Abstract
The legume family (Fabaceae) is the third-largest flowering family with over 18 000 species worldwide that are rich in proteins, oils, and nutrients. However, the production potential of legume-derived food cannot meet increasing global demand. Wild legumes represent a large group of wild species adaptive to diverse habitats and harbor rich genetic diversity for the improvement of the agronomic, nutritional, and medicinal values of the domesticated legumes. Accumulating evidence suggests that the genetic variation retained in these under-exploited leguminous wild relatives can be used to improve crop yield, nutrient contents, and resistance/tolerance to environmental stresses via the integration of omics, genetics, and genome-editing technologies.
Introduction
It is predicted that the global crop productivity must be doubled by 2050 to meet growing global demand [1]. However, the current increase rate of crop production cannot meet the challenge of food security because of two main reasons: 1) Modern crop cultivars are unable to adapt to harsh environments or changing climates due to low genetic variation after the domestication bottleneck; 2) Agricultural land is continuously decreasing due to urbanization, industrialization, and as well as the increasing demand for animal production (land competition) due to human dietary changes [2]. There is an urgent need in developing diverse crop cultivars that can grow in harsh environments with broad-spectrum biotic stress resistance. On the other hand, crop wild relatives (CWRs), the ancestor of modern crops, thrive in diverse and challenging environments, are under-utilized. They harbor rich genetic diversity that can provide novel genes/alleles for the development of crop cultivars more resistant and resilient to harsh growing conditions. The CWRs are usually classified into G1-G4 groups based on its relationship to existing crops. G1 is the most closely related group, while G4 is the most distant one [3,4]. In some cases, when the immediate wild relatives are unknown or extinct, the relatively distant wild species could be used as the alternative gene pools to enrich the reduced genetic diversity of cultivated crops. Over the past decades, tremendous efforts have been made to explore and utilize the genetic diversity in CWRs for crop improvements, such as pest resistance and abiotic stress tolerance [3,5]. Thus, CWRs serve as a vast reservoir of agriculturally-important genes. However, the majority of CWRs are still under-exploited, under-utilized, and not preserved. Figure 1 shows the strategies that can be used for the exploration, utilization, and conservation of leguminous wild relatives.
Figure 1.
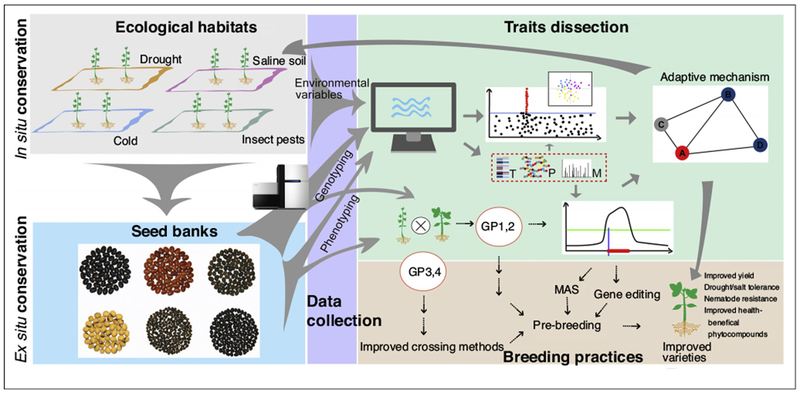
A flowchart depicting the exploration and use of environment-adaptive leguminous wild relatives in developing climate resilient varieties. In situ and ex situ conservation are two major methods to maintain biodiversity. The in-situ conservation retains the species in their adaptive natural habitats, while ex situ conservation preserves the seeds from diverse habitats in a seed bank offering excellent opportunities for research and breeding. Applying high-throughput genotyping and phenotyping (including phenotypic characterization, transcriptomics (T), proteomics (P), metabolomics (M)) technologies on these in situ conserved natural wild populations may advance the dissection of ‘genotype-phenotype’ associations using various bioinformatics approaches. The use of the environmental variables is useful in identifying adaptation-associated genomic regions. For breeding, successful crossing allows the transfer of a superior trait from wild species into an elite variety for new cultivar improvement. Vigorous and fertile seeds produced between species can facilitate linkage mapping development for identifying candidate genes/loci associated with traits of interest. The molecular mechanisms elucidated by association and linkage analyses would shed light on the efficient management of in situ conservation of the leguminous wild relatives. These strategies aided with high-throughput biotechnologies could facilitate the identification of closely-linked markers for marker-assisted selection (MAS) or causal genes that could be edited for favorite traits.
The legume family (Fabaceae) is the third-largest plant family, of which many species are cultivated worldwide. Leguminous plants also fix nitrogen by interacting with symbiotic nitrogen-fixing bacteria [6], and produce usable forms of nitrogen that can be used by other organisms. However, when grown under stressful environmental conditions, many legume yields are significantly decreased. One reason is that cultivated crops have lost a considerable amount of genetic diversity during domestication process [3,7–9]. Additionally, compared to cereals, relatively less funding available for legume community could be another major barrier constraining extensive studies for legume improvement. In turn, there is increasing evidence indicating that leguminous wild relatives retain considerable novel alleles useful for crop improvement [10], such as salt tolerance [11••] and yield-related traits [12,13].
Here, we first review recent progress in the utilization of genetic variation in wild legume species involving biotic stress resistance, abiotic stress tolerance, nutrition, and phytocompounds. We then discuss the challenges and opportunities with the surge of genomics and metabolomics data, as well as cutting-edge genome-editing technologies. We provide a few examples for each trait, which are summarized in Table 1.
Table 1.
Recent studies exploiting the exotic genetic resources of leguminous wild relatives for legume crop improvement using various biotechnological approaches
| Trait classification | Specific traits of interest | Wild leguminous species | Research strategy | References |
|---|---|---|---|---|
| Abiotic stress tolerance | Salt tolerance | Wild Vigna species | Phenotype characterization, genetic mapping | [14–17] |
| Glycine soja | Gene cloning, metabolomics | [11••,18–22] | ||
| Cold tolerance | Wild Cicer species | Phenotype characterization | [81,82] | |
| Drought tolerance | Wild Cicer, Arachis, common bean, and Lentil species | Phenotype characterization, GWAS | [8,26••,28,81] | |
| Biotic stress resistance | Nematodes (Heterodera glycines, Meloidogyne arenaria) resistance | G. soja, perennial Glycine species | Phenotype characterization, RNA-Seq, GWAS, KASP, genetic mapping | [32,33,75,83] |
| Wild Arachis species | Phenotype characterization | [36,37] | ||
| Pathogen, late leaf spot and rust resistance | Wild Arachis spieces | Gene cloning | [84••] | |
| Stemphylium, Ascochyta, anthracnose, and Stemphylium blight resistance | Wild Lentil species | Genetic mapping | [38–41] | |
| Aphid resistance | G. soja | Genetic mapping | [3,43–45] | |
| Butterfly and Plume moth resistance | Wild Cajanus species | Genetic mapping | [46,47] | |
| Yield-related | 100-seed weight | G. soja | Genetic mapping, RNA-seq, gene function analysis | [12,50,51,65] |
| Seed composition | Protein | G. soja, perennial Glycine species | 1-DE, GWAS | [64,85] |
| Oil | G. soja | RNA-seq, phenotype characterization | [64,65] | |
| Domestication | Seed weight, pod dehiscence, flowering, plant height, seed coat color | Wild soybean, lupin, pigeonpea, cowpea species | NGS, microarray | [2,86,87,88•,89•,90] |
| Metabolomics | Flavonoids, phenolics, isoflavones | G. soja | Genetic mapping | [91,92] |
| Saponin | G. soja | LC–MS | [66,67] | |
| Genome sequencing | Whole genome, plastome | G. latifolia, G. soja, G. gracilis | NGS | [93,94,95•] |
| Ecology | Adaptive traits, drought, salt | G. soja, wild chickpea species | NGS, GWAS | [26••,73•,74] |
Note: GWAS, genome-wide association study; RNA-Seq: RNA sequencing; KASP assay (Kompetitive Allele Specific PCR assay): is a fluorescence-based genotyping strategy to distinguish two different alleles; 1-DE: 1-dimensional gene electrophoresis; NGS: next-generation sequencing; LC–MS: liquid chromatography–mass spectrometry.
