Abstract
The initial phase of influenza infection occurs in the upper respiratory tract and the trachea. Yet, little is known about the initial events whereby the immune system recognises the virus and controls viral dissemination. Here, we report that inflammatory dendritic cells (IDC) are recruited to the trachea early on following influenza infection through type I interferon-mediated production of the chemokine CCL2. We further show that recruited IDC express the C-type lectin receptor SIGN-R1 that mediates direct recognition of the virus by interacting with the N-linked glycans present in the glycoproteins of the virion envelope. Activation of IDC via SIGN-R1 triggers the production of the chemokines CCL5, CXCL9 and CXCL10 that initiate the recruitment of protective natural killer (NK) cells in the infected trachea. In the absence of SIGN-R1, the recruitment and activation of NK cells is impaired, which leads to uncontrolled viral proliferation. Altogether, our results shed light on the orchestration of the early cellular and molecular events involved in the immune protection against influenza.
Keywords: SIGN-R1, Trachea, IDC, Influenza virus, NK, CCL2, CCL5, CXCL9, CXCL10
How the innate immune system initially recognises the replicating influenza virus is an important determinant of the infection outcome1. However, there is limited knowledge about these early immune defence mechanisms and how they affect disease progression. Influenza is first encountered by the host immune system in the upper airways in which the initial virus replication is restricted2. Later, progression of the infection to the lower respiratory tract occurs through the airway epithelium of the trachea and is essential for the development of the acute phase of the disease3. Most of the knowledge about the immune response in the trachea has been gained from studies focused on the pulmonary phase of the infection2 and, therefore, a better characterisation on the early response in the trachea is needed.
The immune response to airborne pathogens involves their recognition by macrophages and dendritic cells (DC) located underneath the mucosal cell layer4. Tracheal DC have been divided in different subsets according to their anatomic location and expression of surface markers4,5. One of the best-studied subtypes is the resident conventional DC (cDC) that play an important role in initiating the adaptive response by migrating to regional lymph nodes and presenting antigen to T cells6. Conversely, a second group of DC, namely the inflammatory DC (IDC), perform their function at the site of infection7. IDC are known to be an important source of cytokines, such as tumour-necrosis factor, and inflammatory molecules, such as nitric oxide8. However, the precise function of IDC during influenza infection have not been yet characterised.
The activation of these cells is associated with the detection of viral components by pattern-recognition receptors (PRR) commonly expressed by DC9. C-type lectin receptors are PRR known to bind specific pathogen-associated carbohydrate structures10. Amongst them, the DC-SIGN murine homologue SIGN-R1 is known to directly recognise bacterial11, viral12 and fungal13 pathogens. Previous studies have described the presence of cells expressing SIGN-R1 in the skin and the lungs, and in secondary lymphoid organs such as the spleen14 and the lymph nodes12. However, the specific function of this receptor in the response against infectious virus is not known.
In this study, we found that IDC are recruited into the tracheal epithelium and recognise influenza virus via SIGN-R1. The production of inflammatory chemokines by these cells induces the recruitment of NK cells, which is essential for the control of the viral replication in the trachea.
Results
Influenza infection induces the recruitment of IDC to the trachea
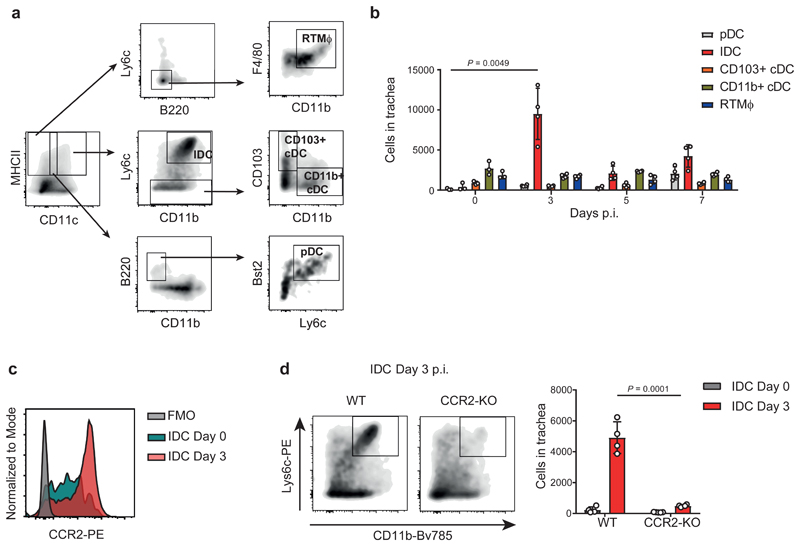
To characterise the inflammatory response that occurs in the trachea following influenza infection, we infected mice with 200 PFU of the mouse-adapted influenza virus strain PR8. We confirmed, using confocal microscopy, that the virus was able to initially infect the tracheal epithelial cell layer (Supplementary Fig. 1a). Next, we characterised by flow cytometric analysis tracheal cell populations according to the expression of different surface markers4,7,15: Inflammatory dendritic cells (IDC), plasmacytoid DC (pDC), CD103+ conventional DC (CD103+ cDC) CD11b+ conventional DC (CD11b+ cDC) and resident respiratory tract macrophages (RTMϕ) (Fig. 1a). We observed a prominent recruitment of IDC at 3 days post infection (d.p.i.) (Fig. 1b). Previous studies have reported CCR2+ Ly6chigh monocytes as the precursors of IDC16. Therefore, we confirmed the monocytic origin of these cells by studying their expression of CCR2 (Fig. 1c), in contrast to the other subtypes of DC (Supplementary Fig. 1b). Furthermore, we observed that, in IDC, CCR2 is eventually downregulated, as the expression of the maturation marker MHC class II increases (Supplementary Fig. 1c). Next, we studied the specific effect on IDC when infecting CCR2-KO animals. We confirmed by flow cytometry that at 3 d.p.i. IDC were significantly reduced in KO animals, compared to their WT counterparts (Fig. 1d). However, we did not detect any significant variation in the total number of the other cell types analysed (Supplementary Fig. 1d).
Figure 1. Influenza infection promotes early recruitment of IDC to the trachea.
a, Flow cytometric characterisation of inflammatory dendritic cells (IDC; CD45+ / MHCII+ / CD11c+ / CD11b+ / Ly6chi), plasmacytoid DC (pDC; CD45+ / MHCII+ / CD11cInt / B220+ / CD11b- / Bst2+/ Ly6c+), CD103+ conventional DC (CD103+ cDC; CD45+ / MHCII+ / CD11c+ / CD11- / Ly6c- / CD103+), CD11b+ conventional DC (CD11b+ cDC; CD45+ / MHCII+ / CD11c+ / CD11b+ / Ly6c- / CD103-) and resident respiratory tract Macrophages (RTMϕ; CD45+ / MHCII+ / CD11c- / B220- / Ly6c- / CD11b+ / F4/80+) in mouse trachea. b, Flow cytometric analysis of DC subsets and RTMϕ in trachea at 0, 3, 5 and 7 days after intranasal infection with influenza virus PR8 (n = 3, 4, 4 and 4 mice per respective group). c, Representative histogram showing surface expression of the chemokine receptor CCR2 in tracheal IDC at 0 and 3 days p.i.. d, Flow cytometric analysis showing a significant reduction in the number of IDC in tracheas from CCR2-KO mice compared to the WT group at day 3 p.i.. Left panel shows a representative scatterplot at day 3 p.i.. Right panel shows IDC quantification (n = 4 mice per group). The presented data are representative of at least three independent experiments (a-d). Results are given as mean ± SD. Data were analysed using the two-tailed Student’s t-test.
