Abstract
Collagen IV is the major matrix component associated with differentiating adipocytes in adipose tissues, and the understanding of its contribution in adipogenic differentiation could be important for elucidation of mechanisms and processes driving the obesity. Therefore, in the light of our previous findings of differential effects of structural conformation of collagen I matrix on differentiation of bone marrow stromal cells, we investigated whether similar phenomenon occurs on collagen IV matrix in native and denatured structural states. The results of the present study show that native collagen IV is unsupportive of adipogenic differentiation and very little if any adipogenesis occurs on this matrix in the presence of adipogenic stimuli. In sharp contrast to native collagen IV, the same matrix in denatured structural state drives highly efficient adipogenic differentiation suggesting that it might be the major driver of adipogenesis in adipose tissues and that the ratio of native to denatured matrix might regulate the intensity of adipogenesis and possibly underlies the obesity. In contrast to observations that adipogenesis on denatured collagen I (collagen I is the major matrix component in musculoskeletal tissues) is suppressed by stress, adipogenesis on denatured collagen IV appears to be stress-resistant suggesting an explanation for the observed ineffectiveness of physical exercise, i.e. mechanical stress, in the reduction of adipose tissues. The obesity was shown to be associated with overproduction of MMPs and decline in levels of TIMPs. Such a shift in MMP/TIMP balance was considered a consequence of the pathology. In the light of the present study, however, this shift might constitute the primary source of the decease. The findings of the present study suggest strategies for the treatment of obesity, raise significant questions and indicate directions for further experimentation.
INTRODUCTION.
Previously, it was shown that structural conformation of extracellular collagen I matrix plays, along with differentiation stimuli, an essential role in progression of bone marrow stromal cells toward osteogenic or adipogenic lineage (Mauney and Volloch, 2009a; 2009b). Thus, on native collagen I matrix a marginal inefficient adipogenesis of bone marrow stromal cells is p38-independent, whereas on collagen I matrix in denatured structural conformation an efficient adipogenesis is primarily regulated by p38 kinase (Mauney and Volloch, 2009a). Inversely, osteogenic differentiation occurs efficiently on native, but not on denatured collagen I matrix, with a low commencement threshold on the former and a substantially higher one on the latter (Mauney and Volloch, 2009a). The drastic difference in the efficiency of osteogenic differentiation reflects distinctly different regulatory pathways: whereas osteogenesis on collagen I matrix in both structural conformations is fully dependent on ERK, on native collagen I matrix osteogenic differentiation of bone marrow stromal cells is Hsp90-dependent, on denatured collagen I matrix it is Hsp90-independent (Mauney and Volloch, 2009a).
These drastically differential effects are due to different assortments of integrin binding sites presented by collagen I in different structural conformations. In native conformation it presents sites for α1β1 and α2β1 integrins, six per molecule for each type of integrin (Xu et al., 2000). The engagement of these integrins initiate an efficient activation of ERK (Egan et al., 1993; Schlaepfer et al., 1996; Takeuchi et al., 1997; Wary et al., 1996; 1998). In addition, α2β1 integrins mediate matrix contraction (Schiro et al., 1991; Tiollier et al., 1990; Yoshizato et al., 1999); this elicits cellular stress response and elevates levels of Hsp90 (Mauney and Volloch, 2009b) which is crucial for α1β1 and α2β1-initiated ERK activation pathway (Cutforth et al., 1994; van der Straten et al., 1997). In denatured structural conformation collagen I presents sites for αVβ3 integrin, seven per molecule, which are obscured in native but exposed in denatured state (Davis, 1992). The engagement of αVβ3 integrins is less efficient than that of α1β1/α2β1 in ERK activation but initiates an effective activation of p38 (Mauney and Volloch, 2009a; 2009b).
The efficiency of the engaged integrins in promoting ERK and p38 activation and consequently osteogenesis and adipogenesis of bone marrow stromal cells appears to reflect the number of binding sites for specific integrins per matrix molecule. This can be illustrated by osteogenesis of bone marrow stromal cells on native collagen IV matrix. Native collagen IV consists of both, collageneous triple helical domains as well as non-collageneous domains. The engagement of cells with this matrix occurs through α1β1 and α2β1 integrins, three and two binding sites per molecule respectively, as well as through αVβ3 integrin, one binding site per molecule in the non-collageneous domain (Eble et al., 1993; Underwood et al., 1995; Colorado et al., 2000; Sudhakar et al., 2005; Zarate et al., 2004). Native collagen IV matrix doesn’t undergo cell-mediated contraction (Tiollier et al., 1990). Consequently, it does not elicit cellular stress response (Mauney and Volloch, 2009b) and cellular levels of Hsp90 are insufficient to support α1β1/α2β1–initiated Hsp90-dependent osteogenic differentiation (Mauney and Volloch, 2009b). This means that osteogenic differentiation of cells on native collagen IV matrix is driven by αVβ3 integrin engagement with a single site per molecule at the non-collageneous domain and predictably should proceed very inefficiently, as was indeed observed (Mauney and Volloch, 2009b).
