Abstract
Objective:
Describe sex differences in outcomes and response to spironolactone in patients with heart failure with preserved ejection fraction (HFpEF).
Background:
HFpEF affects women more frequently than men. Sex differences in effect of mineralocorticoid antagonists have not been reported.
Methods:
This was an exploratory, post-hoc, non-prespecified analysis of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial (TOPCAT). Subjects with symptomatic HF and an LVEF ≥45% were randomized to spironolactone or placebo. Subjects enrolled from the Americas were analyzed. The primary outcome was a composite of cardiovascular (CV) death, cardiac arrest, or HF hospitalization. Secondary outcomes included all-cause, CV, and non-CV mortality, and CV, HF, and non-CV hospitalization. Sex differences in outcomes and treatment effect were determined using time-to-event analysis.
Results:
In total, 882/1767 (49.9%) subjects were women. Women were older with fewer comorbidities but worse patient-reported outcomes. There were no sex differences in outcomes in the placebo arm or in response to spironolactone for the primary outcome or its components. Spironolactone was associated with reduced all-cause mortality in women (HR 0.66, p=0.01), but not in men (pinteraction=0.02).
Conclusions:
In TOPCAT, women and men presented with different clinical profiles and similar clinical outcomes. The interaction between spironolactone and sex in TOPCAT overall and in our analysis was non-significant for the primary outcome, but there was a reduction in all-cause mortality associated with spironolactone in women with a significant interaction. Prospective evaluation is needed to determine whether spironolactone may be effective for treatment of HFpEF in women.
Keywords: sex differences, heart failure with preserved ejection fraction, spironolactone, women
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) has been consistently observed to affect more women than men, but the underlying reasons for this sex difference are incompletely understood. Despite multiple large clinical trials, no medical therapies have proven beneficial in HFpEF, but potential sex differences in treatment response have not been thoroughly explored. Recently, 6 clinically different HFpEF phenotypes were identified, which differed significantly by sex, rates of adverse outcomes, and nominally significant differences in treatment response suggesting that sex may be one of multiple factors important in defining HFpEF phenotypes. (1) Additional data suggests possible sex differences in the effect of aldosterone on cardiac remodeling and clinical response to mineralocorticoid antagonists (MRA) (2–4) raising the question of whether a sex-specific benefit of MRAs might exist. Sex differences in response to treatment with MRAs among patients with HFpEF have not been reported.
The Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial (TOPCAT) (5) presents the first opportunity to explore sex-specific treatment response to MRAs in HFpEF in a large, randomized placebo-controlled trial. There was no significant interaction in TOPCAT between treatment arm and sex with respect to the primary outcome in pre-specified subgroup analysis of the full TOPCAT cohort (pinteraction = 0.99). However, there has been controversy regarding an apparent benefit of spironolactone in subjects enrolled from the Americas but not from Russia or Georgia, (6, 7) and an analysis of possible sex differences within the Americas cohort only has not yet been reported. The goal of this study was to describe sex differences in characteristics, outcomes and treatment response in subjects in TOPCAT enrolled from the Americas. We hypothesized that women would have lower rates of adverse outcomes and a larger benefit of spironolactone compared to men.
METHODS
This manuscript was prepared using TOPCAT clinical data obtained from the National Heart, Lung, and Blood Institute’s Biological Specimen and Data Repository Information Coordinating Center (BioLINCC). TOPCAT conformed with the principles outlined in the Declaration of Helsinki and was approved by institutional review boards at all sites. (8).Our analysis was approved by the Colorado Multiple Institution Review Board and by BioLINCC.
The design of TOPCAT has been reported previously. (5) Briefly, 3445 patients with a left ventricular ejection fraction (LVEF) ≥45% and ≥ 50 years old with a history of non-adjudicated HF hospitalization in the previous 12 months, a B-type natriuretic peptide (BNP) level ≥ 100 pg/ml, or a N-terminal pro-BNP level ≥ 360 pg/ml were randomized in a double-blind fashion to receive either spironolactone or placebo. The mean follow-up was 3.3 years. The primary outcome was a composite of cardiovascular (CV) mortality, aborted cardiac arrest, or HF hospitalization. Secondary outcomes for our analysis included all-cause, CV, and non-CV mortality, and CV, HF, and non-CV hospitalization. Because of previously described concerns about the veracity of HF diagnosis and poor treatment compliance in subjects from Russia/Georgia, (6, 7, 9) we limited our analysis to the 1767 patients enrolled from the Americas in accordance with multiple secondary analyses recently published by the TOPCAT investigators. (10–14)
Statistical analysis
Data were stratified according to sex and treatment arm. Baseline characteristics in women and men were compared using the chi-square test and Mann Whitney U test for categorical and continuous variables, respectively. To account for the possibility of differential treatment effects in men and women, the presence of sex differences in outcomes was based on comparisons between men and women within the placebo arm. Significance of changes in serum potassium, serum creatinine, and systolic blood pressure (SBP) from baseline to 4 and 12 months was determined using the paired Wilcoxon signed-rank test. Differences in change of serum potassium, serum creatinine, and SBP from baseline between treatment groups were compared using the Mann Whitney U test. Univariate and multivariate associations between sex and outcomes were determined using Cox proportional hazards models. Effects of spironolactone versus placebo on primary and secondary outcomes were analyzed by sex, and interaction terms between sex and treatment arm were calculated. Multivariate associations were adjusted for all patient characteristics that differed in significant between women and men in frequency or magnitude (Table 1a). The proportional hazards assumption was tested for all covariates and outcomes by testing the correlation of scaled Schoenfeld residuals with time. Where a covariate showed a significant correlation with time (p<0.05), a coefficient for the interaction between the covariate and time included in multivariate and interaction analyses. A p-value < 0.05 was considered significant throughout.
