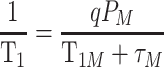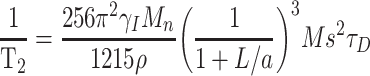Abstract
Background
Beyond magnetic resonance imaging (MRI), which has been widely used clinically, molecular MRI (mMRI) can further provide qualitative and quantitative information at the cellular and molecular levels. However, the diagnostic accuracy may not be satisfactory via single-contrast mMRI due to some interferences in vivo. T1/T2 dual-contrast MRI using the same contrast agent (CA) could significantly improve the detection accuracy. Therefore, in this study, we fabricated poly(ethylene glycol) (PEG)-coated, manganese-doped iron oxide nanocomposites (Mn-IONPs@PEG) as T1/T2 dual-contrast CA, and evaluated its feasibility of T1/T2 dual-contrast MRI in vitro and in vivo.
Methods
Mn-IONPs were prepared by the thermal decomposition of iron-eruciate and manganese-oleate complexes and were coated with 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxy[polyethylene glycol]-2000) (DSPE-PEG 2000). The physicochemical properties and cytotoxicity of the Mn-IONPs were fully characterized, followed by MRI in vitro and in vivo.
Results
Ultrasmall 3 nm-sized nanoparticles were successfully prepared and were identified using transmission electron microscopy (TEM), high-resolution TEM, and X-ray diffraction. After coating with DSPE-PEG, the Mn-IONPs@PEG displayed excellent hydrophilicity and good biocompatibility. Due to the manganese-doping and PEG coating, the Mn-IONPs@PEG showed good relaxivity in vitro. Especially, the Mn-IONPs@PEG coated with DSPE-PEG following a mass ratio to Mn-IONPs of 1:20 showed harmonious longitudinal relaxivity (r1 = 7.1 mM−1s−1) and transversal relaxivity (r2 = 120.9 mM−1s−1), making it a better candidate for T1/T2 dual-contrast mMRI. After administrated via a caudal vein, the Mn-IONPs@PEG can induce significant enhancement in both T1-weighted and T2-weighted MR images and the time at 10 mins after injection was regarded as a suitable time for imaging because both the T1 and T2 enhancement were optimum at that time.
Conclusion
The obtained Mn-IONPs@PEG exhibited good r1 and r2 and was a reasonable candidate for T1/T2 dual-contrast mMRI.
Keywords: magnetic resonance imaging, manganese-doped iron oxide nanoparticles, T1/T2 dual-contrast, DSPE-PEG coating, high relaxivity
Introduction
In recent decades, magnetic resonance imaging (MRI) has played an irreplaceable role in medical diagnosis because of its efficient soft tissue contrast, high spatial resolution, lack of ionizing radiation, and unrestricted signal penetration depth.1,2 Particularly, molecular magnetic resonance imaging (mMRI) has extended beyond traditional MRI technology. mMRI can obtain qualitative and quantitative information about biological processes in vivo at the cellular and molecular levels, greatly expanding the research and application fields of MRI.3–5 In recent years, mMRI has been intensively studied in gene imaging,6 cell tracing,7 drug screening,8 and evaluating early diagnoses and therapeutic effects of disease.9,10 Contrast agent (CA) serving as the signal source of mMRI is the most important ingredient and has reasonably become a hot spot in the field of mMRI.11
Although many types of CA based on magnetic nanoparticles (NPs) with high longitudinal relaxivity (r1)12 or transverse relaxivity (r2)13 have been developed, single-contrast mMRI still possesses insufficient sensitivity and specificity due to its innate properties. Transverse relaxation time (T2) single-contrast CA usually results in MR signal loss and susceptibility artifacts.14 Additionally, the darker areas in MR T2-weighted imaging (T2WI) induced by the T2 single-contrast CA may be confused with other physiological or pathological low-signal regions, such as bleeding, calcification, and metal deposition, which will significantly reduce the sensitivity and specificity of mMRI.15 Similarly, some normal tissues (such as fatty tissue) with hyperintensity in MR longitudinal relaxation time (T1)-weighted imaging (T1WI) may be misunderstood as bright lesions enhanced by T1 CA. T1/T2 dual-contrast MRI using the same CA provides complementary information about a lesion to self-confirm and provide fault-free MR images, proving that it can significantly improve the detection accuracy.16,17
Thus far, multifarious CAs have been reported for T1/T2 dual-contrast MRI. A common strategy for the synthesis of T1/T2 dual-contrast CA is to integrate T1 and T2 signal moieties as hybrid nanoparticles.17–23 However, due to the magnetic coupling effect, the enhancement generated by the T1 and T2 moieties will interact with each other, leading to the compromise of both r1 and r2. Embedding the T1 species into the T2 species can parallel the electronic spins of paramagnetic T1 CA with the direction of the magnetic field induced by the T2 CA, and reasonably enhance both the T1 and T2 relaxivity of the T1/T2 dual-contrast CA.16
Because of good biocompatibility, high relaxivity, and easy and versatile functionalization, superparamagnetic iron oxide nanoparticles (IONPs) have been extensively studied and have emerged as promising T2 CA.24 Benefiting from the size-dependent contrast, the ultrasmall IONPs also have demonstrated satisfactory r1, making them alternative T1/T2 dual-contrast CA.25,26 Nevertheless, the increased T1 relaxivity of the ultrasmall IONPs is the result of the consumption of the size, resulting in a relatively small r2. Thus, ultrasmall IONPs may be not optimal as T1/T2 dual-contrast CA.