For two reasons, it is of special interest to study the progression of adipogenic differentiation on collagen IV matrix in both, native and denatured, structural states. First, as discussed below, the assortments of integrin binding sites presented by collagen IV are drastically different on matrices in native and denatured structural conformations. Second, collagen IV is the major matrix component associated with differentiating adipocytes in adipose tissues (Pierleoni et al., 1998). Therefore, better understanding of adipogenesis on this matrix might have implications for our understanding of processes associated with obesity. The experiments described below were designed to initiate this line of inquiry. In these experiments the procession of adipogenesis in native and denatured collagen IV matrices was compared with that on collagen I matrices.
RESULTS.
Collagen IV matrix in native structural state does not support adipogenesis of bone marrow stromal cells whereas on denatured collagen IV matrix adipogenic differentiation of bone marrow stromal cells proceeds in a highly efficient manner.
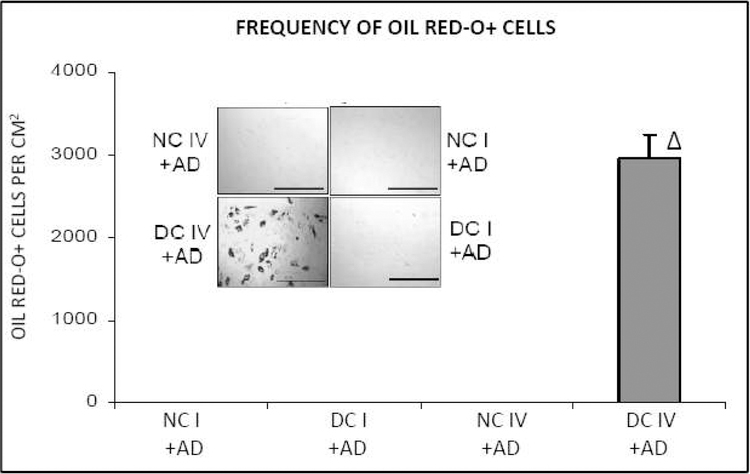
To assess the efficiency of adipogenic differentiation on collagen IV matrices in native and denatured structural states, bone marrow stromal cells were induced to undergo adipogenesis on collagen IV matrices in both structural conformations by the addition of adipogenic stimuli as detailed in Experimental Procedures section. In parallel, for reasons of comparison, similar experiments were carried out on native and denatured collagen I matrices. Adipogenic responses were assessed by monitoring the frequency of lipid-accumulated Oil Red-O positive cells after 9 days. As shown in Figure 1, adipogenic differentiation of bone marrow stromal cells occurs highly efficiently on denatured collagen IV matrix. In fact, adipogenesis of mesenchymal stem cells on denatured collagen IV matrix reached high levels on day nine of the experiment when there is yet no detectable adipogenesis either on native collagen IV or on native or denatured collagen I matrices.
Figure 1. Adipogenic differentiation of bone marrow stromal cells is highly efficient on denatured collagen IV matrix.
NCI: native collagen I matrix; DCI: denatured collagen I matrix; NCIV: native collagen IV matrix; DCIV: denatured collagen IV matrix; AD: adipogenic stimuli. [∆ = p<0.05]: significantly different in comparison to all other experimental groups. Photomicrographs inset: scale bar = 500µm.
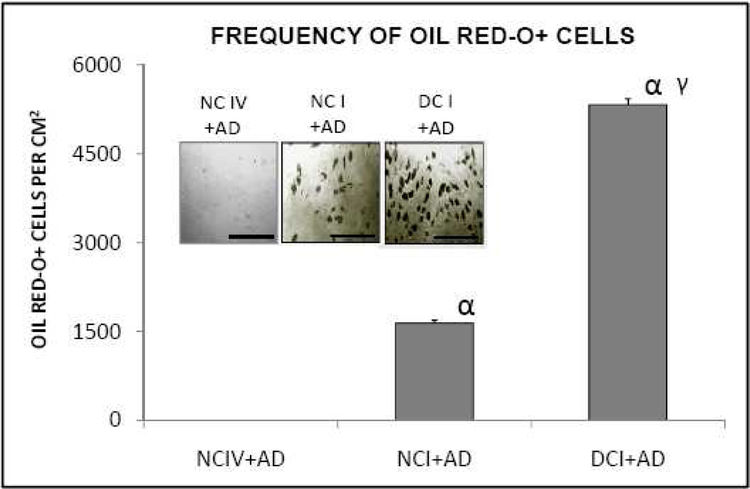
The observation described above raises some questions. We know from the previous studies (Mauney and Volloch, 2009a) that a substantial adipogenesis does eventually, by day 14, occur on denatured collagen I and marginal adipogenesis is seen by the same time on native collagen I matrix. It was, therefore, of interest to measure the extent of adipogenesis at day 14; such a measurement would address a possibility that the lack of detectable adipogenesis at day nine was an artifact of some sort, and serve as a benchmark reference for the extent of adipogenesis on native collagen I. The outcome of such an experiment is shown in Figure 2. Whereas the expected results, comparable with the previous observations (Mauney and Volloch, 2009a), were obtained on native and denatured collagen I matrices, no Oil Red-O positive cells were detected on native collagen IV matrix, indicating that native collagen IV matrix is unsupportive of adipogenesis of bone marrow stromal cells. The 14 days result with cells on denatured collagen IV is not presented because it is not directly compatible with other 14 days measurements. This is because by the fourteenth day of adipogenic differentiation on denatured collagen IV a significant proportion of differentiated cells are dying, in all appearances through bursting, a phenomenon not seen on day nine.
