplot software (version 13.0, Systat Software, Inc., San Jose, CA, USA). The Kolmogorov‐Smirnov test was performed to confirm normality and Mann‐Whitney test was used to compare groups. According to the Clinical and Laboratory Standards Institute (CLSI) guideline C28‐A3, nonparametric analysis was employed in RIs determination regardless the data normality or distribution.12 The 2.5th and 97.5th were used as the lower and upper limits of RIs. The 95% confidence interval (CI) of the two limits was calculated with bootstrap method.13
The Hoffmann indirect RIs estimation was carried out as previously described.8, 9, 10 Chauvenet criteria were used for outlier detection and elimination.9 Briefly, with the Chauvenet criteria, a result is eliminated if the probability of its occurrence is less than 1/(2N), where N is the number of measurements (results) in the data pool and is greater than 4. For a particular result x0, if Prob(X < x 0 )<1/(2N) or Prob(X > x 0 )<1/(2N), then x0 is an outlier of the data pool and excluded in further calculations.9
With outlier results eliminated from the data pool, cumulative frequency graphs for TSH and FT4 were plotted separately. The frequency of a test result is determined as the number of times of a result occurring in the dataset divided by total number of results: F Xi = (Count Xi /Count total ) × 100%. The cumulative frequency is , ordered by Xi.
On the cumulative frequency graph, the data are refined so that only the linear portion was used to determine the best‐fitting linear regression equation with least‐squares method: yi = α*xi + β + εi, where α is the slope, β is the intercept of the line, and εi is the error.
A residual value (ri) was calculated as the difference between the measured result (yi) and the estimated value determined by the linear regression function [f(xi)]: r i = y i ‐ f(x i ). The linear portion of the data was selected when the maximum residual error (MRE) is smaller than the chosen value, which is the within‐subject biological variation in the given test. With an exhaustive method (Cook's distance) programmed in SAS software (SAS Institute Inc., Cary, NC, USA), data points larger than Cook's statistics is eliminated for the iteration. The iteration is repeated until the MRE of best fitting linear curve is equal to or smaller than the chosen value. The RIs will be then calculated from the linear regression equation as follows: RI min =α*2.5 + β, RI max = α*97.5 + β.
To determine the statistical significance of the differences between directly measured RI and the indirectly estimated RI, the reference change value (RCV) was calculated. Their difference is significant only if it is greater than RCV. RCV was calculated as described previously: RCV=2 1/2 *Z*(CV a 2 +CV i 2 ) 1/2, where Z value of 1.96 was selected for 95% probability corresponding to a significant change, CVa is the between‐run analytic variation and CVi is the within‐subject biological variation.10, 14
3. RESULTS AND DISCUSSION
3.1. TSH and FT4 RIs in each trimester
According to the 2017 ATA and the CLSI guidelines for establishing thyroid function tests RIs,1, 12 more than 120 pregnant women that meet the requirements of healthy subjects with normal thyroid functions were recruited in each trimester. Based on the results of Kolmogorov‐Smirnov test, neither serum TSH or FT4 followed a normal distribution (P < .05). The overall data distribution and basic statistics for TSH and FT4 were presented with Box plots in Figure 1. The median (minimum‐maximum) serum TSH levels (mIU/L) in first, second and third trimesters were 1.44 (0.44‐3.92), 1.78 (0.53‐5.48), and 2.10 (0.39‐4.92), respectively. The median (minimum‐maximum) serum FT4 levels (pmol/L) in each trimesters were 14.4 (11.6‐19.2), 14.1 (10.3‐18.7), and 12.4 (9.2‐15.8), respectively. When two groups compared with Mann‐Whitney test, both serum TSH and FT4 levels were significantly different (P < .05) between the first and the second trimesters and the first and the third trimesters. When compared between the second and the third trimesters, only FT4 but not TSH is significantly different. The trimester‐specific RIs for TSH and FT4 determined with nonparametric analysis were shown as follows (Table 1): 0.59‐3.54 mIU/L, 11.8‐18.4 pmol/L (n = 188, the first trimester); 0.80‐4.46 mIU/L, 11.6‐17.4 pmol/L (n = 132, the second trimester); 0.72‐4.19 mIU/L, 9.7‐15.1 pmol/L (n = 157, the third trimester). Our results are distinct from the RIs described in the Siemens reagent package inserts. If the TSH upper reference limit (4.78 mIU/L) were applied in the first trimester women, 6.9% subjects would be erroneously classified as “normal”, possibly resulting in delayed treatment. Same as most other reference studies for pregnant women, subclinical subjects or subjects who were healthy at the time of recruitment but developed complications during pregnancy or after were not excluded. Therefore, the RIs we observed could be potentially wider than they actually are.