Divalent manganese ions (Mn2+) possess five unpaired electrons and are of higher paramagnetism, and Mn2+ doping has been proven to be an effective route to improve the r1 of ultrasmall IONPs.12,27,28 Additionally, doped Mn2+ with a higher magnetic moment (μB=5.92) can occupy both the tetrahedral (Td) and octahedral holes (Oh) in the crystal lattices, resulting in a special mixed spinel structure, a larger saturation magnetization (Ms), and a high r2 of manganese-doped IONPs (Mn-IONPs).2,29 Moreover, based on the embedding rationale, the doped Mn2+ and ultrasmall IONPs show synergetic enhancement, which will further improve both r1 and r2 of Mn-IONPs. Therefore, Mn-IONPs could be better candidates as dual-contrast CA.
Usually, the hydrophobic inorganic nanoparticles need to be modified with either natural macromolecules or synthetic polymers for hydrophilia and further functionalization for biomedical applications. 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxy [polyethylene glycol]) (DSPE-PEG), with various advantages such as a low critical micellar concentration (CMC), excellent biocompatibility, and long blood circulation time has been diffusely employed for surface modification. More importantly, it was reported that DSPE-PEG can significantly improve r2 of the IONPs via the immobilization of nearby water molecules by forming hydrogen bonds.30,31
Herein, Mn-IONPs were prepared through the dynamic simultaneous thermal decomposition (DSTD) method. Strikingly, after coating with DSPE-PEG, the obtained hydrophilic manganese-doped iron oxide nanocomposites (Mn-IONPs@PEG) showed both good r1 and r2 in vitro and in vivo, demonstrating that these nanocomposites comprising ultrasmall Mn-IONPs and DSPE-PEG could be ideal candidates for use as T1/T2 dual-contrast CA.
Materials And Methods
Materials
Oleyl alcohol and dibenzyl ether were purchased from Aldrich Chemical Co. (Saint Louis, MO, USA). Absolute ethyl alcohol was obtained from Chongqing Chuandong Chemical Co. Ltd. (Chongqing, China). Hexane and tetrahydrofuran (THF) were purchased from Adamas Reagent Co. Ltd. (Shanghai, China). DSPE-PEG2000 was obtained from Shanghai Ponsure Biotechnology Co. Ltd. (Shanghai, China). Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Life Technologies Co. (Saint Louis, MO, USA). Fetal bovine serum (FBS) was obtained from Zhejiang Tianhang Biotechnology Co. Ltd. (Zhejiang, China). Penicillin/streptomycin solution and L-glutamine were purchased from Beyotime Biotechnology Co. Ltd. (Shanghai, China). Cell Count Kit-8 (CCK-8) was purchased from Boster Biological Technology Co. Ltd. (Wuhan, China). All of these reagents were used as received.
Synthesis Of Mn-IONPs
The Mn-IONPs were prepared following a previously reported procedure with modifications.12 The iron-eruciate and manganese-oleate were first synthesized and described in the supporting information (SI) in detail. Next, 2 mmol of iron-eruciate, 1 mmol of manganese-oleate and 6 mmol of oleyl alcohol were dissolved in 10 mL of dibenzyl ether at room temperature. The mixture was then heated to 100 °C and maintained for 30 min under a constant argon flow and magnetic stirring. Subsequently, the mixture was heated to 265 °C at a rate of 5 °C/min and was maintained for 1 h. After cooling to room temperature, the synthesized Mn-IONPs were washed with ethanol and centrifuged and were finally dispersed in 20 mL of hexane for storage.
Surface Modification
By coating with the DSPE-PEG-2000, the Mn-IONPs were characterized with hydrophilia. Following the previous method,32 50 mg of Mn-IONPs was first dispersed in 50 mL of THF to form 1 mg/mL of Mn-IONPs solution. Next, DSPE-PEG-2000 was mixed with 1 mL of Mn-IONPs solution with different mass ratios (NPs/PEG = 1:1, 1:5, 1:20, or 1:40) and then was added dropwise into 5 mL of deionized water under ultrasonication. After shaking overnight, the supernatants, including Mn-IONPs@PEG (1:1, 1:5, 1:20, or 1:40), were collected via filtration through a 0.22-μm syringe filter and was stored at 4 °C. Additionally, the hydrophilic Mn-IONPs-TMAH were obtained through ligand exchange with tetramethylammonium hydroxide aqueous solution (TMAH) and were described in the SI in detail.
Characterization
Transmission electron microscopy (TEM; Hitachi HT-7700, Japan) was used to characterize the morphology of the nanoparticles. High-resolution transmission electron microscopy (HRTEM; FEI TECNAI G20, USA) was used to observe the lattice spacing of the nanoparticles. Powder X-ray diffraction (XRD; PANalytical X’Pert Powder, Netherlands) was performed to detect the structure of the nanoparticles. Inductive-coupled plasma optical emission spectrometry (ICP-OES; Thermo Scientific Icap6300 Duo, USA) was carried out to determine the elemental composition of the nanoparticles. The hysteresis loops of the nanoparticles were recorded using a superconducting quantum interference device (SQUID; VSM-Versalab, USA) magnetometer.