Table 1a –
Baseline demographics and comorbidities according to sex, N (%), mean±SD
| Women | Men | |
|---|---|---|
| Characteristic | 882 (49.9) | 885 (50.1) |
| Age* | 72.1±9.9 | 71.0±9.5 |
| White race‡ | 643 (73) | 741 (84) |
| LVEF‡ | 59.8±8.0 | 56.6±7.1 |
| Atrial fibrillation* | 348 (39) | 395 (45) |
| Coronary artery disease‡ | 336 (38) | 479 (54) |
| Angina‡ | 203 (23) | 283 (32) |
| MI‡ | 126 (14) | 233 (26) |
| CABG‡ | 100 (11) | 236 (27) |
| PCI‡ | 139 (16) | 205 (23) |
| Hypertension* | 807 (91) | 781 (88) |
| Diabetes mellitus‡ | 354 (40) | 434 (49) |
| Dyslipidemia‡ | 596 (68) | 654 (74) |
| Tobacco use* | 46 (5) | 71 (8) |
| COPD† | 125 (14) | 166 (19) |
LVEF = Left ventricular ejection fraction HF = Heart failure MI = Myocardial infarction CABG = Coronary artery bypass graft PCI = percutaneous coronary intervention COPD = Chronic obstructive pulmonary disease NYHA = New York Heart Association GFR = glomerular filtration rate SBP = Systolic blood pressure
Men versus women:
p<0.05.
p<0.01.
p<0.001
RESULTS
Baseline characteristics of women and men are summarized in Table 1a–c. Of the 1767 subjects, 882 (49.9%) were women. All baseline demographics and comorbidities were significantly different in women versus men (Table 1a). In general women were older with fewer comorbid conditions than men including coronary artery disease, tobacco use, atrial fibrillation, chronic obstructive pulmonary disease and diabetes mellitus. Women had significantly higher LVEF, blood pressure, and body mass index but lower estimated glomerular filtration rate and serum hemoglobin. Compared with men, women tended had a higher New York Heart Association class, lower overall Kansas City Cardiomyopathy Questionnaire score, and higher Patient Health Questionnaire-9 (Table 1b). Use of anti-hypertensive medications overall was very high in both men and women (99%). There was no significant difference between men and women in use of angiotensin converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB), or diuretics (Table 1c). Women were significantly more likely to be taking calcium channel blockers, whereas men were more likely to be taking beta-blockers or other anti-hypertensive medications.