Recruited IDC are located in close proximity to the tracheal epithelial cell layer during influenza infection
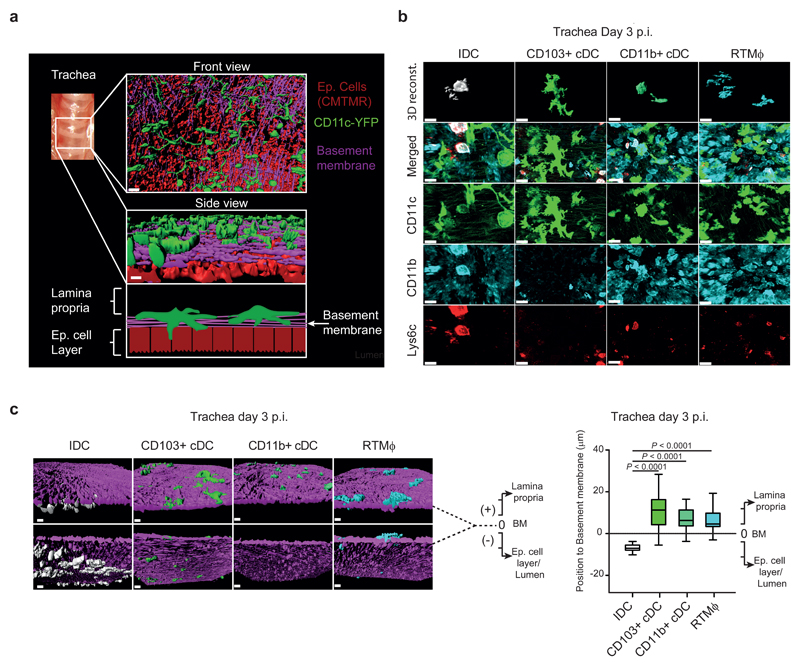
To study the position of the DC network within the mucosal tissue, using confocal microscopy, we produced three-dimensional (3D) reconstructions of mouse tracheas from CD11c-YFP animals. We observed that in uninfected mice, CD11c+ DC were distributed homogeneously above the basement membrane in proximity to the epithelial cell layer (Fig. 2a). To study the specific location of the different subsets of DC and RTMϕ before and after influenza infection, we stained explants from tracheas of CD11c-YFP animals with antibodies against CD11b and Ly6c (Fig. 2b and Supplementary Fig. 2a). Consistent with the flow cytometry data, we could not detect any IDC in uninfected animals (Supplementary Fig. 2b). Instead, in the infected mice, we found that recruited IDC were located towards the lumen of the trachea unlike the other cell types that were mainly located in the lamina propria (Fig. 2c).
Figure 2. Recruited IDC are located in close proximity to the epithelial cell layer during influenza infection.
a, (Top and Middle) 3D surface reconstruction of confocal images from a trachea showing the network of CD11c+ cells (green) located in the lamina propria in close contact with the basement membrane (violet). Epithelial cells (red), located in the luminal part, are stained with CMTMR. (Bottom) Schematic drawing indicating the anatomical distribution of the reconstructed area. Scale bar represents 20 μm. b, Confocal micrographs showing the 3D reconstruction of the DC subsets and RTMϕ, located in the tracheal mucosa at day 3 p.i.. Cells are characterised based on the expression of CD11c (green), CD11b (light blue) and Ly6c (red). IDC are defined as: CD11c+ / CD11b+ / Ly6c+; CD103+ cDC: CD11c+ / CD11b- / Ly6c-; CD11b+ cDC: CD11c+ / CD11b+ / Ly6c-; and RTMϕ: CD11c- / CD11b+ /Ly6c-. Scale bar represents 16 μm in IDC panels and 20 μm in the remaining. c, (Left) representative 3D reconstruction from confocal micrographs of a trachea at day 3 p.i. showing the location of the IDC (red), CD103+ cDC (green), CD11b+ cDC (yellow) and RTMϕ (light blue) with respect to the basement membrane (violet). Scale bar represent 20 μm. (Right) Box plot representing the position of the different group of cells with respect to the basement membrane in the tracheal mucosa at day 3 p.i.. The value of the position of the basement membrane (BM) was considered 0, cells located towards the lamina propria received positive values while cells located towards the luminal side received negative values (data are based on the analysis of 3 confocal reconstructions; IDC, n = 61; CD103+ cDC, n = 54; CD11b+ cDC, n = 40; RTMϕ, n =40). The presented data are representative of at least three independent experiments (a-c). In c, the box plots show 25th to 75th percentiles and median, and the whiskers show 5th to 95th percentiles. Data were analysed using the two-tailed Student’s t-test.