Cytotoxicity
The human liver carcinoma cell line HepG2, purchased from the Chinese Academy of Sciences Cell Bank, was used for cytotoxicity analysis in this study. The cells were routinely cultured in DMEM containing 10% FBS, 2 mM L-glutamine, and 100 U/mL penicillin/streptomycin solution in a humidified incubator with 5% CO2 at 37 °C. CCK-8 was used for the analysis of the cytotoxicity of Mn-IONPs@PEG (1:20). Briefly, the cells were seeded in 96-well plates at a density of 1×104 cells/well and were cultured overnight. Next, fresh mediums containing Mn-IONPs@PEG with serial metal (Fe + Mn) concentrations (0, 1, 5, 10, 20, or 50 µg/mL) were added to replace the previous medium, followed by incubation for an additional 24 h. Each concentration of the samples was repeated five times. Next, the medium was replaced with 100 µL of fresh medium including 10 µL of WST-8 solution. Following 1.5 h of coculture, a spectral scanning multimode reader (Thermo Scientific Varioskan Flash, USA) was used to determine the optical density at the wavelength of 450 nm.
MR Imaging In Vitro
In vitro MR imaging was carried out using a clinical 3.0 T MRI scanner (GE, Signa HDx 3.0T, USA) with a head coil. The Mn-IONPs@PEG and Mn-IONPs-TMAH (shown in SI) solutions with [Fe+Mn] concentrations from 0 to 0.5 mM were imaged using the following parameters: fast spin echo sequence (FSE) T1WI: repetition time (TR) = 240 ms, echo time (TE) = 7.5 ms, field of view (FOV) = 160×160 mm2, matrix = 320×192, slice thickness/spacing =2.0 mm/0.1 mm, number of excitations (NEX) = 4; T1 mapping: TE = 9 ms, TR = 150, 300, 600, 900, 1200 ms, FOV = 160×160 mm2, matrix = 256×256, slice thickness/spacing = 2.0 mm/0.1 mm, NEX = 1; FSE T2WI: TR = 2000 ms, TE = 46.5 ms, FOV = 160×128 mm2, matrix = 192×160, slice thickness/spacing = 2.0 mm/0.1 mm, NEX = 4; T2 mapping: TR = 1500 ms, TE = 7.8, 15.6, 23.5, 31.3, 39.1, 46.9, 54.8, 62.6 ms, FOV = 160×128 mm2, matrix = 160×128, slice thickness/spacing = 2.0 mm/0.4 mm, and NEX = 1.
MR Imaging In Vivo
Animal experiments were conducted in accordance with the National Institutes of Health guidelines on the use of animals in research and were approved by the Laboratory Animal Welfare and Ethics Committee of the Army Medical University. Female C57BL/6 mice with a body weight of approximately 20 g at the age of 8 weeks were purchased from Chongqing TengXin Biotechnology Co. and were housed at the Experimental Animal Center of XinQiao Hospital. The mice were anesthetized with isoflurane and were maintained in a stereotaxic frame (R510IP; RWD Life Science). The T1WI (TE=9.3 ms, TR=580 ms, FOV = 60×60 mm2, matrix = 288×192, slice thickness/spacing =2.0 mm/0 mm, and NEX = 4) and T2WI (TE=69.6 ms, TR=2820 ms, FOV = 60×60 mm2, matrix = 288×192, slice thickness/spacing =2.0 mm/0 mm, and NEX = 6.) Images of mice were acquired using a special rat coil (MS40; Suzhou Medcoil Healthcare Co., Ltd) and a 3.0-T clinical GE MRI scanner before and after injection of the Mn-IONPs@PEG (1:20) with 5 mg [Fe+Mn] per kilogram body weight.
Results
Synthesis And Characterization Of Mn-IONPs
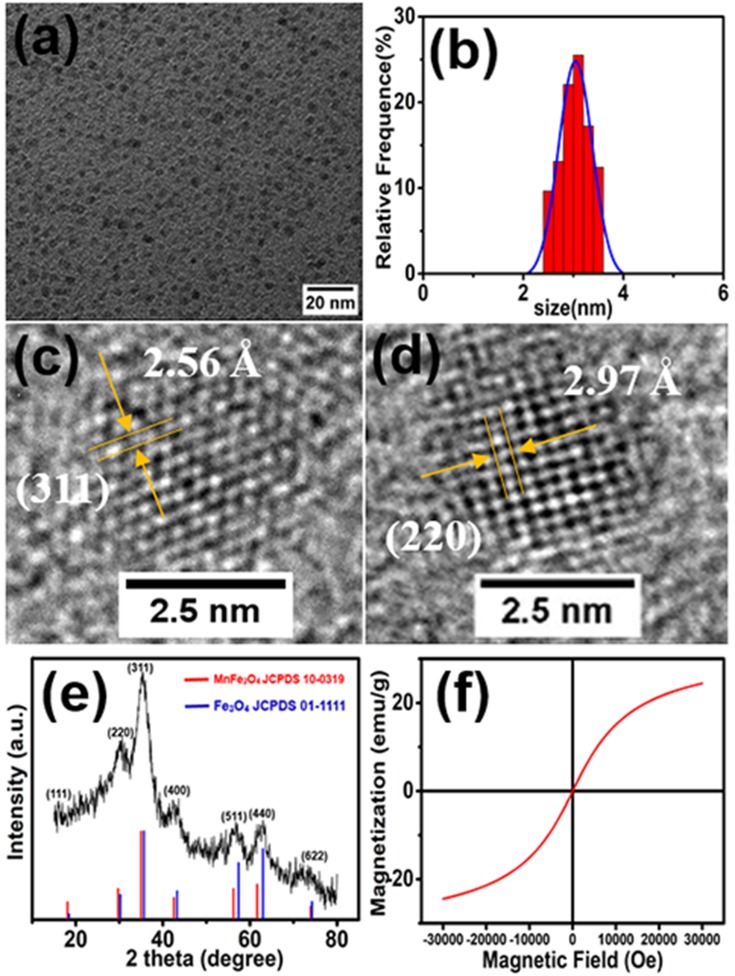