Figure 1.
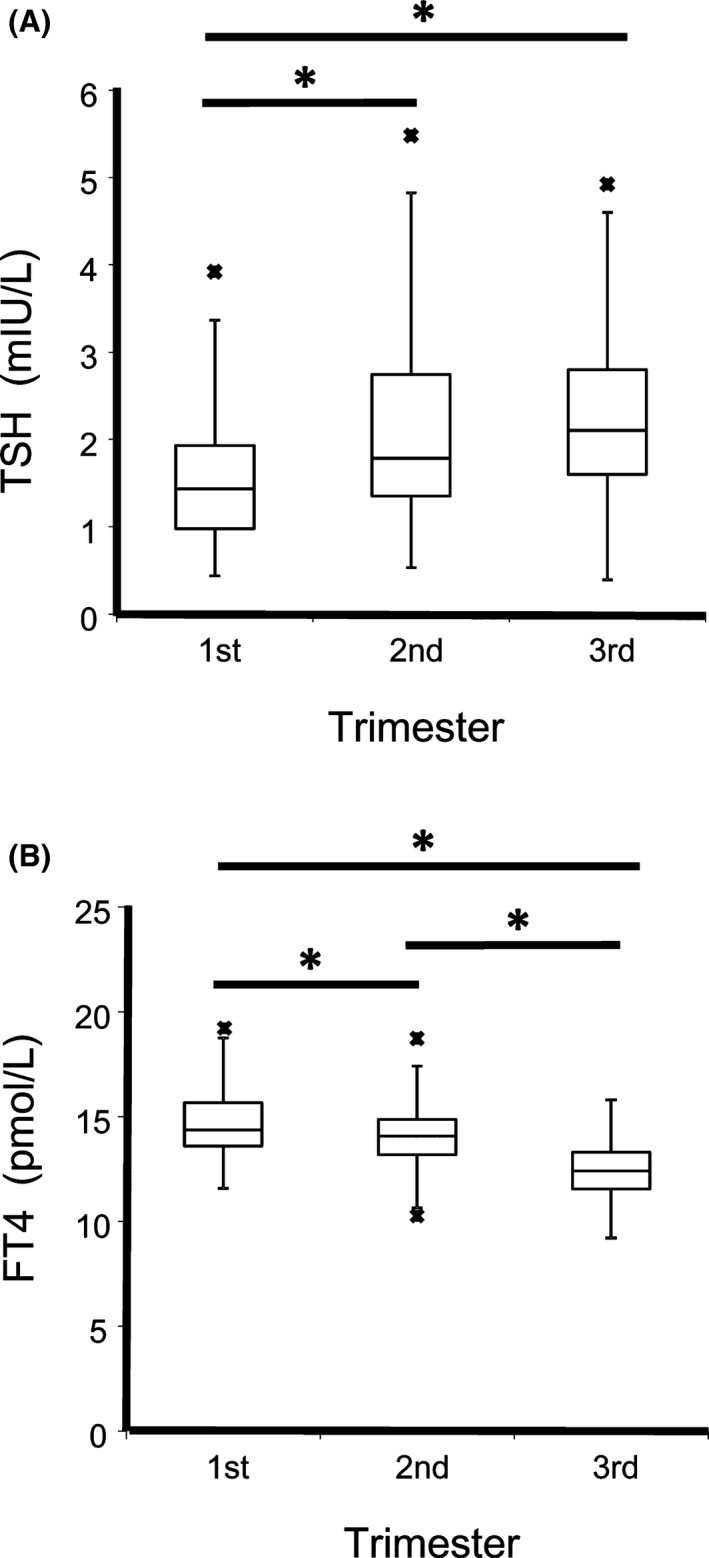
Box plots of serum thyroid stimulating hormone (TSH) (A) and FT4 (B) levels in each gestational trimester. The boxes give the upper and lower quartiles; the vertical and narrow horizontal lines define the results range (including data that are between the 1.5 interquartile range (IQR) of the lower quartile and the 1.5 IQR of the upper quartile). The wide horizontal lines mark the median values. The minimum or maximum values outside the range are presented as asterisks below or above the horizontal bars of each box. TSH and FT4 results of each trimester were compared pair wise by Mann‐Whitney test, with asterisks indicating statistical significance (P < .05) of above comparing groups
Table 1.