Type I interferon (IFN) is required for the production of CCL2 that recruits IDC precursors to the trachea
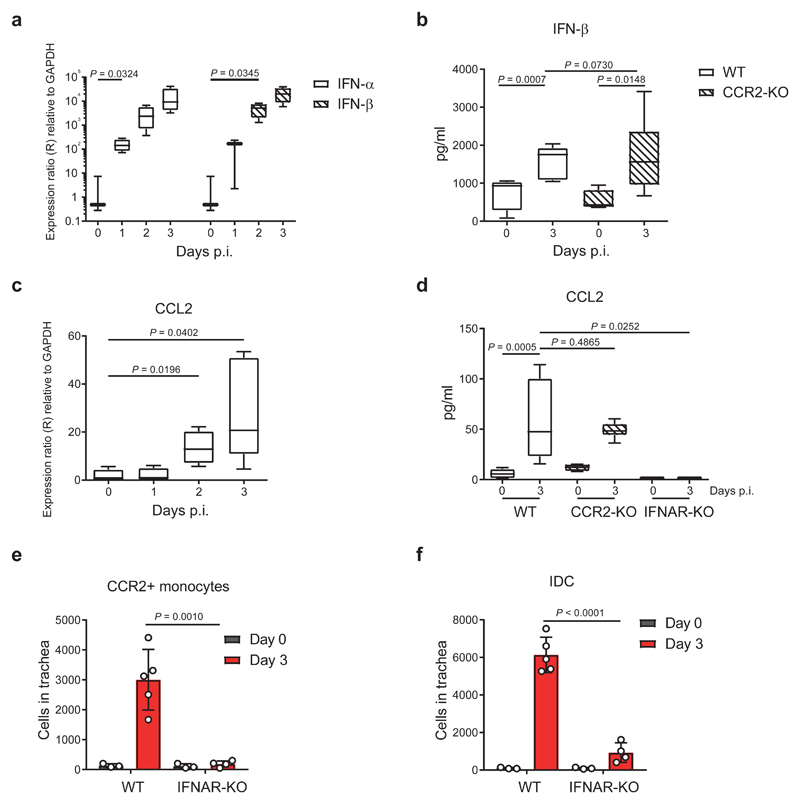
To characterise the anti-viral response that leads to the recruitment of IDC, we assessed the expression of type I IFN in the trachea. We observed a significant upregulation of the transcript levels of both IFN-α and IFN-β during the first 2 d.p.i. (Fig. 3a). Moreover, the cytoplex analysis confirmed that the protein levels of IFN-β were also significantly elevated in both WT and CCR2-KO mice at 3 d.p.i. (Fig. 3b). We also detected a significant increase in the RNA levels of the CCL2 chemokine, a CCR2 ligand, during the first 3 d.p.i. (Fig. 3c), which coincided with an increase in its protein levels (Fig. 3d). Regarding the source of this cytokine we found that RTMΦ, together with pDC and IDC, were the mainly producers of CCL2 (Supplementary Fig. 3a and Supplementary Fig. 3b). Interestingly, we observed a similar pattern of expression of CCL2 and Type I IFN during the first 7 d.p.i. (Supplementary Fig. 3c) concurrent with the IDC recruitment to the trachea. To examine whether there is a connection between the two cytokines, we analysed the CCL2 protein levels in type I IFN receptor (IFNAR)-KO mice, which showed that signalling through IFNAR was required for the secretion of CCL2 (Fig. 3d). In addition, we observed a prominent decrease in the numbers of CCR2+ monocytes in tracheas from IFNAR-KO mice at 3 d.p.i. (Fig. 3e) and a similar reduction in the number of IDC (Fig. 3f), consistent with the role of CCR2+ monocytes as IDC precursors.
Figure 3. Type I interferon (IFN) is required for the production of CCL2, which recruits IDC precursors to the trachea.
a, Time course showing RNA expression of the cytokines IFN-α and IFN-β in mouse trachea during the first 3 days p.i. with influenza virus (IFN-α, n = 3, 4, 4 and4; IFN-β, n = 3, 3, 4 and 4 mice per respective time point). b, Levels of secreted IFN-β in trachea from CCR2-KO mice at day 3 p.i. compared to WT (WT, n = 5 and 5; CCR2-KO, n = 4 and 6 mice per respective time point). c, Time course showing RNA expression levels of the chemokine CCL2 in trachea during the first 3 days p.i. (n = 4, 4, 4 and 5 mice per respective time point). d, Levels of secreted CCL2 in trachea from CCR2-KO and IFNAR-KO mice at day 3 p.i. compared to WT (WT, n = 9 and 10; CCR2-KO, n = 4 and 6; IFNAR-KO, n = 3 and 5 mice per respective time point). e, Flow cytometric quantification of total number of CCR2+ monocytes (CD45+ / MHCII- / CD11c- / CD11b+ / Ly6chi / Ly6g- / CCR2+) in trachea from IFNAR-KO mice at day 3 p.i. compared to uninfected controls (WT, n = 3 and 5; IFNAR-KO, n = 3 and 4 mice per respective time point). f, Flow cytometric quantification of total number of IDC in trachea from IFNAR-KO mice at day 3 p.i. compared to uninfected controls (WT, n = 3 and 5; IFNAR-KO, n = 3 and 4 mice per respective time point). The presented data are representative of at least three independent experiments (a-f). In a-d, the box plots show 25th to 75th percentiles and median, and the whiskers show minimum and maximum values. In e and f, results are given as mean ± SD. Data were analysed using the two-tailed Student’s t-test.
IDC express the lectin receptor SIGN-R1 that recognises glycosylated influenza viral proteins
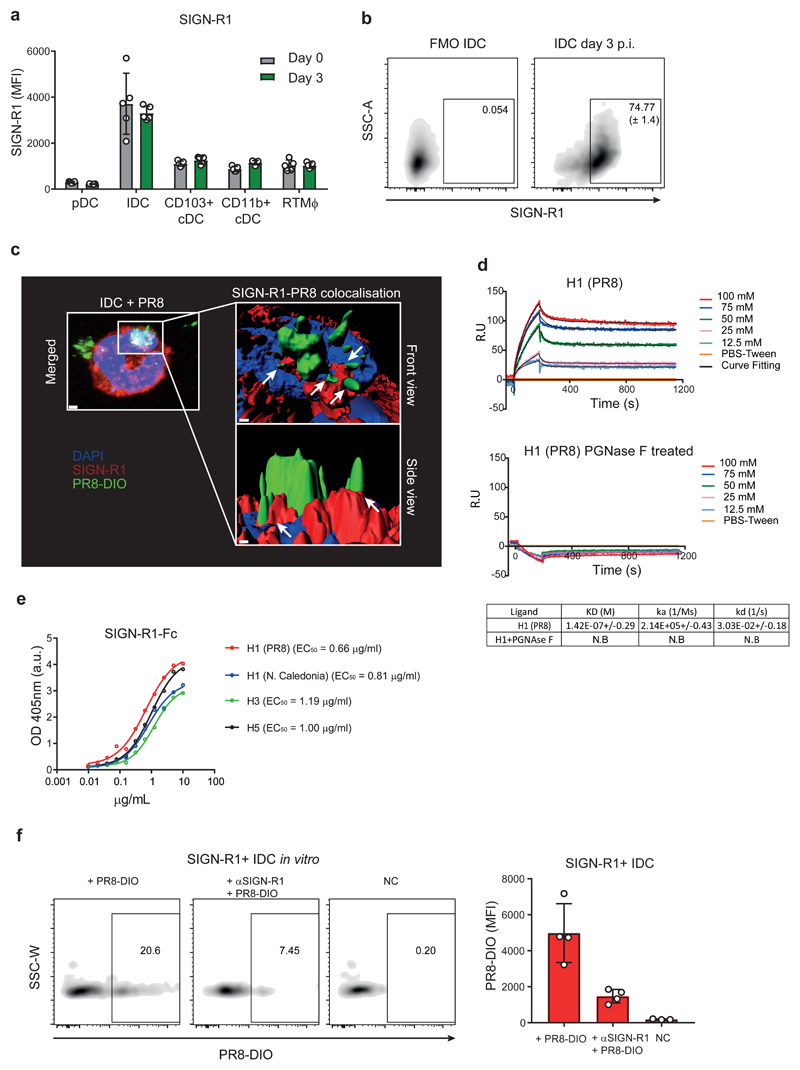
To assess the expression of SIGN-R1 receptor in the mononuclear phagocytic cell compartment of the trachea, we performed flow cytometric analysis. We found that SIGN-R1 was highly expressed by IDC at 3 d.p.i. (Fig. 4a and 4b). Moreover, 3D confocal microscopy revealed that SIGN-R1 colocalised with DIO-labelled influenza virus after 1 h of incubation (Fig. 4c), suggesting that this C-type lectin expressed in IDC is able to bind influenza virus. To examine this possibility, we performed ELISA that confirmed that SIGN-R1 binds with high affinity to whole influenza virus (EC50 = 1.67 μg/ml; Supplementary Fig. 4a). To further investigate this interaction, we performed Surface Plasmon Resonance (SPR) assays to investigate whether SIGN-R1 binds the hemagglutinin (HA) glycoprotein of influenza PR8 virus with a KD of 142 nM (Fig. 4d, upper panel). Next, to evaluate whether the binding of influenza virus to SIGN-R1 could be abrogated by interfering with glycosylation, we removed glycan moieties from the hemagglutinin using different glycosidases. We used Endoglycosidase H (Endo H) to eliminate mannose glycosylation from HA. However, since all later oligosaccharide structures are resistant to Endo H, we also used the Peptide:N-glycosidase F (PNGase F), which is cleaving between the innermost N-Acetylglucosamine and asparagine residues of high mannose regardless of the complexity of the glycan. We monitored glycosylation removal by immunoblot after treatment and we observed that PNGase F was the most efficient enzyme to remove all N-linked glycan (Supplementary Fig. 4b). After N-linked glycan removal of HA, we could not detect any binding between SIGN-R1 and the treated HA (Fig. 4d, lower panel). Of note, binding to the mAb