Mn-IONPs were prepared via thermal decomposition of iron-eruciate and manganese-oleate complexes in the presence of oleyl alcohol in dibenzyl ether. Iron-eruciate and manganese-oleate complexes were manufactured through a simple method and are shown in Figure S1. The characteristic bands of iron-eruciate appeared at 1582, 1557, and 1433 cm−1, while those of manganese-oleate were at 1606, 1549, and 1423 cm−1, which were consistent with the data of a previous report.12 The TEM images and corresponding distribution graphs of the obtained nanoparticles are shown in Figure 1A and B, showing that these nanoparticles were of a size 3.0 ± 0.28 nm with a narrow distribution and good uniformity. The crystal lattice fringes shown in the HRTEM images (Figure 1C and D) demonstrated the high crystallinity of the Mn-IONPs, and the measured interplanar distances of 2.56 and 2.97 Å were equivalent to the (311) and (220) lattice planes of Fe3O4, respectively. The XRD patterns are shown in Figure 1E. Compared with the spinel Fe3O4 powder diffraction data (JCPDS card no. 01–1111), these diffraction peaks shifted slightly toward that of MnFe2O4 (JCPDS card no. 10–0319), which can be attributed to the doped manganese. Coupled with the results of ICP-OES showing that the Mn/Fe molar ratio was approximately 0.20, Mn-IONPs were revealed to be successfully prepared. The hysteresis loop recorded by a SQUID magnetometer using a magnetic field up to 3.0 T at the temperature of 300 K showed that the saturation magnetization (Ms) of Mn-IONPs was 24.45 emu g−1 (Figure 1F). Meanwhile, the negligible coercivity and remanence when the external magnetic field was absent indicate their superparamagnetic behavior. Furthermore, after coating with DSPE-PEG-2000 or TMAH, the obtained hydrophilic Mn-IONPs@PEG or Mn-IONPs-TMAH still maintained size uniformity and showed good stability in water.
Figure 1.
(A) TEM image, (B) size distribution graph, (C, D) HRTEM images, (E) XRD pattern, and (F) hysteresis loop of synthesized Mn-IONPs.
Relaxivity Of Mn-IONPs
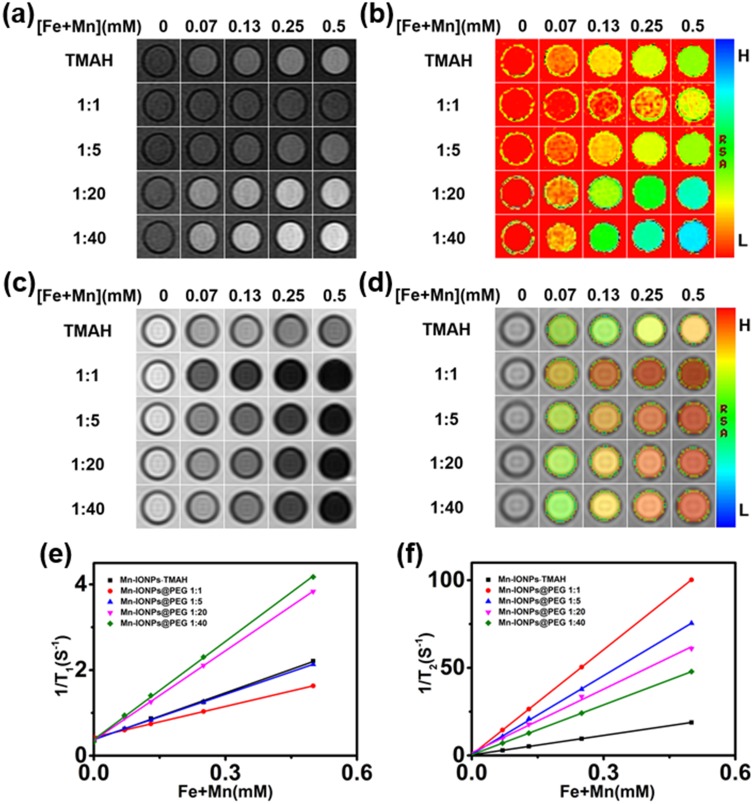
To assess T1 and T2 enhancement, the Mn-IONPs@PEG and Mn-IONPs-TMAH solutions were imaged using a 3.0 T clinical MRI scanner with FSE T1WI, FSE T2WI, T1 mapping and T2 mapping sequences. The two types of Mn-IONPs solutions all showed concentration-dependent signal enhancement (Figure 2A–D). With the concentration increasing, the signal intensity tended to be brighter in T1WI but gradually grew darker in T2WI. The linear fitting of the concentration and 1/T1 or 1/T2 showed that r1 values of Mn-IONPs-TMAH, Mn-IONPs@PEG (1:1), Mn-IONPs@PEG (1:5), Mn-IONPs@PEG (1:20), and Mn-IONPs@PEG (1:40) were 3.7, 2.4, 3.5, 7.1, and 7.8 mM−1s−1, respectively (Figure 2E), and the r2 values were 36.9, 199.5, 149.7, 120.9, and 94.8 mM−1s−1, respectively (Figure 2F).
Figure 2.
In vitro MR images of the Mn-IONPs-TMAH and Mn-IONPs@PEG (1:1, 1:5, 1:20, and 1:40) at 3.0 T: (A) T1-weighted, (B) T1 mapping, (C) T2-weighted and (D) T2 mapping. (E, F) Linear fitting of 1/T1 and 1/T2 over different (Fe+Mn) concentrations of the nanocomposites.