Figure 2. Collagen IV matrix in native structural conformation does not support adipogenesis of bone marrow stromal cells.
NCI: native collagen I matrix; DCI: denatured collagen I matrix; NCIV: native collagen IV matrix; AD: adipogenic stimuli. [α = p<0.05]: significantly different in comparison to NCIV+AD; [γ = p<0.05]: significantly different in comparison to NCI+AD. Photomicrographs inset: scale bar = 500µm.
Thermal stress suppresses adipogenesis of bone marrow stromal cells on denatured collagen I but has no effect on denatured collagen IV matrix.
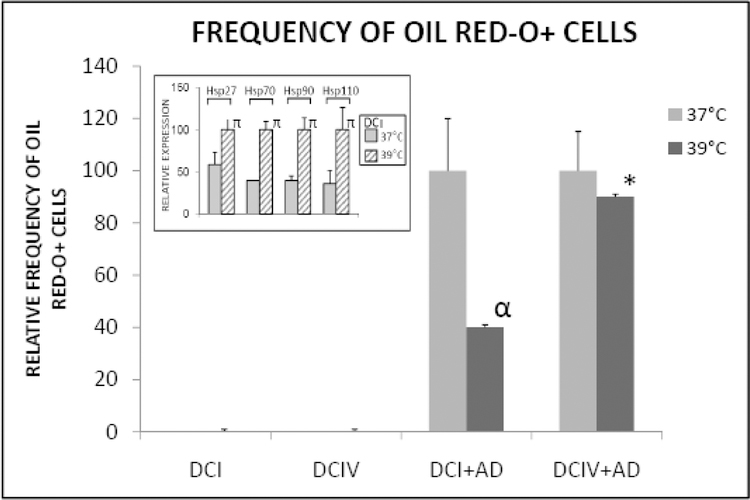
It was shown previously that on denatured collagen I matrix, continuous mild thermal stress suppresses, for the reasons discussed below, adipogenic differentiation of bone marrow stromal cells (Mauney and Volloch, 2009b). In the present study we investigated whether this phenomenon does also occur on denatured collagen IV matrix. To do so, bone marrow stromal cells were induced to undergo adipogenic differentiation on denatured collagen IV and, for reasons of comparison, denatured collagen I matrices at either normal or elevated temperature by the addition of adipogenic stimuli as detailed below in the Experimental Procedures section. As seen in the inset to Figure 3, the thermal treatment elicited in cells on denatured collagen I cellular stress response which manifested itself in elevated levels of major stress response proteins, a phenomenon observed in a previous study (Mauney and Volloch, 2009b). Adipogenic responses were evaluated by monitoring the frequency of lipid-accumulated Oil Red-O positive cells after 14 days. As shown in Figure 3, on denatured collagen I matrix the expected results consistent with the previous observations, namely a drastic reduction in the extent of adipogenesis, were observed. However, on denatured collagen IV matrix, cultivation at the elevated temperature had no effect on the efficiency of adipogenic differentiation of bone marrow stromal cells. The results in Figure 3 are presented in a “relative frequency” format meaning that results (frequency of Oil Red-O positive cells) obtained with cells subjected to thermal treatment are expressed as a proportion of those obtained with thermally untreated cells which are defined as 100%. This format was necessitated by the fact that, as was mentioned above, 14 days measurement on denatured collagens I and IV are not directly compatible. No statistically significant difference was seen in the number of Red-O positive cells per cm2 with thermally treated versus untreated cells on day nine on denatured collagen IV (not shown), when a comparison with cells on denatured collagen I could not be made due to the lack of detectable adipogenesis on the latter at this timepoint.
Figure 3. Adipogenic differentiation of bone marrow stromal cells is suppressed by thermal stress on denatured collagen I matrix but is stress-resistant on collagen IV matrix in denatured state.
AD: adipogenic stimuli; DC1: denatured collagen I matrix; DCIV: denatured collagen IV matrix; Hsp: heat shock protein; 37°C or 39°C: incubation temperature. All experimental groups significantly different from untreated controls, p<0.05. [α= p<0.05]: significantly different in comparison to (DCI+AD) at 37°C. [*= p>0.05]: significantly similar in comparison to (DCIV+AD) at 37°C. Inset: relative expression of Hsps on DCI at 37°C versus 39°C; [π= p<0.05]: compared to cells at 37°C.