H36-717 was preserved before and after N-linked glycan removal (Supplementary Fig. 4c), indicating that HA was correctly folded after PGNAse F treatment. Next, we performed binding assays with different HA subtypes to explore the possibility of SIGN-R1 also recognising other flu strains. In agreement with the SPR affinity measurement, we observed high binding titres; EC50 = 0.66 μg/ml, 0.81μg/ml, 1.19 μg/ml and 1 μg/ml for H1 (PR8 and N. Caledonia), H3 and H5, respectively (Fig. 4e). In addition, SIGN-R1 binding was also observed with neuraminidase subtype 1 (N1) (EC50 = 0.63 μg/ml; Supplementary Fig. 4d). In this assay FI6, an antibody specific against the stem region of HA18, was included to confirm that the observed binding was not due to a HA contamination in the N1 purification. Interestingly, we also observed the binding of SIGN-R1 to other respiratory viruses such as the human respiratory syncytial virus (RSV)19 and the human metapneumovirus (MPV)20 (EC50 = 0.98 μg/ml and EC50=1,14 μg/ml, respectively; Supplementary Fig. 4a). Taken together, these results indicated that SIGN-R1 recognises several viruses by targeting the carbohydrate part of glycoproteins. Moreover, we observed that addition of an αSIGN-R1-blocking antibody significantly reduced the binding of DIO-labelled influenza PR8 to SIGN-R1+ IDC (Fig. 4f), confirming the role of this receptor in the attachment of the virus on IDC.
Figure 4. IDC express the lectin receptor SIGN-R1 that recognises influenza PR8 through binding to glycosylated viral proteins.
a, Flow cytometric analysis showing the expression levels of SIGN-R1 in tracheal DC and RTMϕ at day 3 p.i. (n = 5 mice per group). b, Representative scatterplots showing the percentage of SIGN-R1+ IDC in trachea at day 3 p.i.. c, Representative 3D reconstruction of confocal micrographs showing the colocalisation between DIO-labelled PR8 (PR8-DIO) and SIGN-R1 (white arrows), expressed in an IDC one hour after in vitro culture. Bar indicates 2 μm (left) and 0.5 μm (right). d, Sensogramms of Surface Plasmon Resonance (SPR) analysis of the HA-binding affinity of SIGN-R1 Fc chimera protein (SIGN-R1-Fc.) SIGN-R1-Fc with or without glycosylation (after treatment with N-glycosidase F (PNGase F)), with different concentration of H1, below their respective kinetic parameters, calculated by SPR using purified proteins are shown. N.B., no binding detected. e, ELISA analysis of the HA-binding titre from different subtypes of the recombinant mouse SIGN-R1-Fc (n = 2 replicates per group). f, (Left) Representative scatterplots from flow cytometric analysis showing the capture of PR8-DIO by SIGN-R1+ IDC after an incubation period of 1 h with the virus. In the αSIGN-R1 group, the binding was inhibited by administration of a blocking antibody previous to the incubation with the virus. (Right) Mean fluorescence intensity (MFI) analysis showing differences in the capture of PR8-DIO by IDC treated with αSIGN-R1 blocking antibody with respect to the control groups (n = 4, 4 and 3 replicates per respective group). The presented data are representative of at least three (a-c and f) or two (d-e) independent experiments. Results are given as mean ± SD (a, b and f), as mean± SEM (d) or mean alone (e).
SIGN-R1 is involved the production of inflammatory chemokines by IDC and the recruitment of NK cells into the tracheal mucosa
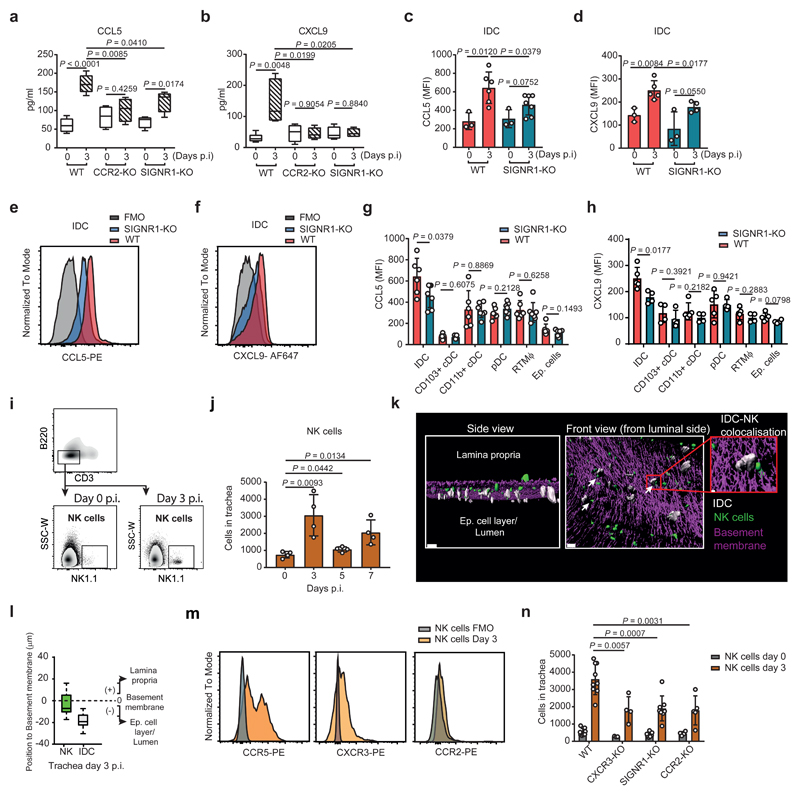
To investigate the role of IDC as inducers of the inflammatory response, we studied the production of different chemokines in tracheal lavage from CCR2-KO and SIGNR1-KO mice at 3 d.p.i.. We found that the absence of IDC or SIGN-R1 resulted in a significant reduction in the levels of the chemokines CCL5, CXCL9 and CXCL10 (Fig. 5a, 5b and Supplementary Fig. 5a, respectively). Furthermore, the reduced chemokine expression on SIGNR1-KO was not related with a deficiency in DC and RTMΦ compared to WT animals (Supplementary Fig. 5b). The specific expression of these chemokines by IDC, as well as their reduced levels in SIGNR1-KO mice, was also confirmed by intracellular flow cytometric staining (Fig. 5c-f). To examine whether the observed differences were due to the direct interaction of SIGN-R1 with the viral proteins, we isolated IDC generated by intranasal administration of Poly (I:C) which had a similar expression levels of SIGN-R1 compared to tracheal IDC at 3 d.p.i. (Supplementary Fig. 5c), and we incubated them in vitro with a vaccine suspension containing HA. The analysis of the secreted chemokine levels at 12 h post stimulation showed that IDC treated with HA were able to increase the production of CCL5, compared to untreated controls, while SIGNR1-KO IDC were not (Supplementary Fig. 5d). We also confirmed that IDC were the major producers of these chemokines in the trachea at 3 d.p.i. and that SIGN-R1 deficiency reduced their expression levels only on IDC (Fig. 5g and 5h, Supplementary Fig.5e). Considering the role of CCL5, CXCL9 and CXCL10 in the recruitment of NK cells to the infection site21, we developed a flow cytometry assay (Fig. 5i) to detect the infiltration of NK in the