In Vitro Cytotoxicity Assay
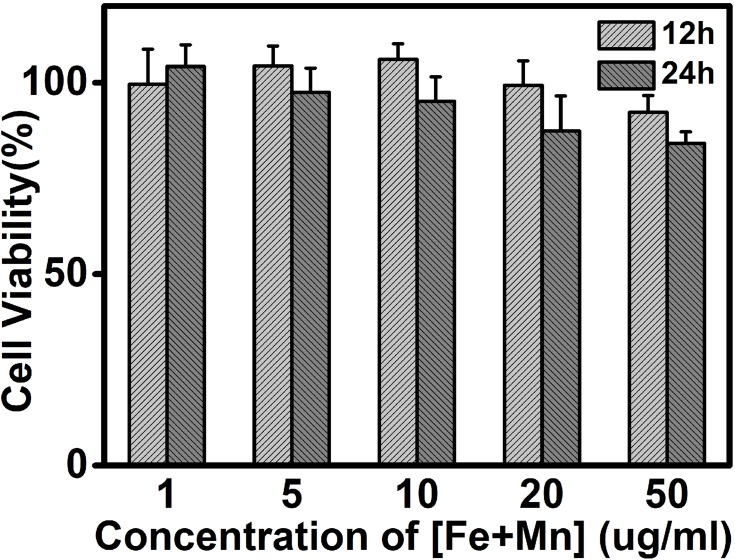
Excellent biocompatibility was considered to be an important requirement of Mn-IONPs@PEG for in vivo application. The in vitro cell cytotoxicity assay of Mn-IONPs@PEG (1:20) was performed on HepG2 cells using CCK-8. Figure 3 shows the viability of HepG2 cells co-cultured with the Mn-IONPs@PEG of [Fe+Mn] concentration ranging from 1 to 50 μg/mL at 37 °C for 12 hrs and 24 hrs. All the cells survived under the current experimental conditions. All Mn-IONPs@PEG samples showed insignificant toxicity with a cell viability greater than 80% at a concentration of less than 50 μg/mL for 12 hrs and 24 hrs. These results demonstrated that Mn-IONPs@PEG have a good biocompatibility.
Figure 3.
Viability of HepG2 cells after incubation with Mn-IONPs@PEG (1:20) at different concentrations (1–50 µg/mL of [Fe+ Mn]) at 37 °C for 12 hrs and 24 hrs.
In Vivo Contrast-Enhanced MRI
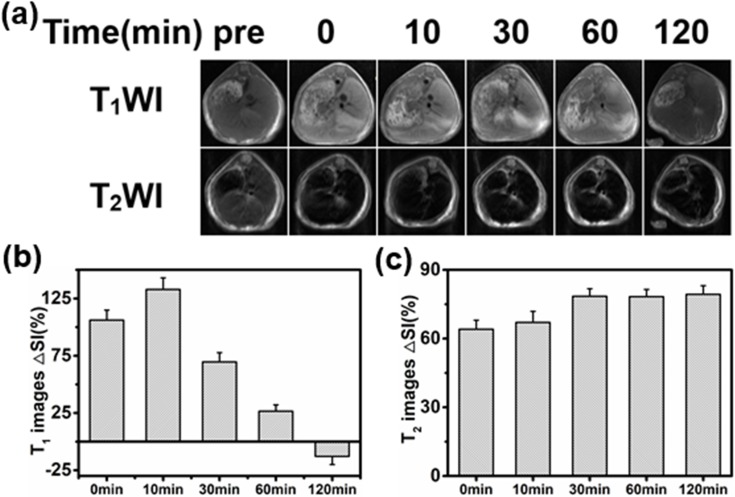
Based on the above results, we further explored the potential of the Mn-IONPs as dual-contrast CA in vivo. Due to the high relaxivity, Mn-IONPs@PEG (1:20) were employed for contrast-enhanced MRI of the liver. Figure 4A shows the axial T1WI and T2WI MR images acquired preinjection and postinjection (0, 10, 30, 60, and 120 min) of Mn-IONPs@PEG. The significantly brighter T1WI MR images and darker T2WI MR images suggest good r1 and r2 values of Mn-IONPs@PEG in vivo. The contrast-enhancement ratios of the MR signal intensity ΔSI (ΔSI = (SIpost − SIpre)/SIpre × 100%) are shown in Figure 4B and C. The ΔSI peak of 133% in T1WI appeared at 10 min after injection followed by a gradual decrease, unexpectedly becoming negative at 120 min. By contrast, the T2WI images showed obvious enhancement at 10 min after injection but increased to a plateau at 30 min and maintained for a long period.
Figure 4.
(A) T1-weighted and T2-weighted MR images of transverse planes of the liver using the FSE sequence acquired at 0, 10, 30, 60, or 120 min after intravenous administration of Mn-IONPs@PEG (1:20). (B, C) Enhancement ratio of the MR signal ΔSI of the liver after contrast-enhancement by Mn-IONPs@PEG (1:20).