DISCUSSION.
The results of the present study suggest that structural conformation of collagen IV matrix plays crucial role in adipogenesis of bone marrow stromal cells. Whereas native collagen IV does not support adipogenesis of bone marrow stromal cells, collagen IV in denatured structural state drives highly efficient adipogenic differentiation. The results obtained on native collagen IV are somewhat surprising in the light of our previous findings with collagen I matrices. On native collagen IV p38 activation and adipogenesis would be driven by the engagement with one αVβ3 site and two α2β1 sites per molecule (there is no p38 activation from α1β1 integrins). The levels of p38 and the extent of adipogenesis would be expected to be lower than on denatured collagen I matrix with its seven αVβ3 sites per molecule. In the worst case scenario, p38 levels would be insufficient for p38-dependent adipogenesis but in this case the extent of adipogenesis could be expected to be comparable with that of p38-independent adipogenesis on native collagen I matrix. Instead, very little if any adipogenesis of bone marrow stromal cells was seen on native collagen IV matrix. There are two possible explanations for this observation. One is that that p38-independent adipogenesis is driven by the integrins’ engagement with α1β1 and/or α2β1 binding sites of which there are six each per molecule on native collagen I but only two of the former and three of the latter per molecule of native collagen IV. This might underlie differential efficiency of adipogenesis on native collagen I and IV matrices and constitute another example where physiological processes reflect the density of specific integrin binding sites. Another possible explanation is that native, but not denatured, collagen IV contains a feature that suppresses adipogenesis of bone marrow stromal cells; additional experimentation is required to address this issue.
In a sharp contrast to the native collagen IV matrix, adipogenic differentiation of bone marrow stromal cells on collagen IV matrix in denatured structural state is driven by the engagement of αVβ3 integrins with 15 binding sites per matrix molecule. This results in a highly efficient adipogenesis, much more so than on denatured collagen I which presents only 7 binding sites per matrix molecule. This observation provides an “extreme” example for the regulatory power of structural conformation of collagen matrices. As was mentioned above, collagen IV is the major matrix component associated with adipocytes in adipose tissues. The results of the present study suggest that in those tissues adipogenic differentiation might be driven by the denatured structural state of collagen IV matrix and that the ratio of native to denatured matrix might regulate the intensity of adipogenesis and possibly underlie the obesity. On the other hand, obesity was shown to be associated with overproduction of MMPs and decline in levels of TIMPs (Chavey et al., 2003). Such a shift in MMP/TIMP balance was considered a consequence of the pathology. However, because it would result in a conversion of collagen matrices, including collagen IV, from native into denatured, adipogenesis-driving, state (Birkedal-Hansen et al., 1993; Veis and George, 1994; Kähäri and Saarialho-Kere, 1997; Knäuper et al., 1997; Messent et al, 1998; Maquoi et al., 2002; Riley et al., 2002; Chavey et al., 2003; Monaco et al., 2006), this shift might constitute the primary source of the decease.
Activation of ERK can potentially be initiated from αVβ3 integrins via two distinct pathways: Hsp90-dependent and –independent. It was shown previously (Mauney and Volloch, 2009b) that the former requires elicitation of stress response and elevation of Hsp90 levels and that under normal (non-stressful) conditions only the latter pathway does occur (Mauney and Volloch, 2009a; 2009b). It was also shown (Mauney and Volloch, 2009b) that elicitation of cellular stress response by a thermal stress results in a shift from Hsp90-independent pathway of ERK activation to a dominant mutually exclusive Hsp90-dependent one. During adipogenesis of bone marrow stromal cells on denatured collagen I matrix at the elevated temperature, this results in a drastic reduction in the extent of adipogenic differentiation (Mauney and Volloch, 2009b; present study). This is because αVβ3-initiated Hsp90-independent ERK pathway simultaneously activates p38 and is the major contributor of p38 activity (Mauney and Volloch, 2009b). Under stress conditions, this pathway is excluded and replaced by an Hsp90-dependent mechanism that activates and thus compensates for ERK but not p38 (Mauney and Volloch, 2009b). The same phenomenon could be also expected to occur during adipogenesis on denatured collagen IV matrix: under thermal stress adipogenic differentiation of bone marrow stromal cells should drastically decline. In the present study this phenomenon is confirmed for cells on denatured collagen I; however it does not occur on denatured collagen IV matrix.
There are two possible reasons for the above observation. The first one is that the engagement of αVβ3 integrins with 15 binding sites per matrix molecule results in activation of p38 to saturation, i.e. to the levels which significantly exceed those needed for maximal adipogenesis, and that the exclusion of one of p38-activating pathways under stressful conditions would still leave levels of p38 sufficient to sustain maximal rate of adipogenesis (look at it as a shift to lower p38 levels but still on a graphic plateau for the extent of adipogenesis).