Abiotic stress tolerance
Salt tolerance
Most legume crops are glycophytes; therefore, salt stress can significantly limit legume yield productivity. However, some of their wild relatives have adapted to various coastal and saline areas. Chankaew et al. [14] identified genetic variation associated with salt tolerance in the halophyte Vigna marina. The quantification of Na+ and K+ concentrations with improved methods has indicated different salt tolerance mechanisms among wild Vigna species [15–17]. For example, a group of wild Vigna species prevents salt-caused damages by excluding salt from the whole plants, while another group accumulates excessive Na+ in vacuoles [16,17]. Iseki et al. [17] suggested that salt tolerance in the genus Vigna has evolved multiple times independently after diverging from its ancestor. These Vigna plants adaptive to saline coastal areas are useful materials for the dissection of the underlying salt-tolerance mechanisms.
Recent studies in Glycine soja, the wild ancestor of cultivated soybean (Glycine max), have identified a number of genotypes with enhanced salt tolerance [11••,18]. A novel salt tolerance gene, GmCHX1, has been recently identified and cloned in G. soja via whole-genome sequencing [11••]. In addition, many candidate genes [18–22] from a single salt-tolerant G. soja genotype, G. soja07256, demonstrated improved salt tolerance when expressed in model plant species, Arabidopsis and Medicago. Given the importance of this genotype, it is worth pinpointing the causal gene(s) conferring salt tolerance with the genomics-linkage mapping approach as previously described [11••]. It is also important to test how these genes behave in saline soils when transferred to soybean cultivars via breeding or gene engineering. Meanwhile, it remains unclear if these genes will result in tradeoffs when used to develop salt-tolerant cultivars. Recently, metabolomics comparisons have provided new insights into salt tolerance mechanisms. For example, when under salt stress conditions, higher accumulation of carbohydrates, fatty acids, phenylalanine, proline, and palmitic acid was observed in the salt-tolerant G. soja plant than that in salt-sensitive soybean cultivar [23,24].
Drought tolerance
Unlike salt tolerance with relatively well-understood mechanisms, drought tolerance is more complex and usually found associated with many loci and each with small effects. Wild legumes are excellent materials for studying drought tolerance because most of them grow in arid regions [25,26••]. For example, many drought-tolerant wild lentil species (such as Lens odemensis, Lens tomentosus) [8] and wild chickpeas [26••] adapt to drought-prone areas. Under moisture-controlled soil conditions, these wild lentil genotypes can increase their capacity to avoid or tolerate drought stress by reducing transpiration rates, and some due to its deep root system. Meanwhile, various phenotypic trade-offs have been observed, such as delayed flowering, reduced plant height and growth rate [8]. Other than deep rooting and increased lateral roots in grain legumes for drought tolerance [27], wild Arachis duranensis maintains relatively high levels of transpiration and photosynthesis rate under dehydration treatment [28]. Even though physical differences exist among these wild species, the drought tolerance mechanisms remain similar: for example, enhanced water uptake, and reduced water loss by growing smaller leaves to maintain water balance under water deficits [28].
Plant root systems are crucial in drought tolerance and they affect aboveground yields. To date, many QTLs associated with root-related traits have been identified in legumes using interspecific mapping populations [27]. More QTLs will likely be identified in the near future as more advanced biotechnologies, such as 2D/3D root imaging systems, as well as more affordable sequencing platforms, are applied to root-related traits studies. Further determination of their genetic relevance to drought response could be helpful for root trait-directed breeding for drought management. Alternatively, a global transcriptomics analysis of drought-stressed wild A. duranensis revealed osmotic regulation as a primary mechanism in drought tolerance [28]. From an ecological adaptation perspective, drought tolerance in wild relatives could also be dissected with landscape genomics, that is, association analysis of their environmental factors combined with genomic variation [25,29,30].
Biotic stress resistance
Nematode resistance
Biotic stresses, such as phytoparasitic nematodes, can cause significant yield loss in a wide range of leguminous hosts. For example, soybean cyst nematode (Heterodera glycines, SCN) is the most devastating soybean pest, which causes over one billion yield losses per year in US [31]. Interactions between SCN, as well as root-knot nematode (Meloidogyne spp., RKN), and their hosts have been intensively studied because of their agricultural importance. To identify new sources of SCN resistance to widen the existing limited resistance gene pool (mainly from soybean cultivar PI88788), Zhang et al. [32] evaluated over 200 wild soybean (G. soja) genotypes and suggested to prioritize the screening of Japanese G. soja accessions for increased chances of finding resistant ones. While most G. max and G. soja are SCN-susceptible, 97.3% of 223 perennial soybean relatives, subgenus Glycine, exhibited varying levels of resistance to three SCN types [33], which makes this subgenus a valuable resource for breeding new cultivars with durable resistance to SCN. Despite the challenges due to cross-incompatibility between Glycine perennials (GP-3) and soybean, recent inspiring studies have shown that it is feasible to produce fertile seeds between Glycine tomentella (polyploidy) and soybeans [34,35••], indicating that the superior traits retained in polyploidy perennial could be potentially used to improve soybeans after significant improvements in hybridization techniques.
The applications of nematode-resistant wild resources in other legume species lag behind but examples are emerging. Arachis stenosperma, a wild relative of peanut (Arachis hypogaea), was identified to be a new source of RKN resistance [36]. It was then used to cross with peanut to develop mapping populations for identifying RKN-resistance genes [36]. A recent study on an expansin superfamily from A. duranensis and Arachis ipaensis, the wild progenitors of cultivated peanut, found that expansin-like B gene (AraEXLB8) shows RKN resistance by overexpressing it in soybean hairy roots [37]. With further functional validation, this gene could be useful in developing RKN-resistant peanut cultivars by transferring resistant alleles to peanut.
Disease resistance
Wild legumes also represent a genetic reservoir by providing exotic genes/alleles conferring broad-spectrum resistance to many different diseases, such as pathogen and fungi. Many wild relatives are resistant to various diseases. For example, Lens orientalis is resistant to stemphylium blight (Stemphylium botryosum) [38], and Len ervoides and Len nigricans are resistant to ascochyta blight (Ascochyta lentis) [39–41]. Wild lentils were also found to be resistant to anthracnose (Colletotrichum truncatum), fusarium wilt (Fusarium oxysporum), powdery mildew (Erysiphe polygoni), and rust (Uromyces fabae) [42]. Further assessment of these resistance sources is needed to evaluate the breadth of the resistance spectrum and to discover the resistance loci for lentil crop improvement.