Table 1c –
Baseline medications according to sex
| Female | Male | ||
|---|---|---|---|
| (N=882) | (N=885) | P-value | |
| Any anti-hypertensive | 874 (99) | 878 (99) | 0.40 |
| ACE-I or ARB | 688 (78) | 707 (80) | 0.29 |
| Beta-blocker | 669 (76) | 718 (81) | 0.005 |
| Calcium channel blocker | 361 (41) | 321 (36) | 0.048 |
| Diuretic | 782 (89) | 791 (90) | 0.54 |
| Other anti-hypertensive | 118 (13) | 177 (20) | <0.001 |
| Aspirin | 477 (54) | 550 (62) | <0.001 |
| Nitrate | 153 (17) | 152 (17) | 0.94 |
| Any hypoglycemic | 322 (37) | 397 (45) | <0.001 |
| Statin | 513 (58) | 635 (72) | <0.001 |
| Other CV medication | 466 (53) | 571 (65) | <0.001 |
| Selective serotonin reuptake inhibitor | 151 (17) | 103 (12) | 0.001 |
ACE-I = Angiotensin converting enzyme inhibitor ARB = Angiotensin receptor blocker CV = Cardiovascular SSRI = Selective serotonin reuptake inhibitor
Table 1b –
Vital signs and health status according to sex
| Female | Male | ||
|---|---|---|---|
| Characteristic | (N=882) | (N=885) | p-value |
| Heart rate, bpm | 69.7±11.4 | 68.4±11.0 | 0.033 |
| SBP, mm Hg | 129.0± 16.4 | 126.1± 15.2 | <0.001 |
| DBP, mm Hg | 72.0±11.8 | 70.7±11.1 | 0.02 |
| Body mass index, kg/m2 | 34.4± 8.8 | 33.2± 7.4 | 0.025 |
| Serum potassium, mmol/L | 4.17±0.43 | 4.21±0.43 | 0.047 |
| Blood urea nitrogen, mg/dL | 24.0±12.3 | 25.8±12.5 | <0.001 |
| Creatinine, mg/dL | 1.06±0.30 | 1.28±0.34 | <0.001 |
| Estimated GFR, ml/min/1.73 m2 | 63.0±22.3 | 66.0±20.5 | <0.001 |
| Hemoglobin, g/dL | 12.4± 1.6 | 13.2± 1.8 | <0.001 |
| NYHA class III-IV | 336 (38) | 284 (32) | 0.008 |
| KCCQ overall score | 54.8±22.5 | 61.4±23.8 | <0.001 |
| PHQ-9 score | 8.3± 6.7 | 7.4± 6.3 | 0.003 |
| Estimated METs/week | 9.3±14.4 | 10.4±22.1 | 0.338 |
SBP = Systolic Blood Pressure DBP = Diastolic Blood Pressure GFR = Glomerular Filtration Rate NYHA = New York Heart Association KCCQ = Kansas City Cardiomyopathy Questionnaire PHQ = Patient Health Questionnaire MET = Metabolic equivalent
Trends in serum potassium, serum creatinine, and SBP are illustrated in Figures 1a–b, and summary statistics are provided in Supplement Table 1. Serum potassium and creatinine increased and SBP decreased significantly at 4 and 12 months in the spironolactone arms for both men and women with the majority of change occurring between baseline and 4 months. Compared to men, women demonstrated a significantly greater increase in creatinine at 4 months (pinteraction=0.03) and in potassium at 12 months (pinteraction=0.04).
Figure 1 – Sex- and treatment-specific trends in serum potassium, creatinine, and systolic blood pressure.
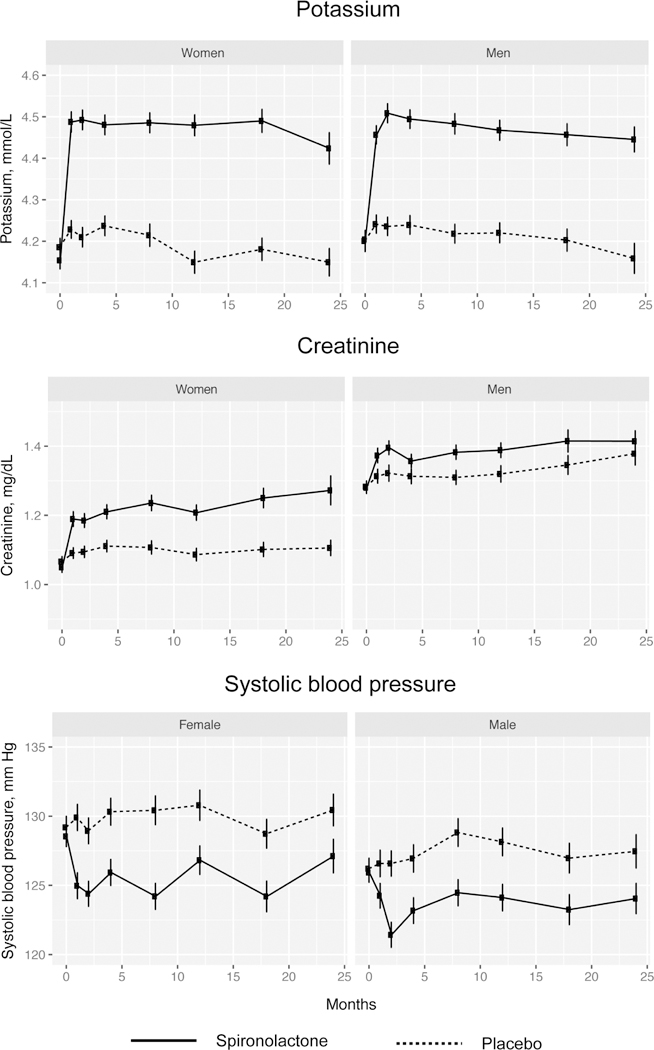
Compared to spironolactone was associated with significant increases in serum potassium and creatinine as well as decrease in systolic blood pressure in both men and women. In women there was a significantly greater increase in creatinine at 4 months (pinteraction=0.03) and serum potassium at 12 months (pinteraction=0.04).