The second possible reason is the presence of two unconventional α1β1 sites located in the noncollageneous domain 1 (NC1) of collagen IV. Indeed, denatured collagen IV is very similar to denatured collagen I; the only two differences are the number of αVβ3 sites (15 versus seven) and the presence of two NC1α1β1 sites on collagen IV. These two binding sites are unique among other members of α1β1 family in that they are not triple helix-specific but located in the noncollageneous part of the molecule, moreover, their binding with α1β1 integrins occurs in a manner different from that used by conventional triple helix-specific binding sites (Marcinkiewicz et al., 2003). More to the point, the engagement of α1β1 integrins with these two sites in the NC1 domain of collagen IV was shown to suppress Raf activation (Sudhakar et al., 2005). Hsp90-dependent pathway of ERK activation is also Raf-dependent; in fact, in this pathway Hsp90 is needed solely for Raf activation. The suppression of Raf activation would prevent stress-mediated shift to Raf-dependent mechanism of ERK activation. This mechanism and one mentioned above might operate simultaneously; because of the potential importance of the observed stress resistance role in energy processing and storage, the mechanistic redundancy is not only possible but could be expected.
The presumed NC1α1β1-mediated stress resistance of adipogenesis of bone marrow stromal cells on denatured collagen IV matrix could be explained by two possible mechanisms: (i) because of inability to proceed through Hsp90-dependent ERK activation pathway the stress-mediated switch from Hsp90-independent mechanism is cancelled, and (ii) the switch does occur but the Hsp90-depended ERK pathway is inactivated through suppression of Raf and the loss of one of p38 activation pathways is compensated for by the reduction of levels of ERK which would allow higher levels of p38 (Mauney and Volloch, 2009a; 2009b); alternatively, even if Raf is not suppressed, the resulting level of p38 activity might still be on the plateau due to its initial oversaturation. Additional investigation is needed to distinguish between these two possibilities. The mechanism of NC1α1β1-mediated suppression of Raf activation is not clear. One interesting possibility to be tested is that the engagement of the α1β1 integrins with these sites triggers a suppression of cellular stress response, possibly through interference with HSF1, for example by inhibiting activating phosphorylation at serine 230 (Holmberg et al., 2001), or by promoting inhibitory phosphorylation at serines 303 and 307 (Chu et al., 1996; 1998; Knauf et al., 1996; Kline and Morimoto, 1997), this, in turn, would prevent the elevation of Hsp90 levels and subsequent activation of Raf.
The results of the present study clearly suggest the prevention of the conversion of native collagen IV into denatured structural state in adipose tissues as the major strategy for the treatment of obesity. This can be achieved by the suppression of MMPs capable of cleaving collagen triple helix and/or by the induction of corresponding TIMPs. As for stress resistance of adipogenesis of bone marrow stromal cells on denatured collagen IV matrix, both scenarios described above, namely excessive activation of p38 and NC1α1β1-mediated mechanism, are consistent with a notion that elicitation of stress response in adipose tissue might not be effective in the suppression of adipogenesis (in contrast to observations on denatured collagen I, the major matrix component in musculoskeletal tissues, where elicited stress response causes switch to Hsp90-dependent pathway and suppresses adipogenesis, see Mauney and Volloch, 2009b). Both scenarios support the change in the MMP/TIMP balance as the major strategy to address obesity. The NC1α1β1 scenario, in addition, also opens potential stress-based strategies that remain to be explored. In conclusion, the findings of the present study raise significant questions and suggest directions for further experimentation.
EXPERIMENTAL PROCEDURES.
Bone marrow stromal cells preparation.
Human bone marrow stromal cells were obtained from commercially available bone marrow aspirates (Cambrex, Walkersville, MD) from five male donors; similar trends in cell responses were observed with all donors. Cells were expanded on tissue culture plastic to passage 1 (P1) utilizing previously reported methods (Altman et al., 2002; Mauney et al., 2004; 2005). Briefly, whole bone marrow aspirates were plated at 8–10 μl aspirate/cm2 on 185 cm2 tissue culture plates and cultivated until confluency (~12–14 days) in 40 ml of expansion medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), 100U/ml penicillin, 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, and 5 ng/ml of basic fibroblast growth factor (bFGF) (Life Technologies, Rockville, MD). Cultures were maintained in a humidified tissue culture incubator at 37°C with 5% CO2 and 95% air. Bone marrow stromal cells were selected on basis of their ability to adhere to tissue culture plastic (TCP); non-adherent hematopoietic cells were removed during medium replacement after approximately 7 days in culture. Medium was changed twice per week thereafter. P1 cells were frozen in liquid nitrogen at 5 mln/ml in 90% fetal calf serum and 10% DMSO and utilized in subsequent experiments. Viable cell recovery after thawing was over 80%. Differentiation potentials of so obtained cell populations for both osteogenic and adipogenic lineages have been verified both in vitro and in vivo (Mauney et al., 2006; 2007). These differentiation-competent cells are commonly referred to either as adult mesenmchymal stem cells, defined as adult bone marrow-derived nonhemopoietic stromal cells capable of differentiation into various lineages, or as bone marrow stromal cells, a designation adopted in the present manuscript.