The observed trimester‐specific reference intervals for TSH and FT4
| Trimester | n | P2.5 | 95% CI (P2.5) | P97.5 | 95% CI (P97.5) |
|---|---|---|---|---|---|
| TSH, mlU/L | |||||
| 1st | 188 | 0.59 | 0.52‐0.65 | 3.54 | 3.03‐3.79 |
| 2nd | 133 | 0.80 | 0.71‐0.89 | 4.46 | 4.08‐5.00 |
| 3rd | 157 | 0.72 | 0.44‐0.90 | 4.19 | 3.97‐4.87 |
| FT4, pmol/L | |||||
| 1st | 188 | 11.8 | 11.7‐12.2 | 18.4 | 17.4‐19.1 |
| 2nd | 133 | 11.6 | 11.2‐11.8 | 17.4 | 16.3‐18.3 |
| 3rd | 157 | 9.7 | 9.4‐10.1 | 15.1 | 14.6‐15.5 |
RI: reference interval; P2.5/P97.5: percentile 2.5%/97.5%; CI: confidence interval.
In early pregnancy, dramatically increased hCG leads to increased FT4 and suppressed TSH levels.1 As pregnancy progresses into second and third trimesters accompanied with hCG leveling off, TSH level gradually increases and FT4 level decreases. Similar changes happened in our study, where an upward shift of TSH and a downward shift of FT4 were observed as gestational age increases. In the 2011 ATA guidelines for thyroid diseases in pregnancy, earlier studies from United States and Europe led to recommendations for TSH upper reference limit of 2.5 mIU/L in the first trimester and 3.0 mIU/L in the second and third trimesters.15 However, recent studies from Asia, including ours in present work, have shown rather modest reduction in the upper reference limit of TSH, 7, 16, 17, 18 compared with that of nonpregnant women.
As reviewed in the 2017 ATA guidelines, for first trimester pregnant women, the lower limit of TSH RIs are ranged from 0.02 to 0.41 mIU/L depending upon the ethnicity background of subjects and assay methodology.1 Even in Chinese population, the TSH lower reference limit from this study with Siemens ADVIA Centaur platform were distinct from that reported with Beckman Coulter DxI 600 (0.06, 0.07, and 0.15 mIU/L for first, second, and third trimester, respectively),18 suggesting the impact of manufacturer's methodology and reagents in RI establishment. Interestingly, in a study where the median TSH and the lower limit of TSH RI were recorded for each gestational week, it was found that they were both continuously decreased as the gestation week increases in first trimester.7 For instance, the lower limit of TSH RI was decreased from 0.65 to 0.06 mIU/L, with gestational week increased from 4 to 12.7 In this study, the majority of selected subjects were recruited at 5‐8 weeks of gestation for their scheduled thyroid function screening, which might partially explain why the observed lower limit of TSH RI was higher than previously reported.