Sap-sucking pest resistance
Wild legumes are also rich exotic resources for sap-sucking pest resistance. The soybean aphid (Aphis glycines Matsumura) causes severe yield loss annually in legume crops. It colonizes on soybean and other legumes such as Medicago and Trifolium [43]. For effective aphid management, aphid resistant genotypes have been identified in wild leguminous species, such as annual G. soja and perennial Glycine species (Glycine falcate, Glycine clandestina) [43,44]. Novel loci have been identified in these wild soybean relatives by genetic mapping [3,45]. Likewise, the source of resistance to other sap-sucking pests was also identified in wild legume relatives such as Cajanus scarabaeoides (a pigeon pea wild relative) for Plume moth (Exelastic atomosa) [46], Cicer wild relatives (Cicer reticulatum) for Helicoverpa armigera [47]. Next generation sequencing of segregating populations as used in wild soybean G. soja [11••,45] may significantly accelerate the dissection of the underlying mechanisms in these under-exploited legumes.
Combination and broad-spectrum resistance and tolerance
In nature, plants usually experience a combination of biotic and abiotic stressors rather than an individual stressor. Therefore, enhancing the legume crops with tolerance/resistance to multiple stresses has become increasingly important. It is a primary goal of legume breeding in the light of future climate instability. Interestingly, wild leguminous relatives are currently identified either resistant to broad-spectrum biotic stresses or tolerant to multiple abiotic stresses. However, wild legumes that combine biotic stress resistance and abiotic stress tolerance are rarely studied. For example, wild pigeon pea genotypes, C. scarabaeoides, were reported to be resistant to both blue butterfly and plume moth [46], the wild lentil (L. ervoides) is resistant to both anthracnose and Stemphylium blight [48], and wild common bean is tolerant to both drought and subzero temperatures [49•]. Ideally, some wild relatives such as wild chickpeas, adaptive to local habitats and resistance to biotic stress, could be an ideal system for analyzing the combination of stress resistance [26••]. In addition, the transfer of multiple desired traits from a wild relative to its elite descendant is still challenging, unless they are controlled by a single or closely linked loci [46]. Nevertheless, interspecific introgression of multiple abiotic stresses into cultivated peanut cultivars has been successful [49•], making this strategy feasible and promising.
Yield-related traits
One of the main contributions of legume wild relatives in yield-related traits is that they are often used as one of the two parents when developing mapping populations for identifying candidate genes associated with seed size, weight, and number, which are essential yield-related traits [50,51,52•]. Interestingly, alleles from wild relatives benefiting yield improvement have also been reported [e. g. Ref. [53]]. For example, Li et al. [53] identified a QTL for seed yield in a backcross population between wild soybean G. soja and cultivated soybean G. max. The lines carrying G. soja alleles demonstrated a 6.3% yield increase than lines homozygous for the G. max allele. Considering that the yield traits are complex and mostly attributed to additive and epistatic effects of many loci [12], transcriptomics-based comparisons between wild and cultivated soybeans resulted in the identification of a WRKY gene correlated to seed size [54•]. Candidate genes, such as WRKY, phosphatase 2C-1 (PP2C-1), as well as the environmentally stable yield-enhancing QTLs, identified in the leguminous wild relatives [12,50,52•,55,56] merit further efforts for function validation and field testing. Their potential applications in legume yield improvements make pre-breeding practices worthwhile.
Nutrition and phytocompounds
Seed composition
A high proportion of proteins, fats, carbohydrates, dietary fibers, B-group vitamins, and minerals can be obtained from legumes [57]. A study on all-cause mortality found out that a daily intake of 20 g of legumes can reduce the rate of mortality [58]. According to the Dietary Guidelines for Americans, an intake of three cups of legumes weekly is recommended for people who consume about 2000kcal/day [59]. These nutrient-related traits were selected during domestication, for example, starch and fat in adzuki bean (Vigna angularis) [60], and protein and oil in soybeans [61]. Intensive studies on these traits have uncovered a number of novel loci within the same genomic regions [62,63•,64]. For example, in soybean, the environmentally stable QTLs on chromosome 20 for seed protein and oil have been repeatedly identified among many different interspecific populations [63•,65]; thus deserving further identification of useful haplotypes/alleles to facilitate seed improvement. Functional markers developed from these important loci, such as dCAPS [62], may significantly accelerate the improvement of seed composition.
In turn, human selection of agriculturally important traits resulted in unintended accumulation of secondary compounds in legume seeds, such as phenolics, flavonoids [66], and saponins [66,67]. High accumulation of these compounds in G. soja might confer resilience to environmental stresses. Meanwhile, they have also been demonstrated to be beneficial to human health, which holds great potential in preventing human disease and developing alternative medicine. Figure 2 demonstrates this ‘two birds, one stone’ scenario of phytocompounds.
Figure 2.
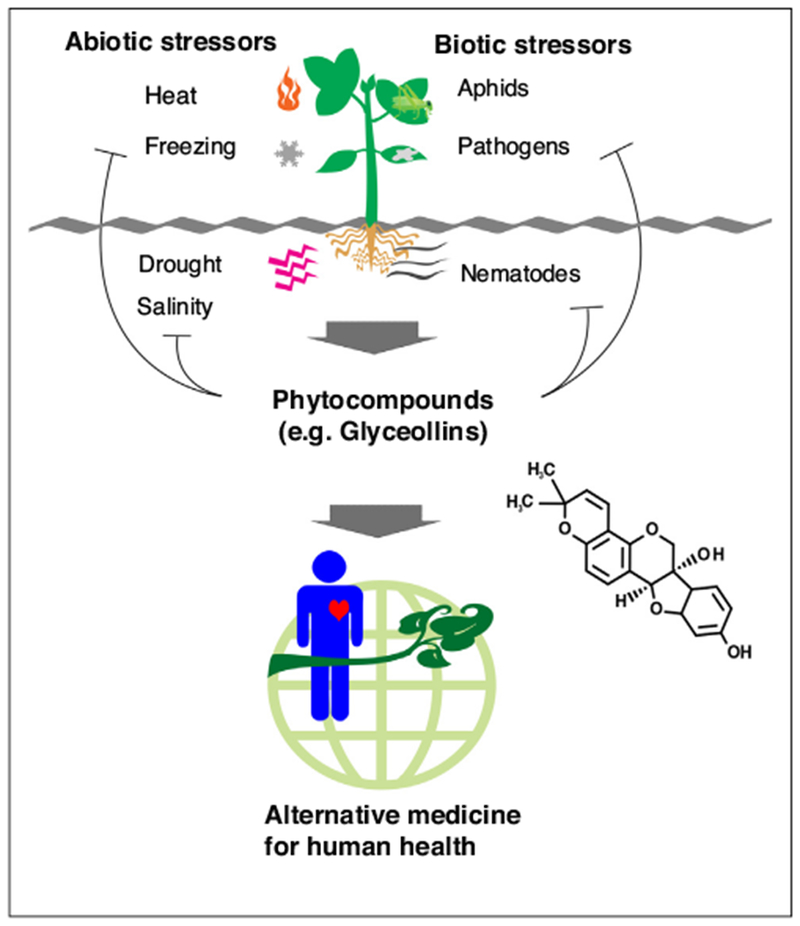
The ‘two birds, one stone’ scenario of phytocompounds: high accumulation of phytocompounds under biotic and abiotic stress conditions can help plants confer resistance to environmental stress, and they are also beneficial to human health.
Phytocompounds
Legumes are a crucial source of many phytochemicals that serve as human-health promoting ingredients in alternative medicines [68]. For example, isoflavonoid formononetin, common in wild legume species, has anti-hyperglycemic activity [69], and reduces insulin resistance and hyperglycemia [70], holding great potential for assisting in the treatment of diabetes. Phytoalexin glyceollins can be induced in soybean and wild soybean, G. soja, (Song lab, unpublished data). They showed anti-proliferative effects on breast cancer cells [71]. Von Wettberg et al. [26••] reported that chickpea wild relatives are rich in health-beneficial phytochemicals, such as polyphenolics, flavonoids, etc. Table 2 summarizes the recent studies on health-beneficial phytocompounds in wild legume species.