Preparation of collagen matrices.
Collagen I solutions were prepared and utilized to cast collagen films using methods previously described (Volloch and Kaplan, 2002). Briefly, sterile rat tail-derived collagen type I (Roche, Indianapolis, IN; cat. #1179179) was dissolved at 0.5 mg/ml in 0.1% acetic acid and either maintained in its native conformation (NC) or denatured (DC) by incubation at 500C for 8 hrs as previously reported (Volloch and Kaplan, 2002); at these conditions collagen was shown to be denatured and have only a small degree of fragmentation (Volloch and Kaplan, 2002). To prepare films, native or denatured collagen solutions were added to 96 well tissue culture plates at 250 µg/cm2, dried under a laminar flow hood for 4–5 days and lyophilized for 1–2 days to enhance film stability during cell cultivation. Similar trends in cell responses were observed when rat tail collagen was compared with human placenta-derived collagen type I (Sigma-Aldrich, cat. #C7774) or when films were cast in 6 or 24 well tissue culture plates. Reported results were obtained with rat tail-derived collagen, except when human placenta-derived collagen type IV (Sigma-Aldrich, cat. #C7521) was used and compared with human collagen I mentioned above.
Adipogenic differentiation of bone marrow stromal cells.
Cells on native or denatured collagen matrices were treated with AD or maintained as untreated controls as previously described (Mauney et al., 2004; 2005), and analyzed for adipogenic differentiation. Briefly, cells were plated at 5×103 cells/cm2 on native or denatured collagen I and collagen IV matrices in medium consisting of DMEM supplemented with 10% FCS, 100U/ml penicillin, 100 μg/ml streptomycin and 0.1 mM nonessential amino acids. 12 hours later adipogenic stimulants (AD) were added and subsequently replaced with each medium change every 3–4 days. Adipogenic stimulants consisted of 0.5 mM 3-isobutyl-1-methyl-xanthine, 1 μM dexamethasone, 5 μg/ml insulin, and 50 μM indomethacin as previously reported (Mauney et al., 2005). Unless otherwise stated, experiments continued for 14 days. Cultures were maintained as described above and medium exchange was carried out twice per week. Parallel control cultures were maintained similarly in the absence of stimulants.
Continual thermal stress.
Cultures were maintained as described above, except in a designated incubator adjusted to 39°C. No changes in the rate of proliferation or in cell behavior were observed. Levels of stress response were measured in cells on DC1 matrix.
Oil Red-O Staining and Frequency of Lipid Accumulating Cells.
Following AD treatment, cultures were analyzed for the frequency of lipid accumulating cells by Oil Red-O staining as previously described (Mauney etal.,2005). Briefly, cultures were fixed in 4% neutral buffered formalin for 12 hours and stained for 45 minutes with a filtered 60% Oil Red-O solution in PBS, made from a stock solution of 0.70g Oil Red-O powder in 200 ml of isopropanol as previously reported (Stewart et al., 2004). Cultures were then washed with PBS to remove background Oil Red-O stain and photomicrographs were taken with a Zeiss Axiovert S100 light microscope and a Sony Exwave HAD 3CCD color video camera utilizing Scion Image software. The number of Oil Red-O positive cells was then manually counted in each culture well to determine the frequency of lipid accumulating cells.
Real time RT-PCR Analysis.
Markers of interest were analyzed by real time RT-PCR as previously described (Mauney etal.,2004; 2005). Briefly, RNA was extracted using Trizol reagent (Life Technologies), and cDNA were synthesized using High-Capacity cDNA Archive kit (ABI Biosystems) following the manufacturers instructions. Reactions were performed and monitored using an ABI Prism 7000 Sequence Detection System (Applied Biosystems). The PCR master mix was based on AmpliTaq Gold DNA polymerase (Applied Biosystems). For stress-response, heat shock proteins 90 (hsp90), 110 (hsp110), 27 (hsp27), and 70 (hsp70) were evaluated. Analysis was performed using commercially available primers and probes from ABI Biosystems Assays-on-Demand™ Gene Expression kits (hsp90, cat.# Hs00743767_sH; hsp110, cat.# Hs00198379_m1; hsp27, cat.# Hs00356629_g1; hsp70, cat.# Hs00271244_s1). For each cDNA sample, the Ct value was defined as the cycle number at which the fluorescence intensity of each target gene was amplified within the linear range of the reaction. Relative expression levels for each gene of interest were calculated by normalizing the quantified gene of interest transcript level (Ct) to the GAPDH transcript level (Ct) as described previously (2∆Ct formula, Perkin Elmer User Bulletin #2).