trachea (Fig. 5j). We observed an early peak in the number of NK cells in infected trachea, which coincided with the aforementioned peak of IDC at 3 d.p.i.. Moreover, confocal analysis indicated that the majority of NK cells were positioned towards the luminal side, in close proximity to IDC (Fig. 5k and 5l) with an average distance of 66.2 µm (Supplementary Fig. 5f). We could also observe that on average, 25.5% of the NK cells analysed were establishing contacts with IDC (Supplementary Fig. 5g). Furthermore, we observed that NK cells expressed the receptors CCR5 and CXCR3, which bind to the chemokines CCL5 and CXCL9/10 respectively22, but not CCR2 (Fig. 5m). The recruitment of tracheal NK cells was partially dependent on the IDC, as demonstrated by the reduced number of these cells in CCR2-KO and SIGNR1-KO mice (Fig. 5n).
Figure 5. SIGN-R1 is involved the production of inflammatory chemokines by IDC and the recruitment of NK cells into the tracheal mucosa.
a,b, Cytokine levels of secreted CCL5 (a) and CXCL9 (b) in tracheas (WT, n = 5; CCR2-KO, n = 4; SIGNR1-KO, n = 4 mice per group). c-f, Representative histogram and mean fluorescence intensity (MFI) analysis showing CCL5 (c,e; WT, n = 3 and 6; SIGNR1-KO, n = 3 and 7 mice per respective time point) and CXCL9 (d,f; WT, n = 3 and 5; SIGNR1-KO, n = 3 and 4 mice per respective time point) expression by tracheal IDC at day 3 p.i.. g,h, Mean fluorescence intensity (MFI) analysis showing expression levels of CCL5 (g; WT, n = 6; SIGNR1-KO, n = 7 mice per group) and CXCL9 (h; WT, n = 5; SIGNR1-KO, n = 4 mice per group) by different cell types at day 3 p.i.. i,j, Flow cytometric quantification of NK cells (CD3- / B220- / NK1.1+) in mouse trachea (n = 4 mice per group). k, Representative 3D reconstruction from confocal micrographs of a trachea at day 3 p.i. showing the proximity between the IDC (white) and NK cells (green) with respect to the basement membrane (violet). White arrows indicate IDC interaction with NK cells. Scale bar represents 40 μm. Magnified area bars, 5 μm. l, Box plot representing the anatomical position of IDC (white) and NK cells (green) relative to the basement membrane in the tracheal mucosa at day 3 p.i. The value of the position of the basement membrane was considered 0, cells located towards the lamina propria received positive values while cells located towards the luminal side received negative values (data are based on the analysis of 3 confocal reconstructions; IDC, n = 63; NK cells, n = 102). m, Representative histograms showing surface expression of different chemokine receptors in tracheal NK cells at day 3 p.i. (n = 3 mice per group). n, Flow cytometric quantification of total number of NK cells in tracheas at day 0 and 3 p.i. (WT, n = 8 and 9; CXCR3-KO, n = 3 and 4; SIGNR1-KO, n = 7 and 8; CCR2-KO, n = 4 and 4 mice per respective time point). The presented data are representative of at least three independent experiments (a-m) or at least two independent pooled experiments (n). In a,b and l, the box plots show 25th to 75th percentiles and median, and the whiskers show minimum and maximum values (a, b) or 5th to 95th percentiles (l). In c, d, g, h, j and n, results are given as mean ± SD. Data were analysed using the two-tailed Student’s t-test.
SIGN-R1+ IDC are required to control influenza infection
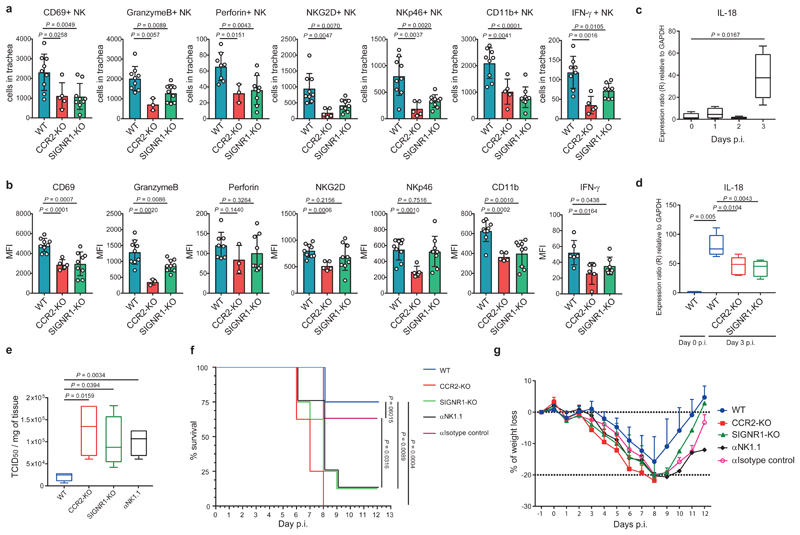
Next, we investigated the involvement of IDC in the activation and functional properties of the recruited NK cells. We detected a significant decrease in the number of cells positive for the markers CD69, granzymeB, perforin, NKG2D, NKp46, CD11b and IFN-γ in CCR2-KO and SIGNR1-KO mice, with respect to the WT at 3 d.p.i. (Fig. 6a). The observed reduction also correlated with a significant decrease in the frequency of positive NK cells for the examined markers and the expression levels of the same markers in NK cells from CCR2-KO and SIGNR1-KO mice, with the exception of perforin, NKG2D and NKp46 (Supplementary Fig. 6a, Fig. 6b,). Considering the role of IL-18 in the activation of NK cells, we detected a significant increase in the transcription levels of these molecule in WT animals during the first 3 d.p.i (Fig. 6c), which were yet significantly reduced in the CCR2-KO and SIGNR1-KO animals (Fig. 6d). To study how the absence of IDC, NK cells or SIGN-R1 affected the progression of influenza infection, we measured viral titres in tracheas from CCR2-KO, SIGNR1-KO and in mice treated with αNK1.1 depletion antibody at 3 d.p.i.. We observed an increase in the influenza titres in all of the examined groups, in relation to the WT control (Fig. 6e). These results correlated with an increased mortality (Fig. 6f) and weight loss (Fig. 6g) in all of the deficient groups. Finally, we analysed the presence of influenza-specific antibodies in WT and SIGNR1-KO mice at day 5 and 10 p.i. (Supplementary Fig. 6b). The results indicated that at the time of death (5-6 d.p.i.) mice had very low or undetectable titers of influenza-specific antibodies and that SIGNR1-KO mice showed no deficiency in antibody production at later time points p.i.