Rates of primary and secondary outcomes as well as documented cause of CV deaths stratified by sex for the placebo arm are summarized in Table 2 and for the spironolactone arm in Supplement Table 2. Kaplan-Meier curves for primary and secondary outcomes stratified by sex are shown in Supplement Figures 1a–d. In the placebo arm, rates of all events were numerically lower in women than in men, but there were no statistically significant differences. In the spironolactone arm, event rates were also numerically lower in women than in men, of which the differences in all-cause mortality and non-CV mortality were statistically significant. There were no significant differences in rates of specific CV causes of death (pump failure versus sudden death versus other) between men and women in either the placebo (chi-square p=0.43), or spironolactone arms (chi-square p=0.16).
Table 2 –
Outcomes in placebo arm according to sex
| Women | Men | ||
|---|---|---|---|
| Outcome* | 440 | 441 | p-value |
| Primary outcome | 130 (30) | 150 (34) | 0.15 |
| CV mortality | 58 (13) | 69 (16) | 0.49 |
| HF hospitalization | 102 (23) | 114 (26) | 0.36 |
| All-cause mortality | 98 (22) | 107 (24) | 0.49 |
| Non-CV mortality | 25 (6) | 31 (7) | 0.41 |
| CV hospitalization | 173 (39) | 175 (40) | 0.91 |
| Non-CV hospitalization | 186 (42) | 191 (43) | 0.76 |
| Causes of CV mortality | |||
| Pump failure | 18 (31) | 17 (25) | 0.44* |
| Sudden death | 15 (26) | 25 (36) | |
| Other cause | 25 (43) | 27 (39) |
CV = Cardiovascular HF = Heart failure
chi-square test (pump failure/sudden death/other cause, women versus men)
Sex-specific multivariate hazard ratios (HR) in the placebo and spironolactone arms for all outcomes adjusted for age, race, LVEF, atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, hypertension, diabetes mellitus, body mass index, heart rate, SBP, estimated glomerular filtration rate, and New York Heart Association are summarized in Supplement Figure 2. There were no significant differences between men and women in the hazard of any outcomes in the placebo arm, whereas in the spironolactone arm, women had a significantly lower multivariate risk of the primary outcome, CV mortality, all-cause mortality, and non-CV mortality.
Kaplan-Meier survival estimates and event rates for the primary and secondary outcomes stratified by sex and treatment for subjects from the Americas are shown Figure 2a–b. Multivariate HRs for outcomes comparing spironolactone versus placebo are summarized in Fig 3, and comprehensive results of univariate and multivariate analyses including interaction terms are found in the Supplement Table 3. The rate of the primary outcome and HF hospitalization not significantly different between patients receiving spironolactone and placebo in either women or men, although there was a trend toward less CV mortality associated with spironolactone in women (9.0 versus 13.2%, p=0.051). Women receiving spironolactone had lower all-cause mortality compared to placebo (15.8 versus 22.3%, p=0.015). There was no significant reduction in any outcomes between treatment arms in men. In multivariate analysis, the association between spironolactone treatment and the primary outcome was similar in women (HR 0.81 [0.63–1.05], p=0.12) and men (HR 0.85 [0.67–1.08], p=0.18, pinteraction=0.84). There was a reduced likelihood of CV mortality associated with spironolactone in women (HR 0.63 [0.42–0.95], p=0.03 respectively) and not in men (p=0.37), but the interaction term was non-significant (pinteraction=0.31). Reductions in HF hospitalization with spironolactone were nonsignificant in both women and men.
Figure 2a – Kaplan-Meier survival curves for primary outcomes and components according to treatment arm and stratified by sex.