Statistical Analysis.
All measurements were collected with N=3–5 independent samples per data point and expressed as means ± standard deviations. Data were analyzed with Microsoft Excel software utilizing a Student’s two tailed t-test assuming equal levels of variance. Statistically significant values were defined as p<0.05.
ACNOWLEDGMENTS.
This research was supported by NIH, NIBIB, through P41-EB002520 grant.
Abbreviations.
- NC I
native collagen I matrix
- DC I
denatured collagen I matrix
- TP
tissue culture plastic
- ERK
extracellular signal-regulated kinase
- p38
p38 mitogen-activated protein kinase
- Raf or Raf-1
serine/threonine-specific kinase in ERK activating cascade
- Hsp
heat shock protein
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of metalloproteinase
- AD
adipogenic stimuli
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES.
- Altman G, Horan R, Martin I, Farhadi J (2002). Cell differentiation by mechanical stress. FASEB J 16, 270–2. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore W, Bodden M, Windsor L, Birkedal-Hansen B, DeCarlo A, Engler JA 1993. Matrix metalloproteinases: a review. Crit. Rev. Oral Biol. Med 4, 197–250. [DOI] [PubMed] [Google Scholar]
- Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E and Tartare-Deckert S (2003). Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J. Biol. Chem 278, 11888–11896. [DOI] [PubMed] [Google Scholar]
- Chu B, Soncin F, Price B, Stevenson M and Calderwood S (1996) Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor 1. J Biol Chem, 271, 30847–30857. [DOI] [PubMed] [Google Scholar]
- Chu B, Zhong R, Soncin F, Stevenson M and Calderwood S (1998) Transcriptional activity of heat shock factor 1 at 37°C is repressed through phosphorylation on two distinct serine residues by glycogen synthase kinase 3α and protein kinases Cα and Cζ. J Biol Chem, 273, 18640–18646. [DOI] [PubMed] [Google Scholar]
- Colorado PC, Torre A, Kamphaus G, et al. (2000). Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res 60, 2520–6. [PubMed] [Google Scholar]
- Cutforth T and Rubin GM (1994). Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell 77, 1027–36. [DOI] [PubMed] [Google Scholar]
- Davis GE (1992). Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun 182, 1025–31. [DOI] [PubMed] [Google Scholar]
- Eble JA, Golbik R, Mann K and Kuhn K (1993). The alpha 1 beta 1 integrin recognition site of the basement membrane collagen molecule [alpha 1(IV)]2 alpha 2(IV). EMBO J 12, 4795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan SE, Giddings BW, Brooks MW, Buday L, Sizeland AM and Weinberg RA (1993). Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature 363, 45–51. [DOI] [PubMed] [Google Scholar]
- Holmberg C, Hietakangas V, Mikhailov A, Rantanen J, Kallio M, Meinander A, Hellman J, Morrice N, MacKintosh C, Morimoto R, Eriksson J, Sistonen L (2001) Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J, 20, 3800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri V, Saarialho-Kere U 1997. Matrix metalloproteinases in skin. Exp. Dermatol 6, 199–213 [DOI] [PubMed] [Google Scholar]
- Knäuper V, Cowell S, Smith B, López-Otín C, O’Shea M, Morris H, Zardi L, and Murphy G (1997). The Role of the C-terminal Domain of Human Collagenase-3 (MMP-13) in the Activation of Procollagenase-3, Substrate Specificity, and Tissue Inhibitor of Metalloproteinase Interaction. J. Biol. Chem 272, 7608–16 [DOI] [PubMed] [Google Scholar]
- Kline M and Morimoto R (1997) Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol, 17, 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf U, Newton E, Kyriakis J and Kingston R (1996) Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev, 10, 2782–2793. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C, Weinreb P, Calvete J, Kiziel D, Mousa S, Lobb R (2003) Obtustatin: A potent selective inhibitor of α1β1 integrin in vitro and angiogenesis in vivo. Cancer Res 63, 2020–23. [PubMed] [Google Scholar]
- Maquoi E, Munaut C, Colige A, Collen D, Lijnen H (2002). Modulation of adipose tissue expression of matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes 51, 1093–101. [DOI] [PubMed] [Google Scholar]
- Mauney J and Volloch V (2009a). Progression of human bone marrow stromal cells into both osteogenic and adipogenic lineages is differentially regulated by structural conformation of collagen I matrix via distinct signaling pathways. Online, Matrix Biology, DOI: 10.1016/j.matbio.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauney J and Volloch V (2009b). Collagen I matrix contributes to determination of adult human stem cells lineage via differential, structural conformation-specific elicitation of cellular stress response. Online, Matrix Biology, DOI: 10.1016/j.matbio.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauney JR, Kaplan DL and Volloch V (2004). Matrix-mediated retention of osteogenic differentiation potential by human adult bone marrow stromal cells during ex vivo expansion. Biomaterials 25, 3233–43. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Volloch V and Kaplan DL (2005). Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion. Biomaterials 26, 6167–75. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Kirker-Head C, Abrahamson L, Gronowicz G, Volloch V and Kaplan DL (2006). Matrix-mediated retention of in vitro osteogenic differentiation potential and in vivo bone-forming capacity by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion. J. Biomed. Mater. Res 79, 464–75. [DOI] [PubMed] [Google Scholar]