Table 2.
Recent studies exploiting the health-beneficial phytocompounds from leguminous wild relatives
| Legume wild relatives | Phytocompounds | Nutritional values/alternative medicine | References |
|---|---|---|---|
| Glycine soja (wild soybean) | Soyasaponins, Soybean agglutinin Bioactive peptides, Lunasin, Genistein Formononetin | Anti-inflammatory; Natural antioxidant agent; Vasodilation; Anticancer activity | [96,97•,98] |
| Medicago sativa and Medicago lupulina | Flavonoids (Formononetin, Biochanin A, Daidzein, Genistein), Polyols, Coumestrol | Hypocholesterolemic effects and can prevent associated diseases; Antimicrobial activity | [99,100] |
| Medicago trunculata | Proanthocyanidins (PAs), | PAs reduce risks of cardiovascular disease and Alzheimer’s disease; Anticancer activity | [101] |
| Butea monospermea (Flame-of-the-forest and bastard teak) | Genistein (flavonoid) | Antioxidant; Anti-inflammatory; Vasodilation; Antimalarial activity | [97•,102] |
| Trifolium pretense (red clover) and Trifolium medium (zigzag clover) | Formononetin, Daidzein, Genistein, Biochanin A | Nutritional, mineral and bioactive values, antimicrobial activity | [99] |
| Glycyrrhiza uralensis (Chinese liquorice) | Flavonoids (flavanones, chalcones, and isoflavones), Triterpene saponin | Anti-inflammatory, antiviral, antimicrobial, antioxidative, antidiabetic, antiasthma, antiallergic and anticancer activities as well as immunomodulatory, gastroprotective, hepatoprotective, neuroprotective and cardioprotective effects | [103] |
| Clitoria ternatea (Butterfly pea) | Phenolic metabolites, Hydroalcoholic extracts, Steroids, Saponins, Flavonoids, Lectins, Tocopherols, Phytosterols | Antifungal; Anti-inflammatory; Antibacterial; Anti-hyperlipidemia; Antiasthmatic; Antidiabetic, nootropic, anxiolytic, anticonvulsant, sedative, antipyretic, and analgesic functions; Antioxidant; Decrease laryngeal cancer cell (HEp-2) viability | [98,104,105] |
| Pueraria thunbergiana (Kudzu bean) | Puerarin (isoflavone), Saponin | Effective in the treatment of osteoporosis (OP) and osteoarthritis (OA); Highly beneficial in treating liver damage; Inhibits HIV-1 initial attachment and inhibits HIV-1 replication; Inhibits HIV-2 and simian immunodeficiency virus; Anti-inflammatory; Antioxidant activity | [106–108] |
| Lupinus albus (Lupine) | Polyphenols (Quercetin, Caffeic acid, Ferulic acid, p-Coumaric acid); Tannins | Antioxidant activity | [68,109] |
Emerging studies have shown that health-beneficial nutrients and phytocompounds in wild legumes could be used to develop alternative medicines for treating human disease. However, only a small fraction of the immensely diverse plant metabolites have been explored for the production of new medications or health supplements. Studies on dissecting the genetic basis of compound production/induction are even more scarce. The isolation of key enzymes for undefined steps in the modification of secondary metabolites, such as triterpenoid saponins in legumes, will shed light on the metabolic engineering of useful phytocompounds [72].
Uncover adaptive loci from an ecological perspective
Given that CWRs grow in diverse environments with broad ecological and geographic distributions, the environmental variations may drive population differentiation for local adaptation, as shown in G. soja [32,73•,74,75], wild Yigna [17], wild common beans [25,76], and wild chickpeas [26••]. During the past few years, landscape genomics has been an emerging integrative research field in identifying adaptation-related loci/SNPs by association studies using environmental variables as quantitative traits. Anderson et al. [73•] recently identified candidate SNPs in G. soja associated with environmental variables, such as temperature, soil, and precipitation. It is expected that more adaptation-associated loci will be identified in other wild legumes once the high-throughput genomic data and ecological variables are available. On the other hand, comparative transcriptome analyses between locally adaptive wild legume populations could lead to the identification of ecological adaptation-related transcripts or allelic variants. By linking the variants with its origin information, breeding scientists may prioritize the locally adapted relatives to advance the improvement of abiotic tolerance through marker-assisted selection.
Challenges and perspectives
Trait transfer
Traditional genetic crossing remains one of the primary strategies for transferring the target traits from a wild relative to an elite variety, despite the fact that the linkage drag may have negative effects on the elite genotype. The recent successful cross of polypoid G. tomentella [34,35••] and diploid soybean showed the feasibility of transferring genes/alleles from the gene pool of GP3 or GP4 to elites after efficient crossing methods are developed. Alternatively, the useful alleles identified in wild species could be ‘duplicated’ into cultivated varieties using the CRISPR/Cas9 system, which is the most powerful tool to date used to make genetic changes in a DNA fragment [77].
Trait dissection
The use of the genetic variation in wild relatives to benefit crop improvements relies largely on the accurate measurements of the trait variation. However, large-scale and accurate measurements of trait variation in legume wild relatives are still challenging because most phenotypically look like weeds and are difficult to handle. Along with the high-throughput phenotyping strategies [78], high-resolution SNP data are indispensable for the identification of the associated loci by genome-wide association studies (GWAS) and linkage mapping. Such analyses can be extended to use the expressed transcriptome, proteome, and metabolome to identify the regulatory/expressed QTLs, considering epistatic effects on complex traits variation. Given that either genomic or regulatory variation may cause trait variation, rather than focusing on a single wild genotype, a pan-genome approach is an effective strategy to capture the genomic variation associated with diverse traits within species [78,79••,80].
A long way to go
Although immense progress has been made, it is just the beginning of using these untapped genetic resources. The knowledge obtained from what we currently know on CWRs is likely the tip of the iceberg; thus, more systematic efforts are needed to increase our understanding and advance legume breeding. These efforts include efficient conservation of these resources in-situ or ex-situ, integration of multiple-dimensional data generated from different platforms, systematic data curation, data/germplasm sharing, advanced biotechnology development, high throughput genotyping and phenotyping, and more affordable technologies quantifying human-beneficial phytocompounds.