Figure 6. SIGN-R1+ IDC are required to control influenza infection in the trachea.
a,b, Flow cytometric quantification of total numbers and mean fluorescence intensity (MFI) of CD69+, GranzymeB+, Perforin+, NKG2D+, NKp46+, CD11b+ and IFN-γ+ NK cells at day 3 p.i (WT, n = 9, 9, 8, 9, 9, 9 and 8; CCR2-KO, n = 5, 3, 3, 5, 5, 5, and 5; SIGNR1-KO, n = 9, 9, 9, 9, 9, 9, and 8 mice per respective marker). c,Time course showing RNA expression levels of IL-18 in trachea during the first 3 days p.i. (n = 4 mice per group). d, RNA expression levels of IL-18 in trachea form WT, CCR2-KO and SIGNR1-KO mice at day 3 p.i. (WT, n= 3 and 5; CCR2-KO, n = 6; SIGNR1-KO, n = 6 mice per group). e, Virus titre in trachea form WT, CCR2-KO, SIGNR1-KO and αNK1.1-treated mice determined by TCID50 at day 3 p.i. with influenza virus (n = 4 mice per group). f, g, Mortality and morbidity analysis of WT, CCR2-KO, SIGNR1-KO, αNK1.1-treated and isotype control-treated mice after infection with influenza virus (n = 8 mice per group). The presented data are representative of at least two independent pooled experiments (a,b) or at least three independent experiments (c-g). In a, b and g, results are given as mean ± SD. In c-e, the box plots show 25th to 75th percentiles and median, and the whiskers show minimum and maximum values. Data were analysed using the two-tailed Student’s t-test (a-e) and the Mantel-Cox method for the survival curve (f).
Discussion
In this study, we aimed to elucidate the mechanism by which IDC contribute to the control of influenza infection. Initially, we characterised the origin of the recruited IDC. Former studies had confirmed Ly6chi CCR2+ monocytes as the precursors of IDC at the site of infection16. In this work, we observed that, following their recruitment to the trachea, monocytes differentiated into IDC, exhibited a high expression of MHCII and CD11c and a downregulation of CCR2. The prominent expression of CCR2 in IDC progenitors suggested a critical role of its ligands, CCL2 and CCL7, in the regulation of their migration to the inflamed tissue23,24. The observed increase in the expression of CCL2 described in this study is probably associated with the recruitment of monocytes from the bone marrow to the bloodstream25 and facilitates monocyte trafficking across an epithelial cell monolayer26.
Previous studies demonstrated that type I IFN signalling is necessary for the enhancement and sustainment of CCL2 levels27. Accordingly, we observed an early and pronounced expression of type I IFN in the trachea upon influenza infection. Moreover, we showed that this signalling pathway was necessary for CCL2 production in the trachea, as IFNAR-KO mice presented a severe reduction in the levels of this chemokine. This correlated with a complete inhibition in the recruitment of CCR2+ monocytes and IDC in the infected trachea. A similar effect on CCL2 production was previously reported in the draining LN of IFNAR-KO mice after vaccination with a non-replicative UV-inactivated PR828. However, we cannot discard the possible effect that lack of viral control in IFNAR-KO mice29 might have on the production of CCL2 and cell recruitment in the infected trachea.
Furthermore, we demonstrated that, following their recruitment in the trachea, IDC are positioned in close proximity to the epithelial cell layer, which suggests a role of IDC in sensing viral particles generated by infected epithelial cells. Moreover, it has been shown that IDC from lungs have the capacity to internalise viral particles during influenza infection30. Therefore, we might expect a similar function of IDC capturing influenza virus in the trachea.
C-type lectin receptors have recently emerged as inducers of the expression of specific genes in response to pathogens31. During the course of our investigation, we studied the expression of the C-type lectin receptor SIGN-R1 in IDC in the trachea. Our data showed that SIGN-R1 participates in the recognition of influenza virus, as blocking of this receptor affected the capture of PR8 by IDC. We confirmed this hypothesis using SPR and demonstrated that SIGN-R1 binding to HA is glycosylation-dependant. Moreover, SIGN-R1 was able to recognise another surface protein of influenza virus, the neuraminidase, and other viruses with a comparable glycosylation pattern. These results are related with the capacity of SIGN-R1 to bind the mannose present on HA from all influenza virus subtypes. Thus, we demonstrated that SIGN-R1 is a key receptor able to bind a number of viral glycoproteins by targeting the carbohydrate moiety. This property could be used for the development of therapeutic agents that might block the infection of a wide range of influenza virus subtypes. In addition, it has been previously demonstrated that SIGN-R1 recognises other airborne pathogens such as Streptococcus pneumoniae11 or Mycobacterium tuberculosis32, suggesting its potential therapeutic use to control airborne bacterial infections.
We have also demonstrated that SIGN-R1 is necessary for the secretion of inflammatory cytokines after binding to influenza virus, as expression in IDC enhanced the production of CCL5, CXCL9 and CXCL10. Furthermore, we observed that CCL5 expression was not increased in SIGNR1-KO IDC when they were stimulated in vitro with HA. However, we did not observe a significant production of CXCL9 and CXCL10. We believe that this might be related to the technical limitations of the in vitro model that prevents IDC from additional activation signals such as IFN-γ, which are known to be required for the expression of these two chemokines33.