3.2. RI estimation by Hoffmann method in first trimester pregnancy
For RI estimation of TSH and FT4 in the first trimester, the indirect Hoffmann method was employed. As shown in Figure 2A, cumulative frequency versus TSH concentration in log scale was graphed, where 9924 TSH results were plotted after eliminating 129 outliers with Chauvenet criteria. A scatter frequency graph of TSH in log scale was presented in Figure 2B. Similarly, cumulative frequency graph and scatter plot of FT4 without outliers were shown in Figure 3A,B, respectively (n = 10039, with 12 outliers removed). The dotted straight lines in Figures 2A and 3A were the linear regression lines derived from the linear data portion determined with the Cook's distance exhaustive method. And the resulting linear equation coefficients (α and β) were applied in lower and upper reference limits calculation as described in Methods and Materials section.
Figure 2.
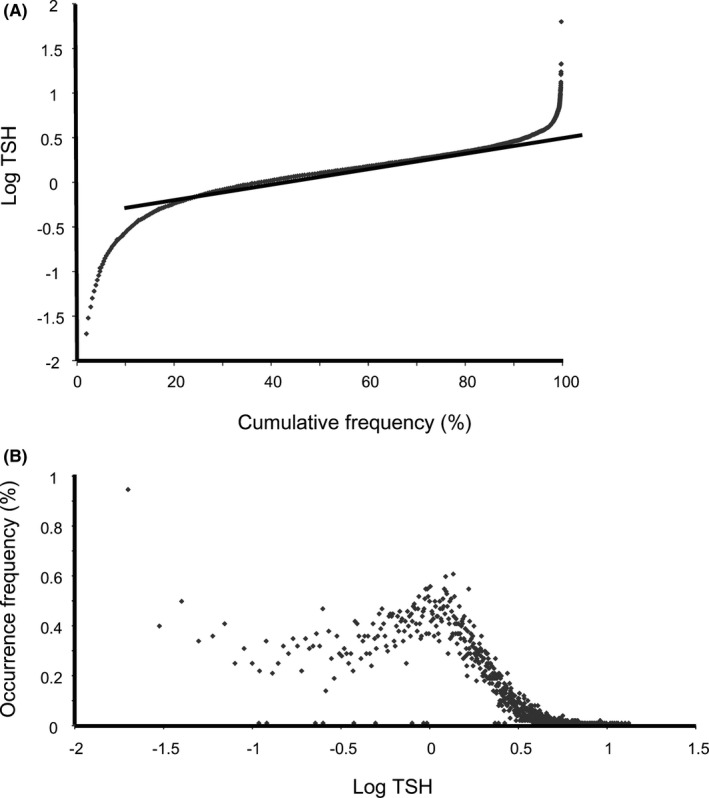
Frequency output graphs for thyroid stimulating hormone (TSH) indirect RI analysis with outpatient results. A, Cumulative frequency graph versus TSH levels (logarithm scale) and regression line (straight dotted line) with outliers removed. The linear regression equation is y = 0.01142x ‐ 0.62653 (n = 9924). B, Scatter graph of TSH levels (logarithm scale) versus occurrence frequency
Figure 3.
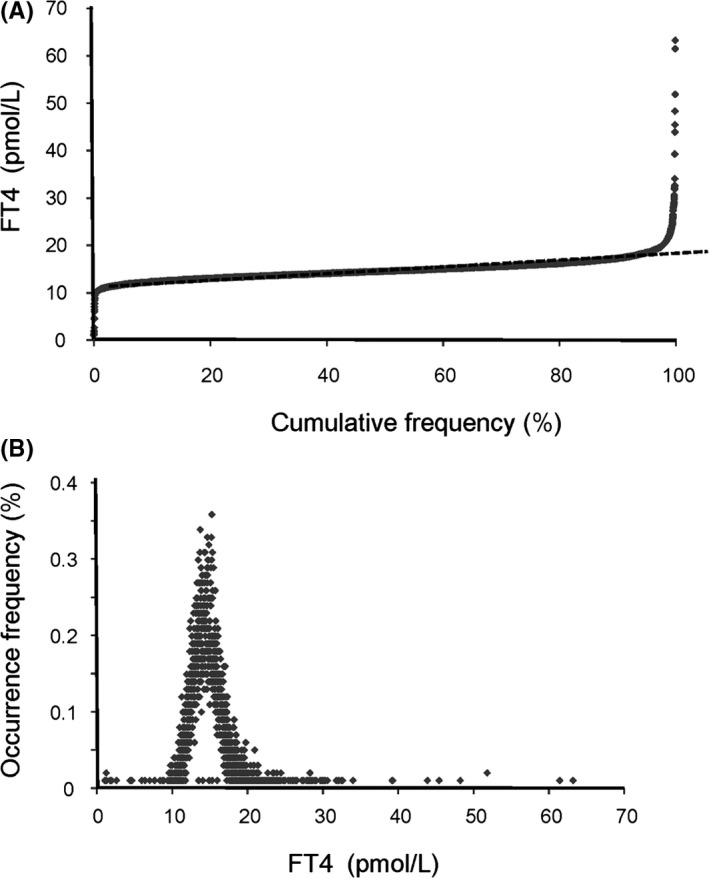
Frequency output graphs for FT4 indirect RI analysis with outpatient results. A, Cumulative frequency graph versus FT4 levels and regression line (straight dotted line) with outliers removed. The linear regression equation is y = 0.06189x + 11.53740 (n = 10039). B, Scatter graph of FT4 levels versus occurrence frequency
Hoffmann indirect method for estimating RIs have been developed since 1963 by Dr. Robert G. Hoffmann and proven to be valid in some clinical tests.8, 9, 10 The biggest advantage of Hoffmann method in RI study is that it no longer requires recruiting “healthy” subjects which do not always have clear definition and standardization. In addition, it eliminates the difficulties of looking for specimens that are not readily available from healthy subjects, such as cerebrospinal fluid. In this study, to our knowledge, for the first time, we applied this indirect statistical method in pregnant women for their thyroid function tests RI estimation. As shown in Table 2, the Hoffman‐calculated RIs of TSH and FT4 were listed together with those derived from direct sampling in this study. None of the absolute differences between calculated and observed RIs was greater than the theoretical RCA, suggesting statistical insignificance between the two groups.