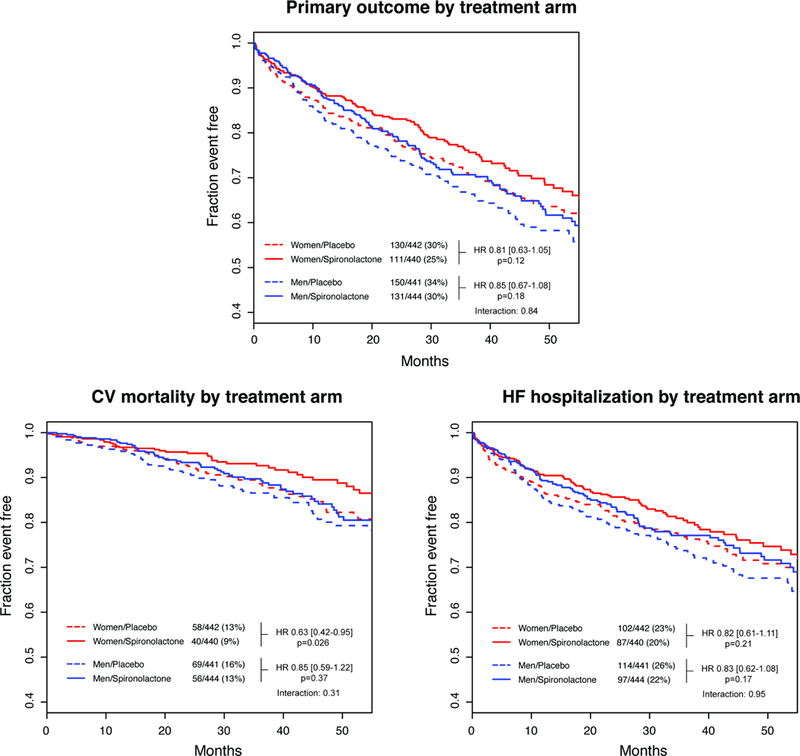
There was no significant association between spironolactone and the primary outcome or HF hospitalization in either women or men. Spironolactone was associated with a significantly reduced risk of CV mortality in multivariate analysis, but the interaction between sex and treatment arm was non-significant.
Figure 2b – Kaplan-Meier survival curves for secondary outcomes according to treatment arm and stratified by sex.

Spironolactone was associated with a significantly reduced likelihood of all-cause mortality with a significant sex-treatment arm interaction. There was no association between spironolactone and likelihood of non-CV mortality, CV hospitalization, or non-CV hospitalization.
Figure 3 – Multivariate Cox proportional hazard ratios associated with spironolactone for all outcomes, men versus women.

Spironolactone was associated with a reduced likelihood of all-cause mortality in women but not men with a significant sex-treatment interaction. There were no other significant associations between spironolactone and primary or clinical outcomes.
There was a significant reduction in all-cause mortality associated with spironolactone in women (HR 0.66 [0.48–0.90], p=0.01) but not in men (p=0.68) with a significant sex-treatment interaction (pinteraction=0.024). To estimate the impact of deaths of unknown cause on the associations between all-cause mortality, CV mortality and spironolactone treatment, analysis was repeated where deaths of unknown cause were combined with CV deaths. The multivariate HR for mortality was significant in women (HR 0.57 [0.39–0.83], p=0.003) but not in men (p=0.80) with a significant interaction (pinteraction=0.049). There were no significant differences associated with spironolactone for either sex in non-CV mortality, CV hospitalization or non-CV hospitalization (women 0.43, men p=0.63).
DISCUSSION
In this secondary analysis of TOPCAT, we found several sex differences in HFpEF patient characteristics and evidence of a possible sex-specific treatment response to spironolactone. Women generally had fewer comorbidities but had worse patient-reported outcomes than men. There were no sex differences in rates of primary or secondary outcomes in patients receiving placebo and no significant interaction between sex and treatment arm with respect to the primary outcome or its components. Treatment with spironolactone in women was associated with a lower multivariate risk of all-cause mortality with a statistically significant interaction between sex and treatment arm suggesting a possible sex-specific benefit of spironolactone in women. This finding is hypothesis-generating only and require substantial validation prior to clinical application. However given the paucity of effective therapies in patients with HFpEF, a prospective study of the effect of spironolactone in women with HFpEF.
Patient characteristics and outcomes
Sex differences in patient characteristics and outcomes have been described previously in three clinical trials in HFpEF: Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE), (15) the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM)-Preserved program, (16) and the Digitalis Investigation Group-Preserved Ejection Fraction study (DIG-PEF). (17, 18) Directionality of sex differences in baseline characteristics between our analysis, I-PRESERVE, and DIG-PEF are summarized in Table 3a. Differences in baseline characterstics observed in the present study were concordant in all 3 studies except for diabetes mellitus, which was less common in women in TOPCAT, the same between men and women in I-PRESERVE, and more common in women in DIG-PEF.