CCL5, CXCL9 and CXCL10 are known to be involved in the recruitment of T cells and NK cells to the site of infection34,35. In the present study, we concluded that the reduction in the number of NK cells observed in SIGNR1-KO animals was due to the lower expression of these cytokines as a result of the lack of activation of tracheal IDC. However, due to limitations in the in vivo model of infection, such as the absence of a mutant influenza virus lacking glycosylation, we cannot rule out that an alternative mechanism, independent of SIGN-R1 binding to viral proteins, might be responsible for the observed phenotype. The importance of NK cells during viral infection has been previously demonstrated in humans36, but also in mice, where the absence of NK cells was associated with a higher susceptibility to influenza virus37. In this study, we postulate that newly recruited NK cells perform effector functions intended to control the viral spread of the virus. In support of this hypothesis, we detected a robust expression in the recruited NK cells of the cytotoxic receptors NKG2D and NKp46, which are involved in the recognition of viral-associated molecules and the activation of these cells38.
Furthermore, in CCR2-KO and SIGNR1-KO we observed a reduced infiltration of NK cells expressing IFN-γ, granzyme B and perforin. These molecules are critical for the effector function of NK cells39–42. However, we also observed a decrease in the expression level of some of the previous markers, which indicates that SIGN-R1+ IDC are contributing to the activation of NK cells during influenza infection. Regarding the way in which this may occurs, previous studies have demonstrated that IL-18 plays an important role in NK cell activation43 and IDC have the capacity to produce IL-1844. Here, we have reported reduced levels of IL-18 in infected SIGNR1-KO and CCR2-KO mice. Therefore, we believe that IDC, secrete IL-18 that promotes the activation and effector function of NK cells during influenza infection.
The increase in the viral titres observed in CCR2-KO and SIGNR1-KO mice might be associated with a deficiency in the activation and recruitment of NK cells in these mouse models. This data supported the role of IDC in controlling the initial replication of the virus, as suggested by previous studies with influenza-infected CCR2-KO mice45. Interestingly, CCR2-KO mice exhibited increased viral levels and mortality after infection compared to SIGNR1-KO mice, which coincided with a reduced chemokine production and NK cell activation. We believe that this might be related with the severe reduction in IDC numbers in this strain rather than a deficiency in other cell subtypes or immune mechanisms. IDC are known to also produce other cytokines such as TNF-α and NO8 which limit influenza virus infection46,47. Conversely, other reports have observed a lower susceptibility of CCR2-KO mice compared to wild-type animals after infection with influenza virus48,49. This discrepancy has been proposed to be related to the differences in the experimental conditions, such as the concentration of the virus used for the initial infection50.
In conclusion, we demonstrated that IDC play an important role in the initial events of the immune response against influenza infection through the direct recognition of viral particles by SIGN-R1 and the secretion of chemokines that recruit and activate NK cells in the tracheal mucosa. Consequently, NK cells contribute to the early elimination of virus-infected cells in the trachea (Supplementary Fig. 6c).
Methods
Mice
C57BL/6 mice were bred in-house or acquired from Janvier Labs. SIGNR1-KO32 mice were kindly donated by Dr. Olivier Neyrolles. The following transgenic strains with a C56BL/6 background were used: CD11c-YFP51, CCR2-KO52, IFNAR-KO53. Mice were maintained in specific pathogen-free facilities at the Institute for Research in Biomedicine, Bellinzona. Experiments were performed in accordance with the Swiss Federal Veterinary Office guidelines and animal protocols were approved by the local veterinarian authorities.
Antibodies
The fluorescently labelled antibodies for cell surface phenotypic staining, cell sorting, and intracellular staining are listed in Supplementary Table 1. In addition, the following depletion antibodies were used: for NK cells, αNK1.1 (PK136, BioXcell) and mouse IgG2a, κ (C1.18.4, BioXcell) as isotype control. The following antibodies were used for ELISA: FI6 (HUMABS BioMed); alkaline phosphatase AffiniPure goat anti-human IgG, Fcγ fragment specific (Jackson Immunoresearch); human anti-F (RSV) IgG1 (MPE8, HUMABS BioMed) as isotype control; and alkaline phosphate-conjugated goat anti-mouse total IgG, IgG1, IgG2a, IgG2b, IgG3, IgM and IgA antibodies (Southern Biotec). For the SPR assays, mAb H36-717 was used as positive control.
Virus production, generation of recombinant influenza virus, infection and survival assay
The influenza virus strain A/PR/8/34 was grown, purified, inactivated and labelled as described previously12. To generate the recombinant influenza virus PR8 carrying a mCherry reporter (rPR8-NS1-mCherry), a plasmid encoding the NS1 of PR8 fused with mCherry gene was constructed by overlapping fusion PCR. Briefly, the NS1 ORF without the stop codon was fused to the N- terminal of a mCherry via a GSGRRQAGGT linker region (NS1-mCherry). The mCherry was followed by a short GSGSG linker, a 19-aa 2A autoproteolytic site (ATNFSLLKQAGDVEENPGP) derived from porcine teschovirus-1 and by the NEP ORF. The first 147 nucleotides of the NEP were silently mutated. Also, silent mutations in the endogenous splice acceptor site in the NS1 ORF were introduced to prevent splicing. The rPR8-NS1-mCherry virus was rescued using standard reverse genetics techniques. Age-matched (six to eight-week-old) female mice were anesthetised with a mix of ketamine (100 mg/kg bodyweight, Parke Davis) and xylazine (10 mg/kg bodyweight, Bayer) and intranasally inoculated with 40µl (20µl on each nare) containing 200 PFU of influenza virus. In survival studies, mice were monitored daily for up to 12 days and sacrificed when weight loss was superior to 20%. For NK cell depletion 200 µg of the depletion antibodies αNK1.1 (see antibody section) was administered intraperitoneally at day 2 before infection and at day 1 after infection. For analysis of influenza-specific antibodies, mice were infected with 70 PFU of influenza virus. The human RSV virus (A2 strain) was obtained from ViraTree, propagated and expanded in HEp-2 cells. The MPV virus (strain A1/6621/01) was propagated and expanded in LLC-MK2 cells.
Flow cytometry
Trachea was collected from the larynx to the tracheal bifurcation, mechanically disrupted with scissors and digested for 45 min at 37°C in an enzyme mix composed of: DNase I (0.28 mg/ml, Amresco), and 0.26 units / ml of Liberase TL Research Grade (Roche) in RPMI 1640 Medium (Gibco) followed by a stop solution of 2 mM EDTA (Sigma Aldrich) and 2 % heat-inactivated filter-sterilised Fetal Calf Serum (Thermo Fisher Scientific) in PBS (Sigma Aldrich). Single cell populations were obtained by forcing the remaining tissue pieces through a 40-µm strainer followed by lysis of red blood cells. Fc receptors from the isolated cells were blocked (αCD16/32, Biolegend) followed by surface staining and analysis by flow cytometry on a LSRFortessa™ (BD Biosciences). Where indicated, intracellular staining was performed according to eBioscience™. Intracellular Fixation & Permeabilization Buffer Set (eBioscience) following the manufacturer’s instructions. Dead cells were excluded using ZombieAcqua fixable viability dye (Biolegend) and data were analysed using FlowJo software (TriStar Inc).