Discussion
As is well known, T1 enhancement of superparamagnetic NPs depends on the interaction of the paramagnetic ions on the surface and nearby proton. Doping paramagnetic ions into the NPs has proven to be an effective route to improve r1.29 Many kinds of metal ions have been reported to be paramagnetic because of their unpaired electrons. Unlike lanthanide ions, the transition metal ions show both excellent biocompatibility and comparative paramagnetism and should be considered better ions for doping. Mn2+ with five unpaired electrons shows the highest paramagnetism compared with other transition metal ions.2 Additionally, based on the quantum mechanical inner-sphere theory, the T1 relaxivity for NPs-based T1 CA can be given by33
where PM is the mole fraction of metal ions, q is the number of water molecules bound per metal ion, τM is the residence lifetime of the bound water, and T1M, which is related to τM and the rotational correlation time (τR), is the relaxation time of the bound water protons. It was revealed that the τM of Mn(H2O)62+ is 4.3 × 104 ns and is 2 orders of magnitude smaller than that of Fe(H2O)63+ (5.3 × 106 ns) and other transition metal ions.33 Thus, Mn2+ doping will effectively accelerate spin-lattice relaxation and increase r1. Moreover, embedding paramagnetic ions into the superparamagnetic NPs significantly boosts both r1 and r2 of superparamagnetic hybrid NPs by synergetic interaction between the T1 and T2 species.16
In addition to Mn2+ doping, decreasing the size of NPs into the ultrasmall region has also been proven to be an effective approach to boost r1. The smaller the size of superparamagnetic NPs is, the larger the surface-to-volume ratio is, and the more superficial paramagnetic ions are, and the better r1 the NPs possess.12 Thus, sufficiently small Mn2+ doping nanoparticles must be synthesized to guarantee good r1. Theoretically, the key to successfully obtain ultrasmall Mn2+ doping nanoparticles is to decompose both the precursors at a similar temperature to form a multiple metal-containing monomer before nucleation to obtain smaller nanoparticles instead of a single-metal-containing monomer, resulting in the formation of a nondopant-containing nucleus initially and then another metal serving as the host to grow nanoparticles.12 Thus, the Mn-IONPs in this study were prepared by DSTD of the iron-eruciate and manganese-oleate complexes, which had a similar decomposition temperature in the presence of oleyl alcohol in dibenzyl ether instead of iron (III) acetylacetonate and manganese (II) acetylacetonate. From the results of TEM, HRTEM, XRD and ICP, Mn-IONPs with an ultrasmall size of 3.0 ± 0.28 nm were successfully synthesized and the Mn2+ was fully proven to be doped successfully.
It is very important to have both good r1 and r2 to be good T1/T2 dual-contrast CA. However, we decreased the size of the NPs to the ultrasmall range to reach an optimal r1 of the NPs in this study, resulting in a relative thicker spin-canted layer and a much lower saturation magnetization (Ms) and, finally, a much lower r2 as expected. Unlike r1, the relaxation of T2 CA is dominated by the outer-sphere mechanism and can be given by34
where γI, Mn, ρ, a and L are the proton gyromagnetic ratio, molar mass, density, radius of NPs and impermeable thickness of the surface coating layer, respectively. τD is the translational diffusion time, which is determined by r and D according to the equation τD = r2/D, where r and D are the effective radius and diffusion coefficient, respectively. It is obvious that r2 is proportional to a3, while Ms2 and τD are inversely proportional to L3. To increase Ms, we doped Mn2+ into ultrasmall supermagnetic magnetite. Because the doped Mn2+ can occupy both the tetrahedral (Td) and octahedral holes (Oh) in the crystal lattices, Mn-IONPs form a special mixed spinel structure, finally resulting in larger Ms and r2; this finding has been confirmed in this study by showing that the r2 of Mn-IONPs-TMAH (36.9 mM−1s−1) is larger than IONPs with the same size (r2 = 29.2 mM−1s−1).15 Using another approach by coating Mn-IONPs with DSPE-PEG, we further enhance its r2.
According to Equation (2), r2 can be improved by decreasing D. The PEG molecules on the surface of the Mn-IONPs can immobilize surrounding water molecules in a large region by forming hydrogen bonds, resulting in a small D and a large τD.30,31 Thus, it is reasonable that all the DSPE-PEG-coated Mn-IONPs showed a much higher r2 than THAM-coated Mn-IONPs. However, a decreasing trend for r2 was noted with the increase in the molar ratio of Mn-IONPs: DSPE-PEG. This phenomenon can be easily explained because the hydrogen–bond interactions between PEG and water protons have a critical molar ratio. Too many coating molecules may exclude water protons from the induced magnetic field from the Mn-IONPs, forming an impermeable coating layer on the surface of the Mn-IONPs and leading to a lower r2. Interestingly, we also found that r1 can be changed by varying the coating molecules and the molar ratio of these molecules. Mn-IONPs-TMAH showed a higher r1 than Mn-IONPs-PEG (1:1) and Mn-IONPs-PEG (1:5) but possessed a lower r1 than Mn-IONPs-PEG (1:20) and Mn-IONPs-PEG (1:40). Unlike r2, r1 increased significantly with the increase in the molar ratio of Mn-IONPs: DSPE-PEG. This phenomenon can be attributed to τR and q based on Equation (1). As is well known, T1 relaxation relies on a chemical exchange between the paramagnetic center and water molecules in the first coordination sphere. The more water molecules located in the first coordination sphere, the better r1 is. Although NPs become hydrophilic by coating with DSPE-PEG, there is still a DSPE-oleic acid layer that is impermeable on the surface of the NPs, resulting in much fewer water molecules in the first coordination sphere than Mn-IONPs-TMAH, in which the impermeable oleic acid layer has been exchanged. Thus, the r1 of Mn-IONPs-TMAH was higher than that of Mn-IONPs-PEG (1:1) and Mn-IONPs-PEG (1:5). However, why do Mn-IONPs-PEG (1:20) and Mn-IONPs-PEG (1:40) show a higher r1 than Mn-IONPs-TMAH? The cause may be that r1 can also be remarkably influenced by τR, which is proportional to the molecular weight. With the increase in the molar ratio of Mn-IONPs: DSPE-PEG, the molecular weight of NPs increases notably, leading to a higher r1. Over the critical ratio, the shielding effect of the impermeable DSPE-oleic acid layer will be counteracted and τR will be dominant, making the r1 of Mn-IONPs-PEG (1:20) and Mn-IONPs-PEG (1:40) higher than that of Mn-IONPs-TMAH.