Table 2.
Comparison of first trimester RIs calculated by Hoffmann method with RIs observed from direct sampling
| Analyte | Observed RIs | Calculated RIs | Absolute difference (%) |
|---|---|---|---|
| Lower‐Upper | Lower‐Upper | Lower‐Upper | |
| TSH (mlU/L) | 0.59‐3.54 | 0.33‐3.96 | 44.0‐11.9 |
| FT4 (pmol/L) | 11.8‐18.4 | 11.7‐17.5 | 0.8‐4.9 |
RI: reference interval; RCV: reference change value.
It is noteworthy that the calculated RI of TSH appeared to be slightly wider than that obtained from direct sampling, leading to the concern of inclusion of unhealthy subjects in the indirect method. In practice, to maintain the accuracy of RIs derived from Hoffmann method, it is suggested that the percentage of test results outside of reference limits should correlate with a prevalence of abnormal conditions. It has been proven that large number of observations from outpatient settings will negate above concern. In our study, more than ten thousands of test results were used for each calculation of TSH and FT4 RIs. As shown in Table 2, the absolute difference between observed RIs and calculated ones was well below RCA. As reported previously,8, 9, 10 the calculated ranges with Hoffman method were usually slightly narrower than the ranges obtained from direct sampling, similar result was observed for FT4 in our study. However, the calculated TSH RI was wider than the observed one for first trimester pregnant women, leading to the concern that some hyper‐ or hypothyroidism cases could have been misclassified as “normal” subjects. This discrepancy is random and not statistically significant; it could be due to relatively small direct sampling size (n = 188), compared to the patient pool used in calculation method. More importantly, the calculated TSH upper reference limit (3.96 mIU/L) is still distinct from that (4.78 mIU/L) provided by Siemens package insert, highlighting the necessity of establishing the proper RIs for thyroid function tests for pregnant women.