Acknowledgments
We apologize to authors of papers not cited due to space limitations. We thank Dr D. Zhang at College of Agronomy, Henan Agricultural University for generously providing us the images of G. max and G. soja seeds. We thank Dr J. Griffin, Ms. J. Kofsky, and Ms. S. Xiong for proofreading the manuscript. This work was supported by the National Institute of General Medical Sciences, Award Number: R15GM122029; the North Carolina Soybean Producers Association, Award Number: 18-0252, North Carolina Biotechnology Center, Award Number: 2019-BIG-6507, North Carolina State University Plant Pathways Elucidation Project Consortium, Award Number: 18-0919, and University of North Carolina at Charlotte.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ray DK, Mueller ND, West PC, Foley JA: Yield trends are insufficient to double global crop production by 2050. PLoS One 2013, 8:e66428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander P, Rounsevell MDA, Dislich C, Dodson JR, Engstrom K, Moran D: Drivers for global agricultural land use change: the nexus of diet, population, yield and bioenergy. Global Environ Change Hum Policy Dimens 2015, 35:138–147. [Google Scholar]
- 3.Zhang HY, Mittal N, Leamy LJ, Barazani O, Song BH: Back into the wild-apply untapped genetic diversity of wild relatives for crop improvement. Evol Appl 2017, 10:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mammadov J, Buyyarapu R, Guttikonda SK, Parliament K, Abdurakhmonov IY, Kumpatla SP: Wild relatives of maize, rice, cotton, and soybean: treasure troves for tolerance to biotic and abiotic stresses. Front Plant Sci 2018, 9:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maxted N, Kell SP: Establishment of a Global Network for the In Situ Conservation of Crop Wild Relatives: Status and Needs. Rome, Italy: Food and Agriculture Organization of the United Nations Commission on Genetic Resources for Food and Agriculture; 2009. [Google Scholar]
- 6.Bourion V, Heulin-Gotty K, Aubert V, Tisseyre P, Chabert-Martinello M, Pervent M, Delaitre C, Vile D, Siol M, Duc G et al. : Co-inoculation of a pea core-collection with diverse rhizobial strains shows competitiveness for nodulation and efficiency of nitrogen fixation are distinct traits in the interaction. Front Plant Sci 2018, 8:2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai YL, Lindhout P: Domestication and breeding of tomatoes: what have we gained and what can we gain in the future? Ann Bot 2007, 100:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorim LY, Vandenberg A: Evaluation of wild lentil species as genetic resources to improve drought tolerance in cultivated lentil. Front Plant Sci 2017, 8:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasanna BM: Diversity in global maize germplasm: characterization and utilization. J Biosci 2012, 37:843–855. [DOI] [PubMed] [Google Scholar]
- 10.Kofsky J, Zhang HY, Song BH: The untapped genetic reservoir: the past, current, and future applications of the wild soybean (Glycine soja). Front Plant Sci 2018, 9:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.••.Qi XP, Li MW, Xie M, Liu X, Ni M, Shao GH, Song C, Yim AKY, Tao Y, Wong FL et al. : Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing. Nat Commun 2014, 5:4340. [DOI] [PMC free article] [PubMed] [Google Scholar]; A novel G. soja-unique salt tolerant gene, GmCHX1, was uncovered by a combined analysis of whole genome de novo sequencing, genome resequencing, high-density-marker QTL mapping.
- 12.Wang WB, Li XL, Chen SX, Song SY, Gai JY, Zhao TJ: Using presence/absence variation markers to identify the QTL/allele system that confers the small seed trait in wild soybean (Glycine soja Sieb. & Zucc.). Euphytica 2016, 208:101–111. [Google Scholar]
- 13.Wang WB, Liu MF, Wang YF, Li XL, Cheng SX, Shu LP, Yu ZP, Kong JJ, Zhao TJ, Gai JY: Characterizing two inter-specific bin maps for the exploration of the QTLs/genes that confer three soybean evolutionary traits. Front Plant Sci 2016, 7:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chankaew S, Isemura T, Naito K, Ogiso-Tanaka E, Tomooka N, Somta P, Kaga A, Vaughan DA, Srinives P: QTL mapping for salt tolerance and domestication-related traits in Vigna marina subsp. oblonga, a halophytic species. Theor Appl Genet 2014, 127:691–702. [DOI] [PubMed] [Google Scholar]
- 15.Iseki K, Marubodee R, Ehara H, Tomooka N: A rapid quantification method for tissue Na+ and K+ concentrations in salt-tolerant and susceptible accessions in Vigna vexillata (L.) A. Rich. Plant Prod Sci 2017, 20:144–148. [Google Scholar]
- 16.Yoshida Y, Marubodee R, Ogiso-Tanaka E, Iseki K, Isemura T, Takahashi Y, Muto C, Naito K, Kaga A, Okuno K et al. : Salt tolerance in wild relatives of adzuki bean, Vigna angularis (Willd.) Ohwi et Ohashi. Genet Resources Crop Evol 2016, 63:627–637. [Google Scholar]
- 17.Iseki K, Takahashi Y, Muto C, Naito K, Tomooka N: Diversity and evolution of salt tolerance in the genus vigna. PLoS One 2016, 11:e0164711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia BW, Sun MZ, DuanMu HZ, Ding XD, Liu BD, Zhu YM, Sun XL: GsCHX19.3, a member of cation/H+ exchanger superfamily from wild soybean contributes to high salinity and carbonate alkaline tolerance. Sci Rep 2017, 7:9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Y, Liu AL, Duan XB, Wang ST, Sun XL, Duanmu H, Zhu D, Chen C, Cao L, Xiao JL et al. : GsERF6, an ethylene-responsive factor from Glycine soja, mediates the regulation of plant bicarbonate tolerance in Arabidopsis. Planta 2016, 244:681–698. [DOI] [PubMed] [Google Scholar]
- 20.Sun MZ, Jia BW, Cui N, Wen YD, Duanmu HZ, Yu QY, Xiao JL, Sun XL, Zhu YM: Functional characterization of a Glycine soja Ca(2+)ATPase in salt-alkaline stress responses. Plant Mol Biol 2016, 90:419–434. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Duan XB, Ding XD, Chen C, Zhu D, Yin KD, Cao L, Song XW, Zhu PH, Li Q et al. : A novel AP2/ERF family transcription factor from Glycine soja, GsERF71, is a DNA binding protein that positively regulates alkaline stress tolerance in Arabidopsis. Plant Mol Biol 2017, 94:509–530. [DOI] [PubMed] [Google Scholar]
- 22.Cao L, Yu Y, DuanMu HZ, Chen C, Duan XB, Zhu PH, Chen RR, Li Q, Zhu YM, Ding XD: A novel Glycine soja homeodomain-leucine zipper (HD-Zip) I gene, Gshdz4, positively regulates bicarbonate tolerance and responds to osmotic stress in Arabidopsis. BMC Plant Biol 2016, 16:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang DS, Zhang J, Li MX, Shi LX: Metabolomics analysis reveals the salt-tolerant mechanism in Glycine soja. J Plant Growth Regul 2017, 36:460–471. [Google Scholar]
- 24.Zhang J, Yang DS, Li MX, Shi LX: Metabolic profiles reveal changes in wild and cultivated soybean seedling leaves under salt stress. PLoS One 2016, 11:e0159622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes AJ, Monserrate FA, Ramirez-Villegas J, Madrinan S, Blair MW: Drought tolerance in wild plant populations: The case of common beans (Phaseolus vulgaris L.). PLoS One 2013, 8:e62898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.••.von Wettberg EJB, Chang PL, Basdemir F, Carrasquila-Garcia N, Korbu LB, Moenga SM, Bedada G, Greenlon A, Moriuchi KS, Singh V et al. : Ecology and genomics of an important crop wild relative as a prelude to agricultural innovation. Nat Commun 2018, 9:649. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals the ecological adaptation of wild chickpea populations using population genomics and highlights a range of local-adaptation-related agronomic traits for crop improvement.