Excellent biocompatibility is considered a key point for inorganic NPs application in vivo.11 To improve the biocompatibility, we selected DSPE-PEG, a well-established nonimmunogenic and noncytotoxic material, for Mn-IONPs coating. By CCK-8 analysis, the acquired Mn-IONPs@PEG (1:20) showed negligible cytotoxicity and excellent biocompatibility, which were consistent with the results of previous studies.30,31,35 Thus, we further explored the potential of Mn-IONPs@PEG (1:20) for T1/T2 dual-contrast MR imaging in vivo. Obviously, clear signal intensity changes were noted in both T1WI and T2WI over time. The signal intensity changes in the liver may be the result of the biological distribution and metabolism of Mn-IONPs@PEG in vivo. After injection, Mn-IONPs@PEG gradually accumulated in the liver within 10 mins, leading to increased ΔSI of both T1 and T2. However, the ΔSI of T1WI decreased over time because of passive aggregation driven by the phagocytosis of Kupffer cells, resulting in stable and individual Mn-IONPs@PEG gradually aggregating into clusters and causing the larger T2 contrast enhancement compared with the smaller T1 contrast enhancement. Particularly, the ΔSI even turned out to be negative at 120 min because the dominated T2 contrast enhancement significantly affected the T1 contrast. By contrast, the ΔSI of T2WI kept increasing until it reached a plateau and there was no downward trend within the observation period because of no degradation. The results obtained from the in vivo MRI verified that Mn-IONPs@PEG are highly sensitive T1 and T2 dual CA for imaging of the liver.
Conclusion
In summary, we successfully synthesized 3-nm-sized Mn-IONPs via facile thermal decomposition of iron-eruciate and manganese-oleate complexes in the presence of oleyl alcohol in dibenzyl ether. After encapsulation in DSPE-PEG-2000, the Mn-IONPs@PEG displayed good colloidal stability and excellent biocompatibility. More importantly, due to Mn2+ doping and DSPE-PEG-2000 coating, the synthesized NPs showed unexpected high r1 and r2. Confirmed by in vitro and in vivo MR imaging, the Mn-IONPs@PEG are ideal candidates for use as T1/T2 dual-contrast CA.
Acknowledgment
This research was supported by National Science Foundation of China (No. 81471635 and 81501521), Natural Science Foundation of Chongqing (No. cstc2018jcyjAX0321 and cstc2017jcyjBX0038).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Smith BR, Gambhir SS. Nanomaterials for in vivo imaging. Chem Rev. 2017;117(3):901–986. doi: 10.1021/acs.chemrev.6b00073 [DOI] [PubMed] [Google Scholar]
- 2.Ni D, Bu W, Ehlerding EB, et al. Engineering of inorganic nanoparticles as magnetic resonance imaging contrast agents. Chem Soc Rev. 2017;46(23):7438–7468. doi: 10.1039/c7cs00316a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–1171. [DOI] [PubMed] [Google Scholar]
- 4.Weissleder R. Molecular imaging: exploring the next frontier. Radiology. 1999;212(3):609–614. [DOI] [PubMed] [Google Scholar]
- 5.Herschman HR. Molecular imaging: looking at problems, seeing solutions. Science. 2003;302(5645):605–608. [DOI] [PubMed] [Google Scholar]
- 6.Louie AY, Hüber MM, Ahrens ET, et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18:321. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Wang L, Cao J, et al. Functional investigations on embryonic stem cells labeled with clinically translatable iron oxide nanoparticles. Nanoscale. 2014;6(15):9025–9033. [DOI] [PubMed] [Google Scholar]
- 8.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7(7):591–607. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Ren J, Ye Z, et al. Bio-inspired synthesis of PEGylated polypyrrole@polydopamine nanocomposite as theranostic agent for t1-weighted MR imaging guided photothermal therapy. J Mater Chem B. 2017;5:1108–1116. [DOI] [PubMed] [Google Scholar]
- 10.Zhang F, Ni Q, Jacobson O, et al. Polymeric nanoparticles with a glutathione-sensitive heterodimeric multifunctional prodrug for in vivo drug monitoring and synergistic cancer therapy. Angew Chem Int Ed Engl. 2018;57(24):7066–7070. doi: 10.1002/anie.201801984 [DOI] [PubMed] [Google Scholar]
- 11.Na HB, Song IC, Hyeon T. Inorganic nanoparticles for MRI contrast agents. Adv Mater. 2009;21(21):2133–2148. doi: 10.1002/adma.v21:21 [DOI] [Google Scholar]
- 12.Zhang H, Li L, Liu XL, et al. Ultrasmall ferrite nanoparticles synthesized via dynamic simultaneous thermal decomposition for high-performance and multifunctional T1 magnetic resonance imaging contrast agent. ACS Nano. 2017;11(4):3614–3631. doi: 10.1021/acsnano.6b07684 [DOI] [PubMed] [Google Scholar]
- 13.Jang JT, Nah H, Lee JH, et al. Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew Chem Int Ed Engl. 2009;48(7):1234–1238. doi: 10.1002/anie.200805149 [DOI] [PubMed] [Google Scholar]