Table 3a –
Baseline patient characteristics in TOPCAT (placebo arm only), I-PRESERVE, and DIG-PEF
| Characteristic | TOPCAT | I-PRESERVE | DIG |
|---|---|---|---|
| Age | ↑ | ↑ | ↑ |
| LVEF | ↑ | ↑ | ↑ |
| Atrial fibrillation | ↓ | ↓ | Sinus only |
| Coronary artery disease | ↓ | ↓ | ↓ |
| Hypertension | ↓ | ↓ | ↓ |
| Diabetes mellitus | ↓ | ↔ | ↑ |
| Body mass index | ↑ | ↑ | ↑ |
| Tobacco use | ↓ | ↓ | NA |
| COPD | ↓ | ↓ | NA |
| Heart rate | ↑ | ↑ | NA |
| Systolic blood pressure | ↑ | ↑ | ↑ |
| Estimated GFR | ↓ | ↓ | ↓ |
| Hemoglobin | ↓ | ↓ | ↓ |
| NYHA class | ↑ | ↑ | ↑ |
| MLWHF/KCCQ | ⇑ | ⇑ | NA |
- Significantly higher in women
- Significantly lower in women
- No significant sex difference
- Significantly Higher MLWHF/lower KCCQ in women
Event rates for all secondary outcomes in our analysis are summarized in Table 3b. In our analysis there were no significant sex differences in any clinical endpoints in subjects receiving placebo. Event rates for all mortality outcomes and CV hospitalization in our study were similar CHARM-Preserved although formal statistical tests were not reported. (16) Multivariate risk of all outcomes was lower in I-PRESERVE, (15) whereas there was a lower multivariate risk of all-cause mortality in women in DIG-PEF, but no significant difference in HF or CV hospitalization. (17, 18) Larger studies that included observational data such as the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) found reduced mortality in women compared to men with HFpEF using a large individual-patient data meta-analysis. (19) Taken together, these study results reveal mixed evidence of sex differences in both mortality and hospitalization outcomes in patients with HFpEF with somewhat more evidence of reduced mortality in women.
Table 3b –
Comparison of event rates TOPCAT (placebo arm only), CHARM-Preserved, I-PRESERVE and DIG trials, events/100 patient years (5, 16, 32)
| TOPCAT | CHARM-Preserved | I-PRESERVE | DIG-PEF* | |||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | Women | Men | |
| Outcome | n=440 | n=441 | n=1811 | n=1212 | n=2491 | n=1637 | n=341 | n=378 |
| Deaths | ||||||||
| All-cause | 3.64 | 4.15 | 5.68 | 5.3 | 4.32 | 6.72 | 7.88 | 8.07 |
| CV | 2.15 | 2.68 | 3.83 | 3.87 | 2.51 | 4.21 | 5.96 | 5.62 |
| Non-CV | 0.93 | 1.2 | 1.84 | 1.43 | 1.26 | 2.14 | 1.84 | 1.59 |
| Hospitalization | ||||||||
| CV | 6.34 | 6.8 | 20.4 | 20.4 | 17 | 21.8 | 15.2 | 17 |
| HF | 3.79 | 4.43 | 7.32 | 5.75 | 7.1 | 7.8 | 5.26 | 8.53 |
| Non-CV | 6.91 | 7.42 | 19.3 | 14.9 | 14.1 | 17.5 | 15.3 | 13.1 |
CV = Cardiovascular HF = Heart failure
Event rates derived from DIG trial data obtained from BioLINCC
Treatment response to spironolactone
There was no significant interaction between sex and treatment arm in the primary TOPCAT analysis with respect to the primary outcome (pinteraction=0.99). However subjects from the Americas and Russia/Georgia regions were analyzed together for all pre-specified subgroup analyses in TOPCAT including the interaction between treatment and sex. (5) In our analysis of subjects from the Americas only, there was no significant sex/treatment interaction effect for the primary outcome or its components CV mortality and HF hospitalization. We did observe a possible sex difference in treatment response to spironolactone with respect to all-cause mortality (HR 0.66 [0.48–0.90], p=0.01, pinteraction=0.02), although there was no significant sex-specific treatment effect in either CV or non-CV morality alone. This discrepancy may be due to lack of statistical power as well as the inclusion of deaths of unknown cause in the all-cause mortality outcome. Although our findings raise the possibility of a sex-specific response to spironolactone with respect to all-cause mortality, our results are not conclusive, and existing supporting evidence is mixed.
Sex differences in the biologic activity of spironolactone in the kidney and the myocardium could promote sex-specific treatment response to spironolactone. We observed increases in serum potassium and serum creatinine and decreases in SBP associated with spironolactone in both men and women as expected (6) with a greater increase in women in creatinine at 4 months (pinteraction=0.03) and in potassium at 12 months (pinteraction=0.04). Previously, a greater decline in renal function associated with eplerenone in women compared with men was also observed in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study study (EPHESUS). (3) Given that there was no difference between men and women in baseline use of ACE-Is ARBs in either our study or EPHESUS, these findings could reflect greater renal activity of spironolactone in women.