- 27.Ye H, Roorkiwal M, Valliyodan B, Zhou LJ, Chen PY, Varshney RK, Nguyen HT: Genetic diversity of root system architecture in response to drought stress in grain legumes. J Exp Bot 2018, 69:3267–3277. [DOI] [PubMed] [Google Scholar]
- 28.Vinson CC, Mota APZ, Oliveira TN, Guimaraes LA, Leal-Bertioli SCM, Williams TCR, Nepomuceno AL, Saraiva MAP, Araujo ACG, Guimaraes PM et al. : Early responses to dehydration in contrasting wild Arachis species. PLoS One 2018, 13:e0198191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blair MW, Cortes AJ, This D: Identification of an ERECTA gene and its drought adaptation associations with wild and cultivated common bean. Plant Sci 2016, 242:250–259. [DOI] [PubMed] [Google Scholar]
- 30.Cortes AJ, Blair MW: Genotyping by sequencing and genome-environment associations in wild common bean predict widespread divergent adaptation to drought. Front Plant Sci 2018, 9:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Z, Liu S, Noe J, Arelli P, Meksem K, Li Z: SNP identification and marker assay development for high-throughput selection of soybean cyst nematode resistance. BMC Genomics 2015, 16:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang HY, Li CY, Davis EL, Wang JS, Griffin JD, Kofsky J, Song BH: Genome-wide association study of resistance to soybean cyst nematode (Heterodera glycines) HG Type 2.5.7 in wild soybean (Glycine soja). Front Plant Sci 2016, 7:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen L, Yuan C, Herman TK, Hartman GL: Accessions of perennial Glycine species with resistance to multiple types of soybean cyst nematode (Heterodera glycines). Plant Dis 2017, 101:1201–1206. [DOI] [PubMed] [Google Scholar]
- 34.Singh RJ, Nelson RL: Methodology for creating alloplasmic soybean lines by using Glycine tomentella as a maternal parent. Plant Breed 2014, 133:624–631. [Google Scholar]
- 35.••.Singh RJ, Nelson RL: Intersubgeneric hybridization between Glycine max and G. tomentella: production of F-1, amphidiploid, BC1, BC2, BC3, and fertile soybean plants. Theor Appl Genet 2015, 128:1117–1136. [DOI] [PubMed] [Google Scholar]; This study described methods of successful introgression from wild perennial Glycine species into cultivated soybean in producing fertile soybeans with improved resistance to soybean rust.
- 36.Leal-Bertioli SCM, Moretzsohn MC, Roberts PA, Ballen-Taborda C, Borba TCO, Valdisser PA, Vianello RP, Araujo ACG, Guimaraes PM, Bertioli DJ: Genetic mapping of resistance to Meloidogyne arenaria in Arachis stenosperma: a new source of nematode resistance for peanut. G3-Genes Genomes Genet 2016, 6:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guimaraes LA, Mota APZ, Araujo ACG, de Alencar Figueiredo LFD, Pereira BM, de Passos Saraiva MA, Silva RB, Danchin EGJ, Guimaraes PM, Brasileiro ACM: Genome-wide analysis of expansin superfamily in wild Arachis discloses a stress-responsive expansin-like B gene. Plant Mol Biol 2017, 94:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kant P, Materne M, Rodda MS, Slater AT: Screening lentil germplasm for stemphylium blight resistance. Australas Plant Pathol 2017, 46:129–136. [Google Scholar]
- 39.Dadu RHR, Ford R, Sambasivam P, Gupta D: A novel Lens orientalis resistance source to the recently evolved highly aggressive Australian Ascochyta lentis Isolates. Front Plant Sci 2017, 8:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dadu RHR, Ford R, Sambasivam P, Gupta D: Evidence of early defence to Ascochyta lentis within the recently identified Lens orientalis resistance source ILWL180. Plant Pathol 2018, 67:1492–1501. [Google Scholar]
- 41.Tullu A, Banniza S, Tar’an B, Warkentin T, Vandenberg A: Sources of resistance to ascochyta blight in wild species of lentil (Lens culinaris Medik.). Genet Resources Crop Evol 2010, 57:1053–1063. [Google Scholar]
- 42.Singh M, Sharma SK, Singh B, Malhotra N, Chandora R, Sarker A, Singh K, Gupta D: Widening the genetic base of cultivated gene pool following introgression from wild Lens taxa. Plant Breed 2018, 137:470–485. [Google Scholar]
- 43.Hill CB, Li Y, Hartman GL : Resistance of Glycine species and various cultivated legumes to the soybean aphid (Homoptera: Aphididae). J Econ Entomol 2004, 97:1071–1077. [DOI] [PubMed] [Google Scholar]
- 44.Hesler LS, Tilmon KJ: Resistance to Aphis Glycines among wild soybean accessions in laboratory experiments. Crop Prot 2018, 112:74–82. [Google Scholar]
- 45.Zhang SC, Zhang ZN, Wen ZX, Gu CH, An YQC, Bales C, DiFonzo C, Song QJ, Wang DC: Fine mapping of the soybean aphid-resistance genes Rag6 and Rag3c from Glycine soja 85-32. Theor Appl Genet 2017, 130:2601–2615. [DOI] [PubMed] [Google Scholar]
- 46.Mishra RR, Sahu AR, Rath SC, Panigrahi J: Genetic linkage mapping of loci conferring resistance to Blue butterfly (Lampides boeticus L.) and Plume moth (Exelastis atomosa Wals.) on chromosome 2 (CcLG02) in Pigeonpea. Bot Lett 2016, 163:217–230. [Google Scholar]
- 47.Sharma HC, Pampapathy G, Lanka SK, Ridsdill-Smith TJ: Exploitation of wild Cicer reticulatum germplasm for resistance to Helicoverpa armigera. J Econ Entomol 2005, 98:2246–2253. [DOI] [PubMed] [Google Scholar]
- 48.Bhadauria V, Ramsay L, Bett KE, Banniza S: QTL mapping reveals genetic determinants of fungal disease resistance in the wild lentil species Lens ervoides. Sci Rep 2017, 7:3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.•.Souter JR, Gurusamy V, Porch TG, Bett KE: Successful introgression of abiotic stress tolerance from wild Tepary bean to common bean. Crop Sci 2017, 57:1160–1171. [Google Scholar]; This study provides a successful example of introgressing genes from drought and low temperature tolerance relative, tepary bean, into common bean for increased abiotic stress tolerance.
- 50.Kulkarni KP, Asekova S, Lee DH, Bilyeu K, Song JT, Lee JD: Mapping QTLs for 100-seed weight in an interspecific soybean cross of Williams 82 (Glycine max) and PI 366121 (Glycine soja). Crop Pasture Sci 2017, 68:148–155. [Google Scholar]
- 51.Kulkarni KP, Kim M, Shannon JG, Lee JD: Identification of quantitative trait loci controlling soybean seed weight in recombinant inbred lines derived from PI 483463 (Glycine soja) x ‘Hutcheson’ (G. max). Plant Breed 2016, 135:614–620. [Google Scholar]
- 52.•.Lu X, Xiong Q, Cheng T, Li QT, Liu XL, Bi YD, Li W, Zhang WK, Ma B, Lai YC et al. : A PP2C-1 allele underlying a quantitative trait locus enhances soybean 100-seed weight. Mol Plant 2017, 10:670–684. [DOI] [PubMed] [Google Scholar]; This study identified a PP2C allele underlying QTL for soybean 100-seed weight using whole-genome sequencing of a RIL population from an interspecies cross between a wild soybean and a cultivated soybean.
- 53.Li DD, Pfeiffer TW, Cornelius PL: Soybean QTL for yield and yield components associated with Glycine soja alleles. Crop Sci 2008, 48:571–581. [Google Scholar]
- 54.•.Gu Y, Li W, Jiang H, Wang Y, Gao H, Liu M, Chen Q, Lai Y, He C: Differential expression of a WRKY gene between wild and cultivated soybeans correlates to seed size. J Exp Bot 2017, 68:2717–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study integrated QTL mapping, RNA sequencing, haplotype analysis and identified GsWRKY15a controlling seed size in soybean.