4. CONCLUSION
Thyroid function tests RIs can be affected by many factors, such as gestational age, geographical location, ethnicity, and methodology. Therefore, when possible, it is necessary to establish population‐based trimester‐specific and assay method‐specific RIs using local population.1 Here we reported trimester‐specific RIs for TSH and FT4 in Chinese pregnant women with Siemens ADVIA Centaur chemiluminescent platform. The Hoffmann indirect method was also applied in TSH and FT4 RIs estimation using results of first trimester pregnant women who visited our institute as outpatients. The RIs determined by direct sampling and Hoffmann indirect calculation showed no statistical difference. The application of Hoffmann method may be a valid alternative of RI estimation in pregnancy.
ACKNOWLEDGEMENTS
This work was supported by the Specialized Youth Foundation Project of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (grant number FCYYQN‐2015011).
Han L, Zheng W, Zhai Y, et al. Reference intervals of trimester‐specific thyroid stimulating hormone and free thyroxine in Chinese women established by experimental and statistical methods. J Clin Lab Anal. 2018;32:e22344 10.1002/jcla.22344
REFERENCES
- 1. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315‐389. [DOI] [PubMed] [Google Scholar]
- 2. Baloch Z, Carayon P, Conte‐Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3‐126. [DOI] [PubMed] [Google Scholar]
- 3. van Raaij JM, Vermaat‐Miedema SH, Schonk CM, Peek ME, Hautvast JG. Energy requirements of pregnancy in The Netherlands. Lancet. 1987;2:953‐955. [DOI] [PubMed] [Google Scholar]
- 4. Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404‐433. [DOI] [PubMed] [Google Scholar]
- 5. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester‐specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sapin R, D'Herbomez M, Schlienger JL. Free thyroxine measured with equilibrium dialysis and nine immunoassays decreases in late pregnancy. Clin Lab. 2004;50:581‐584. [PubMed] [Google Scholar]
- 7. Li C, Shan Z, Mao J, et al. Assessment of thyroid function during first‐trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. 2014;99:73‐79. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann RG. Statistics in the Practice of Medicine. JAMA. 1963;185:864‐873. [DOI] [PubMed] [Google Scholar]
- 9. Katayev A, Fleming JK, Luo D, Fisher AH, Sharp TM. Reference intervals data mining: no longer a probability paper method. Am J Clin Pathol. 2015;143:134‐142. [DOI] [PubMed] [Google Scholar]
- 10. Katayev A, Balciza C, Seccombe DW. Establishing reference intervals for clinical laboratory test results: is there a better way? Am J Clin Pathol. 2010;133:180‐186. [DOI] [PubMed] [Google Scholar]
- 11. Horowitz GL. Estimating reference intervals. Am J Clin Pathol. 2010;133:175‐177. [DOI] [PubMed] [Google Scholar]
- 12. Horowiz GL, Altaie S, Boyd JC, et al. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratories: Approved Guideline (3rd edn). Wayne, PA, USA: Clinical and Laboratory Standards Institute Document C28‐A3; 2008. [Google Scholar]
- 13. Linnet K. Nonparametric estimation of reference intervals by simple and bootstrap‐based procedures. Clin Chem. 2000;46:867‐869. [PubMed] [Google Scholar]
- 14. Fraser CG, Hyltoft Peterson P, Larsen ML. Setting analytical goals for random analytical error in specific clinical monitoring situations. Clin Chem. 1990;36:1625‐1628. [PubMed] [Google Scholar]
- 15. Stagnaro‐Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marwaha RK, Chopra S, Gopalakrishnan S, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008;115:602‐606. [DOI] [PubMed] [Google Scholar]
- 17. Yan YQ, Dong ZL, Dong L, et al. Trimester‐ and method‐specific reference intervals for thyroid tests in pregnant Chinese women: methodology, euthyroid definition and iodine status can influence the setting of reference intervals. Clin Endocrinol (Oxf). 2011;74:262‐269. [DOI] [PubMed] [Google Scholar]
- 18. Zhang J, Li W, Chen QB, et al. Establishment of trimester‐specific thyroid stimulating hormone and free thyroxine reference interval in pregnant Chinese women using the Beckman Coulter UniCel DxI 600. Clin Chem Lab Med. 2015;53:1409‐1414. [DOI] [PubMed] [Google Scholar]