- Mauney J, Nguyen T, Gillen K, Kirker-Head C, Gimble J, Kaplan D (2007). Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 28, 5280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messent A, Tuckwell D, Knauper V, Humphries M, Gavrilovic J 1998. Effects of collagenase-cleavage of type I collagen on alpha2beta1 integrin-mediated cell adhesion. J. Cell. Sci 111, 1127–35. [DOI] [PubMed] [Google Scholar]
- Monaco S, Sparano V, Gioia M, Sbardella D, DiPierro D, Marini S, Coletta M (2006). Enzymatic processing of collagen IV by MMP-2 (gelatinase A) affects neutrophil migration and it is modulated by extracatalytic domains. Protein Sci 15, 2805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierleoni C, Verdenelli F, Castellucci M, and Cinti S (1998). Fibronectins and basal lamina molecules expression in human subcutaneous white adipose tissue. Eur. J. Histochem 42, 183–188 [PubMed] [Google Scholar]
- Riley G, Curry V, Degroot J, VanEl B, Verzijl N, Halzeman B, Bank R (2002). Matrix metalloproteinase activities and their relationship with collagen remodeling in tendon pathology. Matrix Biol 21, 185–95. [DOI] [PubMed] [Google Scholar]
- Schiro JA, Chan BM, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ and Kupper TS (1991). Integrin alpha 2 beta 1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell 67, 403–10. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD and Hunter T (1996). Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol. Cell. Biol 16, 5623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WC, Baugh JE Jr, Floyd ZE, and Stephens JM (2004). STAT 5 activators can replace the requirement of FBS in the adipogenesis. Biochem. Biophys. Res. Commun 324, 355–359. [DOI] [PubMed] [Google Scholar]
- Sudhakar A, Nyberg P, Keshamouni VG, Mannam AP, Li J, Sugimoto H, Cosgrove D and Kalluri R (2005). Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J. Clin. Invest 115, 2801–10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T and Matsumoto T (1997). Differentiation and transforming growth factor-beta receptor down-regulation by collagen-alpha2beta1 osteoblastic cells. J. Biol. Chem 272, 29309–16. [DOI] [PubMed] [Google Scholar]
- Tiollier J, Dumas H, Tardy M and Tayot JL (1990). Fibroblast behavior on gels of type I, III, and IV human placental collagens. Exp. Cell. Res 191, 95–104. [DOI] [PubMed] [Google Scholar]
- Underwood PA, Bennett FA, Kirkpatrick A, Bean PA and Moss BA (1995). Evidence for the location of a binding sequence for the alpha 2 beta 1 integrin of endothelial cells, in the beta 1 subunit of laminin. Biochem. J 309, 765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Straten A, Rommel C, Dickson B, Hafen E (1997). The heat shock protein 83 (Hsp83) is required for Raf-mediated signaling in Drosophila. EMBO J 16, 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis A, and George A (1994). Fundamentals of interstitial collagen self-assembly. In Extracellular Matrix Assembly and Function Yurchenco PD, Birk DE, and Mecham RP, editors. Academic Press, 15–45. [Google Scholar]
- Volloch V, Mosser D, Massie B, Sherman M (1998). Reduced thermotolerance in aged cells results from a loss of hsp72-mediated control of JNK pathway. Cell. Stress Chaperones 3, 265–71. [PMC free article] [PubMed] [Google Scholar]
- Volloch V and Kaplan D (2002). Matrix-mediated cellular rejuvenation. Matrix Biol 21, 533–43. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, and Giancotti FG (1996). The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87, 733–43. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mariotti A, Zurzolo C, and Giancotti FG (1998). A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell 94, 625–34. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gurusiddappa S, Rich RL, Owens RT, Keene DR, Mayne R, Hook A and Hook M (2000). Multiple binding sites in collagen type I for the integrins alpha1beta1 and alpha2beta1. J. Biol. Chem 275, 38981–9. [DOI] [PubMed] [Google Scholar]
- Yoshizato K, Tsukahara M, Oki T, Hayashi M, Obara M and Morpho Y (1999). The interaction of cellular fibronectin with collagen during fibroblast-mediated contraction of collagen gels. J. Investig. Dermatol. Symp. Proc 4, 190–5. [DOI] [PubMed] [Google Scholar]
- Zarate S, Romero P, Espinosa R, Arias CF and Lopez S (2004). VP7 mediates the interaction of rotaviruses with integrin alphavbeta3 through a novel integrin-binding site. J. Virol 78, 10839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]