- 55.Liu D, Yan Y, Fujita Y, Xu D: Identification and validation of QTLs for 100-seed weight using chromosome segment substitution lines in soybean. Breed Sci 2018, 68:17127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xin DW, Qi ZM, Jiang HW, Hu ZB, Zhu RS, Hu JH, Han HY, Hu GH, Liu CY, Chen QS: QTL location and epistatic effect analysis of 100-seed weight using wild soybean (Glycine soja Sieb. & Zucc.) chromosome segment substitution lines. PLoS One 2016, 11:e0149380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fabbri AD, Crosby GA: A review of the impact of preparation and cooking on the nutritional quality of vegetables and legumes. Int J Gastron Food Sci 2016, 3:2–11. [Google Scholar]
- 58.Foyer CH, Lam H-M, Nguyen HT, Siddique KH, Varshney RK, Colmer TD, Cowling W, Bramley H, Mori TA, Hodgson JM: Neglecting legumes has compromised human health and sustainable food production. Nat Plants 2016, 2:16112. [DOI] [PubMed] [Google Scholar]
- 59.Agriculture: USDoHaHSaUSDo: 2015–2020 Dietary Guidelines for Americans. 2015. [Google Scholar]
- 60.Yang K, Tian ZX, Chen CH, Luo LH, Zhao B, Wang Z, Yu LL, Li YS, Sun YD, Li WY et al. : Genome sequencing of adzuki bean (Vigna angularis) provides insight into high starch and low fat accumulation and domestication. Proc Natl Acad Sci U S A 2015, 112:13213–13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou ZK, Jiang Y, Wang Z, Gou ZH, Lyu J, Li WY, Yu YJ, Shu LP, Zhao YJ, Ma YM et al. : Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat Biotechnol 2015, 33:408–414. [DOI] [PubMed] [Google Scholar]
- 62.Obala J, Saxena RK, Singh VK, Kumar CVS, Saxena KB, Tongoona P, Sibiya J, Varshney RK: Development of sequence-based markers for seed protein content in pigeonpea. Mol Genet Genomics 2018:1–12. [DOI] [PubMed] [Google Scholar]
- 63.•.Patil G, Vuong TD, Kale S, Valliyodan B, Deshmukh R, Zhu CS, Wu XL, Bai YH, Yungbluth D, Lu F et al. : Dissecting genomic hotspots underlying seed protein, oil, and sucrose content in an interspecific mapping population of soybean using high-density linkage mapping. Plant Biotechnol J 2018, 16:1939–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]; Genomic hotspots and unique allelic variation from wild soybean that control seed composition properties were identified using high-density QTL mapping.
- 64.Leamy LJ, Zhang HY, Li CB, Chen CY, Song BH: A genome-wide association study of seed composition traits in wild soybean (Glycine soja). BMC Genomics 2017, 18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu X, Li QT, Xiong Q, Li W, Bi YD, Lai YC, Liu XL, Man WQ, Zhang WK, Ma B et al. : The transcriptomic signature of developing soybean seeds reveals the genetic basis of seed trait adaptation during domestication. Plant J 2016, 86:530–544. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi Y, Li XH, Tsukamoto C, Wang KJ: Identification of a novel variant lacking group A soyasaponin in a Chinese wild soybean (Glycine soja Sieb. & Zucc.): implications for breeding significance. Plant Breed 2016, 135:607–613. [Google Scholar]
- 67.Takahashi Y, Li XH, Tsukamoto C, Wang KJ: Categories and components of soyasaponin in the Chinese wild soybean (Glycine soja) genetic resource collection. Genet Resources Crop Evol 2017, 64:2161–2171. [Google Scholar]
- 68.Grela ER, Samolinska W, Kiczorowska B, Klebaniuk R, Kiczorowski P: Content of minerals and fatty acids and their correlation with phytochemical compounds and antioxidant activity of leguminous seeds. Biol Trace Element Res 2017, 180:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu GZ, Tian W, Huan M, Chen JL, Fu HT: Formononetin exhibits anti-hyperglycemic activity in alloxan-induced type 1 diabetic mice. Exp Biol Med 2017, 242:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oza MJ, Kulkarni YA: Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front Pharmacol 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lecomte S, Chalmel F, Ferriere F, Percevault F, Plu N, Saligaut C, Surel C, Lelong M, Efstathiou T, Pakdel F: Glyceollins trigger anti-proliferative effects through estradiol-dependent and independent pathways in breast cancer cells. Cell Commun Signal 2017, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arendt M, Cairns KM, Ballard JWO, Savolainen P, Axelsson E: Diet adaptation in dog reflects spread of prehistoric agriculture. Heredity 2016, 117:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.•.Anderson JE, Kono TJY, Stupar RM, Kantar MB, Morrell PL: Environmental association analyses identify candidates for abiotic stress tolerance in Glycine soja, the wild progenitor of cultivated soybeans. G3-Genes Genomes Genet 2016, 6:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study applied population genomics to USDA wild soybean population and identified candidate loci associated with local adaptation environments such as temperature, soil, and monthly precipitation.
- 74.Leamy LJ, Lee CR, Song QJ, Mujacic I, Luo Y, Chen CY, Li CB, Kjemtrup S, Song BH: Environmental versus geographical effects on genomic variation in wild soybean (Glycine soja) across its native range in northeast Asia. Ecol Evol 2016, 6:6332–6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang HY, Song QJ, Griffin JD, Song BH: Genetic architecture of wild soybean (Glycine soja) response to soybean cyst nematode (Heterodera Glycines). Mol Genet Genomics 2017, 292:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cerda-Hurtado IM, Mayek-Perez N, Hernandez-Delgado S, Muruaga-Martinez JS, Reyes-Lara MA, Reyes-Valdes MH, Gonzalez-Prieto JM: Climatic adaptation and ecological descriptors of wild beans from Mexico. Ecol Evol 2018, 8:6492–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu DG, Hu RB, Palla KJ, Tuskan GA, Yang XH: Advances and perspectives on the use of CRISPR/Cas9 systems in plant genomics research. Curr Opin Plant Biol 2016, 30:70–77. [DOI] [PubMed] [Google Scholar]
- 78.Shakoor N, Lee S, Mockler TC: High throughput phenotyping to accelerate crop breeding and monitoring of diseases in the field. Curr Opin Plant Biol 2017, 38:184–192. [DOI] [PubMed] [Google Scholar]
- 79.••.Li YH, Zhou GY, Ma JX, Jiang WK, Jin LG, Zhang ZH, Guo Y, Zhang JB, Sui Y, Zheng LT et al. : De novo assembly of soybean wild relatives for pan-genome analysis of diversity and agronomic traits. Nat Biotechnol 2014, 32:1045–1052. [DOI] [PubMed] [Google Scholar]; This study provided a pan-genome of seven reprehensive G. soja genotypes using de novo genome sequencing, identified genome-wide variation between G. soja and G. max, and proposed their divergence time.
- 80.Zhou P, Silverstein KAT, Ramaraj T, Guhlin J, Denny R, Liu JQ, Farmer AD, Steele KP, Stupar RM, Miller JR et al. :Exploring structural variation and gene family architecture with de novo assemblies of 15 Medicago genomes. BMC Genomics 2017, 18:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saeed A, Darvishzadeh R: Association analysis of biotic and abiotic stresses resistance in chickpea (Cicer spp.) using AFLP markers. Biotechnol Biotechnol Equip 2017, 31:698–708. [Google Scholar]
- 82.Singh RK, Singh S, Anandhan S, Shannon LM, Quiroz-Figueroa FR, Ruiz-May E: First insights into the biochemical and molecular response to cold stress in Cicer microphyllum, a crop wild relative of chickpea (Cicer arietinum). Russian J Plant Physiol 2017, 64:758–765. [Google Scholar]
- 83.Zhang HY, Song BH: RNA-seq data comparisons of wild soybean genotypes in response to soybean cyst nematode (Heterodera Glycines). Genomics Data 2017, 14:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.••.Sharma S, Pandey MK, Sudini HK, Upadhyaya HD, Varshney RK: Harnessing genetic diversity of wild Arachis species for genetic enhancement of cultivated peanut. Crop Sci 2017, 57:1121–1131. [Google Scholar]; This study proved evidence of successful introgression of resistance alleles from wild peanut species into cultivated peanut for peanut improvement.