- 14.Shin T-H, Choi J-S, Yun S, et al. T1 and T2 dual-mode MRI contrast agent for enhancing accuracy by engineered nanomaterials. ACS Nano. 2014;8:3393–3401. doi: 10.1021/nn405977t [DOI] [PubMed] [Google Scholar]
- 15.Kim BH, Lee N, Kim H, et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J Am Chem Soc. 2011;133(32):12624–12631. doi: 10.1021/ja203340u [DOI] [PubMed] [Google Scholar]
- 16.Zhou Z, Huang D, Bao J, et al. A synergistically enhanced T1-T2 dual-modal contrast agent. Adv Mater. 2012;24(46):6223–6228. doi: 10.1002/adma.201203169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, You J, Wu C, et al. T1-T2 molecular magnetic resonance imaging of renal carcinoma cells based on nano-contrast agents. Int J Nanomedicine. 2018;13:4607–4625. doi: 10.2147/IJN.S168660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Im GH, Kim SM, Lee DG, et al. Fe3O4/MnO hybrid nanocrystals as a dual contrast agent for both T1- and T2-weighted liver MRI. Biomaterials. 2013;34(8):2069–2076. doi: 10.1016/j.biomaterials.2012.11.054 [DOI] [PubMed] [Google Scholar]
- 19.Tegafaw T, Xu W, Ahmad MW, et al. Dual-mode T1 and T2 magnetic resonance imaging contrast agent based on ultrasmall mixed gadolinium-dysprosium oxide nanoparticles: synthesis, characterization, and in vivo application. Nanotechnology. 2015;26(36):365102. doi: 10.1088/0957-4484/26/36/365102 [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Gao L, Liu K, et al. Characterization of Fe3O4/SiO2/Gd2O(CO3)2 core/shell/shell nanoparticles as T1 and T2 dual mode MRI contrast agent. Talanta. 2015;131:661–665. doi: 10.1016/j.talanta.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Du R, Zhang L, et al. A pH-responsive yolk-like nanoplatform for tumor targeted dual-mode magnetic resonance imaging and chemotherapy. ACS Nano. 2017;11(7):7049–7059. doi: 10.1021/acsnano.7b02675 [DOI] [PubMed] [Google Scholar]
- 22.Cabrera-Garcia A, Checa-Chavarria E, Pacheco-Torres J, et al. Engineered contrast agents in a single structure for T1-T2 dual magnetic resonance imaging. Nanoscale. 2018;10(14):6349–6360. doi: 10.1039/C7NR07948F [DOI] [PubMed] [Google Scholar]
- 23.Gong M, Yang H, Zhang S, et al. Targeting T1 and T2 dual modality enhanced magnetic resonance imaging of tumor vascular endothelial cells based on peptides-conjugated manganese ferrite nanomicelles. Int J Nanomedicine. 2016;11:4051–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee N, Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem Soc Rev. 2012;41(7):2575–2589. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Zhang X, Skallberg A, et al. One-step synthesis of water-dispersible ultra-small Fe3O4 nanoparticles as contrast agents for T1 and T2 magnetic resonance imaging. Nanoscale. 2014;6(5):2953–2963. [DOI] [PubMed] [Google Scholar]
- 26.Pellico J, Ruiz-Cabello J, Fernandez-Barahona I, et al. One-step fast synthesis of nanoparticles for MRI: coating chemistry as the key variable determining positive or negative contrast. Langmuir. 2017;33(39):10239–10247. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Wang SX, Sun Q, et al. Ultrasmall manganese ferrite nanoparticles as positive contrast agent for magnetic resonance imaging. Adv Healthc Mater. 2013;2(7):958–964. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Cao Y, Wang L, et al. Manganese doped iron oxide theranostic nanoparticles for combined T1 magnetic resonance imaging and photothermal therapy. ACS Appl Mater Interfaces. 2015;7(8):4650–4658. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Huh YM, Jun YW, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13(1):95–99. [DOI] [PubMed] [Google Scholar]
- 30.Tong S, Hou S, Zheng Z, et al. Coating optimization of superparamagnetic iron oxide nanoparticles for high T2 relaxivity. Nano Lett. 2010;10(11):4607–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaConte LE, Nitin N, Zurkiya O, et al. Coating thickness of magnetic iron oxide nanoparticles affects R2 relaxivity. J Magn Reson Imaging. 2007;26(6):1634–1641. [DOI] [PubMed] [Google Scholar]
- 32.Lu J, Ma S, Sun J, et al. Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials. 2009;30(15):2919–2928. [DOI] [PubMed] [Google Scholar]
- 33.RB LAUFFER. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging theory and design. Chem Rev. 1987;87:901–927. [Google Scholar]
- 34.Zeng J, Jing L, Hou Y, et al. Anchoring group effects of surface ligands on magnetic properties of Fe3O4 nanoparticles: towards high performance MRI contrast agents. Adv Mater. 2014;26(17):2694–2698. 2016. [DOI] [PubMed] [Google Scholar]
- 35.Johnson NJ, He S, Nguyen Huu VA, Almutairi A. Compact micellization: a strategy for ultrahigh t1 magnetic resonance contrast with gadolinium-based nanocrystals. ACS Nano. 2016;10(9):8299–8307. [DOI] [PubMed] [Google Scholar]