Large studies of ACE-Is and ARBs in HFpEF have failed to show a sex difference in reduction of adverse clinical outcomes, and sex differences in HFpEF outcomes with MRAs have not been reported. (16, 21, 22) Studies of MRA in HFrEF have not shown evidence of a sex-specific treatment effect in the Randomized Aldactone Evaluation Study (23) or the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (24) study, where MRAs were associated with improved outcomes in both studies. There was an apparent reduction in all-cause mortality associated with eplerenone in women but not men with acute myocardial infarction complicated by LV dysfunction (EF < 40%) in EPHESUS, although as in our analysis the interaction between sex and treatment arm was not significant. (25) Animal studies have shown a greater impact of MRAs on cardiac remodeling in females versus males including normalization of gene myocardial gene expression profiles post-myocardial infarction. (4, 29, 30) It is feasible that MRAs might have greater impact on cardiac remodeling in women than in men in HFpEF due to greater myocardial sensitivity to aldosterone in women, but significant additional clinical and translational investigation is required to demonstrate sex-differences in biologic effects of spironolactone sufficient to improve clinical outcomes such as mortality.
Limitations
This is a post-hoc, exploratory subgroup analysis, which stratified the TOPCAT subjects enrolled from the Americas according to sex, and all findings are hypothesis-generating only. An important limitation is the subgroup-within-subgroup analysis plan stratifying the population first by region, then sex, then treatment arm. As has been discussed elsewhere, many subjects enrolled from Russia/Georgia may not have had HF or taken the study medication as reliably, (6, 7, 31) raising questions of the validity of combining the Americas and Russia/Georgia cohorts for an analysis of HFpEF outcomes. Therefore, we analyzed the subjects enrolled from the Americas only despite the potential methodologic ramifications, which include an increased likelihood of both type I and type II errors. (10–14) The reduced sample sizes are relatively underpowered to detect outcomes of interest particularly with respect to formal treatment-subgroup interaction statistics. Increasing numbers of subgroups also increase the risk of false-positive associations due to random chance. These issues reinforce the importance of conducting an appropriately powered, prospective trial examining the role of spironolactone prior to recommending use of spironolactone for management of HFpEF in women.
CONCLUSION
Women with HFpEF enrolled in TOPCAT were older with higher LVEF and fewer comorbidities but worse health status than men. There were no significant sex differences in clinical endpoints. We observed a significant reduction in all-cause mortality associated with spironolactone in women but not in men. Prospective evaluation is warranted to determine whether spironolactone should be recommended for treatment of HFpEF in women.
Supplementary Material
CLINICAL PERSPECTIVES.
Heart failure with preserved ejection fraction represents roughly half of all heart failure cases and predominately affects women. There are currently no proven therapies that clearly improve long-term survival or reduce heart failure hospitalizations. This analysis of subjects with HFpEF enrolled from the Americas in the TOPCAT trial shows that women and men present with distinct clinical profiles, although prognosis is poor for both. It also shows a possible improvement in all-cause mortality outcome associated with spironolactone therapy in women but not in men. Previous studies have also suggested that spironolactone could have a greater protective effect against adverse cardiac remodeling in women compared to men. If future prospective studies confirm our observations, spironolactone may be a new treatment option for HFpEF in women.
Acknowledgements:
The authors wish to thank all of TOPCAT investigators for their efforts in conducting this trial and making these data available.
Funding: This work was supported by funding from the American Heart Association [17IG33660301]; the National Heart, Lung, and Blood Institute at the National Institutes of Health [1K08HL125725 and HH SN268200425207C]; and Jacqueline’s Research Fund, Center for Women’s Health Research, University of Colorado School of Medicine.
Clinical Trials Registration: TOPCAT Clinicaltrials.gov identifier: BioLINCC: https://biolincc.nhlbi.nih.gov/studies/topcat/
ABBREVIATIONS
- ACE-I
Angiotensin converting enzyme inhibitor
- ARB
Angiotensin receptor blocker
- CV
Cardiovascular
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- LVEF
Left ventricular ejection fraction
- MRA
Mineralocorticoid antagonist
- RAAS
Renin-angiotensin-aldosterone system
- SBP
systolic blood pressure
- TOPCAT
Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have no conflicts to disclose.