- 85.Song B, Oehrle NW, Liu SS, Krishnan HB: Characterization of seed storage proteins of several perennial Glycine species. J Agric Food Chem 2016, 64:8499–8508. [DOI] [PubMed] [Google Scholar]
- 86.Wang J, Chu SS, Zhang HR, Zhu Y, Cheng H, Yu DY: Development and application of a novel genome-wide SNP array reveals domestication history in soybean. Sci Rep 2016, 6:20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mousavi-Derazmahalleh M, Nevado B, Bayer PE, Filatov DA, Hane JK, Edwards D, Erskine W, Nelson MN: The western Mediterranean region provided the founder population of domesticated narrow-leafed lupin. Theor Appl Genet 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.•.Varshney RK, Saxena RK, Upadhyaya HD, Khan AW, Yu Y, Kim C, Rathore A, Kim D, Kim J, An S et al. : Whole-genome resequencing of 292 pigeonpea accessions identifies genomic regions associated with domestication and agronomic traits. Nat Genet 2017, 49:1082–1088. [DOI] [PubMed] [Google Scholar]; A genome-wide scan of pigeon pea elite, landrace and wild species for selective sweeps revealed candidate genes associated with agronomically important traits.
- 89.•.Han YP, Zhao X, Liu DY, Li YH, Lightfoot DA, Yang ZJ, Zhao L, Zhou G, Wang ZK, Huang L et al. : Domestication footprints anchor genomic regions of agronomic importance in soybeans. New Phytol 2016, 209:871–884. [DOI] [PubMed] [Google Scholar]; This study proposed the geographic origin of soybean and analyzed the evolutionary relationship between cultivated, wild, and semi-domesticated soybean.
- 90.Valliyodan B, Dan Q, Patil G, Zeng P, Huang J, Dai L, Chen C, Li Y, Joshi T, Song L et al. : Landscape of genomic diversity and trait discovery in soybean. Sci Rep 2016, 6:23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li MW, Munoz NB, Wong CF, Wong FL, Wong KS, Wong JW, Qi X, Li KP, Ng MS, Lam HM: QTLs regulating the contents of antioxidants, phenolics, and flavonoids in soybean seeds share a common genomic region. Front Plant Sci 2016, 7:854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nawaz MA, Golokhvast KS, Rehman HM, Tsukamoto C, Kim HS, Yang SH, Chung G: Soyisoflavone diversity in wild soybeans (Glycine soja Sieb. & Zucc.) from the main centres of diversity. Biochem Syst Ecol 2018, 77:16–21. [Google Scholar]
- 93.Gao CW, Gao LZ: The complete chloroplast genome sequence of wild soybean, Glycine soja. Conserv Genet Resources 2017, 9:329–331. [Google Scholar]
- 94.Gao CW, Gao LZ: The complete chloroplast genome sequence of semi-wild soybean, Glycine gracilis (Fabales: Fabaceae). Conserv Genet Resources 2017, 9:343–345. [Google Scholar]
- 95.•.Liu Q, Chang SY, Hartman GL, Domier LL: Assembly and annotation of a draft genome sequence for Glycine latifolia, a perennial wild relative of soybean. Plant J 2018, 95:71–85. [DOI] [PubMed] [Google Scholar]; This study provided 939-Mb draft genome assembly and annotation of Glycine latifolia and identified G. latifolia-specific 92-bp putative centromeric repeats that was absent in G. max.
- 96.Jing C, Yuan Y, Tang Q, Zou P, Li Y, Zhang C: Extraction optimization, preliminary characterization and antioxidant activities of polysaccharides from Glycine soja. Int J Biol Macromol 2017, 103:1207–1216. [DOI] [PubMed] [Google Scholar]
- 97.•.Sureda A, Silva AS, Sanchez-Machado DI, Lopez-Cervantes J, Daglia M, Nabavi SF, Nabavi SM: Hypotensive effects of genistein: from chemistry to medicine. Chem-Biol Interact 2017, 268:37–46. [DOI] [PubMed] [Google Scholar]; This study described the effect of genistein acts as a useful anti-hypertensive agent in different experimental models.
- 98.Zhu F, Du B, Xu B: Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Crit Rev Food Sci Nutr 2018, 58:1260–1270. [DOI] [PubMed] [Google Scholar]
- 99.Butkutė B, Padarauskas A, Cesevičcienė J, Pavilonis A, Taujenis L, Lemežzienė N: Perennial legumes as a source of ingredients for healthy food: proximate, mineral and phytoestrogen composition and antibacterial activity. J Food Sci Technol 2017, 54:2661–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sedighi M, Bahmani M, Asgary S, Beyranvand F, Rafieian-Kopaei M: A review of plant-based compounds and medicinal plants effective on atherosclerosis. J Res Med Sci 2017, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu C, Wang X, Shulaev V, Dixon RA: A role for leucoanthocyanidin reductase in the extension of proanthocyanidins. Nat Plants 2016, 2:16182. [DOI] [PubMed] [Google Scholar]
- 102.Rudrapal M, Chetia D: Plant flavonoids as potential source of future antimalarial leads. Syst Rev Pharm 2017, 8:13. [Google Scholar]
- 103.Mochida K, Sakurai T, Seki H, Yoshida T, Takahagi K, Sawai S, Uchiyama H, Muranaka T, Saito K: Draft genome assembly and annotation of Glycyrrhiza uralensis, a medicinal legume. Plant J 2017, 89:181–194. [DOI] [PubMed] [Google Scholar]
- 104.Morris JB, Wang ML: Updated review of potential medicinal genetic resources in the USDA, ARS, PGRCU industrial and legume crop germplasm collections. Ind Crops Prod 2018, 123:470–479. [Google Scholar]
- 105.Shen Y, Du L, Zeng H, Zhang X, Prinyawiwatkul W, Alonso-Marenco JR, Xu Z: Butterfly pea (Clitoria ternatea) seed and petal extracts decreased HEp-2 carcinoma cell viability. Int J Food Sci Technol 2016, 51:1860–1868. [Google Scholar]
- 106.Chang BY, Lee DS, Lee JK, Kim YC, Cho HK, Kim SY: Protective activity of kudzu (Pueraria thunbergiana) vine on chemically-induced hepatotoxicity: in vitro and in vivo studies. BMC Complement Altern Med 2016, 16:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luo Y, Zheng S, Ding Y, Dai Y, Zhou Y, Xiang R, Bay-Jensen AC, Karsdal MA, Qvist P, Zheng Q: Preventive effects of kudzu root on bone loss and cartilage degradation in ovariectomized rat. Am J Transl Res 2017, 9:3517–3527. [PMC free article] [PubMed] [Google Scholar]
- 108.Mediouni S, Jablonski JA, Tsuda S, Richard A, Kessing C, Andrade MV, Biswas A, Even Y, Tellinghuisen T, Choe H et al. : Potent suppression of HIV-1 cell attachment by Kudzu root extract. Retrovirology 2018, 15:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lima AI, Mota J, Monteiro SA, Ferreira RM: Legume seeds and colorectal cancer revisited: protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem 2016, 197:30–38. [DOI] [PubMed] [Google Scholar]