REFERENCES
- 1.Kao DP, Lewsey JD, Anand IS, et al. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur. J. Heart Fail 2015;17:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasan RS, Evans JC, Benjamin EJ, et al. Relations of Serum Aldosterone to Cardiac Structure Gender-Related Differences in the Framingham Heart Study. Hypertension 2004;43:957–962. [DOI] [PubMed] [Google Scholar]
- 3.Rossignol P, Cleland JGF, Bhandari S, et al. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study. Circulation 2012;125:271–279. [DOI] [PubMed] [Google Scholar]
- 4.Kanashiro-Takeuchi RM, Heidecker B, Lamirault G, Dharamsi JW, Hare JM. Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin Transl Sci 2009;2:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Claggett B, Assmann SF, et al. Regional Variation in Patients and Outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 7.de Denus S, O’Meara E, Desai AS, et al. Spironolactone Metabolites in TOPCAT — New Insights into Regional Variation. N. Engl. J. Med 2017;376:1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rickham PP. Human Experimentation: Code of Ethics of the World Medical Association. Br. Med. J 1964;2:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bristow MR, Enciso JS, Gersh BJ, et al. Detection and Management of Geographic Disparities in the TOPCAT Trial: Lessons Learned and Derivative Recommendations. JACC Basic Transl Sci 2016;1:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegde SM, Claggett B, Shah AM, et al. Physical Activity and Prognosis in the TOPCAT Trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist). Circulation 2017;136:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvaraj S, Claggett B, Shah SJ, et al. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: an analysis of the TOPCAT trial. Eur. J. Heart Fail 2017. [DOI] [PubMed]
- 12.Vaduganathan M, Claggett BL, Chatterjee NA, et al. Sudden Death in Heart Failure with Preserved Ejection Fraction: A Competing Risks Analysis from the TOPCAT Trial. JACC Hear. Fail 2018. [DOI] [PubMed]
- 13.Cikes M, Claggett B, Shah AM, et al. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction: The TOPCAT Trial. JACC Hear. Fail 2018;6. [DOI] [PubMed]
- 14.Sanders NA, Supiano MA, Lewis EF, et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur. J. Heart Fail 2018:570–577. [DOI] [PubMed]
- 15.Lam CSP, Carson PE, Anand IS, et al. Sex differences in clinical characteristics and outcomes in elderly patients with heart failure and preserved ejection fraction: The Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ. Hear. Fail 2012;5:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Meara E, Clayton T, McEntegart MB, et al. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure - Results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007;115:3111–3120. [DOI] [PubMed] [Google Scholar]
- 17.Deswal A, Bozkurt B. Comparison of Morbidity in Women Versus Men With Heart Failure and Preserved Ejection Fraction. Am. J. Cardiol 2006;97:1228–1231. [DOI] [PubMed] [Google Scholar]
- 18.Alla F, Al-Hindi AY, Lee CR, Schwartz TA, Patterson JH, Adams KF. Relation of sex to morbidity and mortality in patients with heart failure and reduced or preserved left ventricular ejection fraction. Am. Heart J 2007;153:1074–1080. [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Sellés M, Doughty RN, Poppe K, et al. Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta-analysis. Eur J Hear. Fail 2012;14:473–479. [DOI] [PubMed] [Google Scholar]
- 20.Adams KF, Sueta CA, Gheorghiade M, et al. Gender differences in survival in advanced heart failure. Insights from the FIRST study. Circulation 1999;99:1816–1821. [DOI] [PubMed] [Google Scholar]
- 21.Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur. Heart J 2006;27:2338–2345. [DOI] [PubMed] [Google Scholar]
- 22.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–67. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 24.Zannad F, McMurray JJV., Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21. [DOI] [PubMed] [Google Scholar]
- 25.Pitt B, Remme W, Zannad F, et al. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction Bertram. New Eng J Med 2003;348:1309–21. [DOI] [PubMed] [Google Scholar]
- 26.Edelmann F, Tomaschitz A, Wachter R, et al. Serum aldosterone and its relationship to left ventricular structure and geometry in patients with preserved left ventricular ejection fraction. Eur Hear. J 2012;33:203–212. [DOI] [PubMed] [Google Scholar]
- 27.Schunkert H, Hense HW, Muscholl M, et al. Associations between circulating components of the renin-angiotensin-aldosterone system and left ventricular mass. Heart 1997;77:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regitz-Zagrosek V, Lehmkuhl E. Heart failure and its treatment in women: Role of hypertension, diabetes, and estrogen. Herz 2005;30:356–367. [DOI] [PubMed] [Google Scholar]
- 29.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 2002;53:672–677. [DOI] [PubMed] [Google Scholar]
- 30.Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog Cardiovasc Dis 2007;49:241–251. [DOI] [PubMed] [Google Scholar]
- 31.Bristow MR, Enciso JS, Gersh BJ, et al. Detection and Management of Geographic Disparities in the TOPCAT Trial: Lessons Learned and Derivative Recommendations Public Access. JACC Basic Transl Sci 2016;1:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam CSP, Brutsaert DL. Endothelial dysfunction: a pathophysiologic factor in heart failure with preserved ejection fraction. J Am Coll Cardiol 2012;60:1787–1789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