SIGN-R1 blocking assays
Tracheas were processed as for flow cytometric analysis and tracheal IDC in single-cell suspensions were sorted using a FACSAria IIIu (BD Biosciences) accordingly with their expression markers (CD45+ / CD11c+ / MHCII+ / CD11b+, Ly6chi). IDC dispensed into a round-bottom 96 well-plate containing 5x104 cells in 100 µl of RPMI 1640 Medium (Gibco) per plate and cultured for 2 h at 37°C. Following this, cell suspensions were incubated with 1 µg of fluorescently labelled αSIGN-R1-blocking antibody or isotype control antibody for 30 min on ice (see antibody section). After washing, cell suspensions were (apart from Negative Control (NC) wells) incubated with 5x105 PFU of DIO-fluorescently labelled UV-inactivated PR8 (PR8-DIO) for 1 h on ice. Finally, after washing, IDC suspensions were surface stained using 1 µg of fluorescently labelled αSIGN-R1-blocking antibody, fixed and analysed by flow cytometric analysis.
Immunohistology/microscopy of tracheal explants
Immunofluorescence confocal microscopy was performed using a Leica TCS SP5 confocal microscope, using sequential acquisition to limit the signal crosstalk. For immunofluorescence images shown in Fig. 2, 5, Supplementary Fig. 1 and Supplementary Fig. 2, tracheas were collected and fixed in 4% paraformaldehyde at 4°C for 1 h. Then, in case of that the epithelial cell layer was stained, a solution containing 10 µM of CellTracker™ Orange CMTMR (Thermo Fisher Scientific) or 5 µM Cell Trace™ Violet (Thermo Fisher Scientific), was placed in the intraluminal space of the trachea and explants were incubated for 30 min at 7°C and washed 3 times with PBS. After that, they were blocked with proper sera and stained with the indicated antibodies in Triton 0.01% + BSA 1% PBS for 4 h at 4°C with shaking (see antibody section). Tracheas were cut in two halves along the long axis. Pieces were placed on a microscopy slide and embedded in Fluoromount™ Aqueous Mounting Medium (Sigma Aldrich). 3D images were acquired using a HCX PL APO CS 20X/0.7 oil immersion objective, z-step was 1 μm for a total depth ranging from 50 μm to 112 μm. The cell populations were identified assessing the signal intensity in the different channels as follow (or explained in the relative caption for Fig. 2 and Supplementary Fig. 2). In Fig. 5k IDC were defined as CD11c+ / Ly6c+ / NK1.1-, NK cells were defined as CD11c- / NK1.1+. The basement membrane was identified (in the absence of additional specific staining) by manually localising the position of collagen fibres that generated autofluorescence signals detectable in the 412-464 nm channel after excitation with a 405 nm laser during the imaging process. For every reconstruction 10-40 cells of each cell type were analysed. 3D surfaces shown in Fig. 2, 5 and Supplementary Fig. 2 were reconstructed using the Imaris software (Bitplane). Counting and localisation with respect to the collagen layer of basement membrane (Fig. 2d, 5k and Supplementary Fig. 2b) was performed manually using a Cell Counter plugin in FIJI software54. In Supplementary Fig. 6a, the average distance between NK and the nearest neighbour cell in the IDC population was calculated measuring the euclidean distance of the centres of the identified cells. In Supplementary Fig. 6b, contact between cells was calculated as described55 and was considered when their distance (closest voxels) is less than or equal to 5 µm.
SINGR1-PR8 microscopy colocalisation
In Fig. 4, IDC were isolated from tracheas as mentioned in the previous section and cultured in RPMI 1640 medium in μ-Slide 8 well ibiTreat (ibidi) for 3 h at 37°C to let them attach to the bottom of the slide wells. Cell suspensions were subsequently incubated with 5x105 PFU of PR8-DIO for 1 h on ice. After washing, IDC suspensions were surface stained using 1 µg of fluorescently labelled αSIGN-R1 antibody. Finally, cell were fixed, embedded with Vectashield® mounting media with DAPI (Vector Laboratories) and examined by immunofluorescence confocal microscopy. 3D images were acquired using a HCX PL APO lambda blue 63.0 x 1.40 oil immersion objective, z-step was 1 μm for a total depth 20 μm. 3D surfaces shown in Fig. 4 was reconstructed using the Imaris software (Bitplane).
Recombinant proteins and enzymatic treatment
Recombinant mouse SIGN-R1 Fc chimera protein (SIGN-R1-Fc), extracellular domain was obtained from R&D systems. HA from Influenza A H1N1 (A/Puerto Rico/8/34), Influenza A H1N1 (A/New Caledonia/20/99), Influenza A H3N2 (A/Victoria/361/2011) and Influenza A H5N1 (A/Vietnam/1194/2004), and Neuraminidase from Influenza A H5N1 (A/Hubei/1/2011) were obtained from Sino Biological. Hemagglutinin HA from Influenza A H1N1 (A/Puerto Rico/8/34) was used for glycosylation assay using Endo Hf (NEB) or PNGase F (NEB) using the manufacturer’s instructions.
Western blotting
Recombinant HA were treated or not by glycosidase and boiled in reducing SDS-PAGE loading buffer and subjected to SDS-PAGE and western blotting. Detection was performed with an anti-His tag antibody (Thermo Fisher Scientific, clone 4E3D10H2/E3) in 1% BSA for 2 hours and secondary Goat anti-Mouse IgG (H+L)-HRP conjugated (Thermo Fisher Scientific).
SIGN-R1 ELISA
Maxisorp (Nunc) of ELISA plates where coated with 50ul of UV-inactivated virus (1mg/ml of PR8, RSV and MPV), recombinant HA (2µg/ml) or N1 (2µg/ml) at 4 °C overnight. The plates were washed with PBS, blocked with 1 % BSA in PBS for 1 h at room temperature, then dilutions of recombinant mouse SIGN-R1 Fc chimera protein (SIGN-R1-Fc, R&D systems). For plates coated with N1, Fl6 antibody and the isotype control human anti-F (RSV) IgG1 (see antibody section) were added to the wells. After incubation for 1 h at room temperature, plates were washed, and 50 μl of alkaline phosphatase AffiniPure goat anti-human IgG, Fcγ fragment specific (see antibody section) diluted 1:1000 in 1% BSA/PBS was added to each well. After 1 h incubation at room temperature and washing, 50 μl of the solution (1.0 mg/ml pNPP, 0.2 M Tris buffer) prepared with SIGMAFAST™ p-Nitrophenyl (Sigma-Aldrich) phosphate tablets in deionized water was added to each well, followed by incubation at room temperature for 45 min. Absorbance was read on a microplate reader Biotel PoweWave 340 (Biotek) at 405 nm. EC50 were inferred from fitting binding curves by GraphPad Prism 7 software.
Influenza-specific serum antibodies ELISA
After infection, blood was collected at 5 and 10 d.p.i. and serum was obtained. Enzyme-linked immunosorbent assays were performed as previously described56. Absorbance was read on a microplate reader Biotel PoweWave 340 (Biotek) at 405 nm.
Tracheal Cytoplex assay
LEGENDPlex™ assays (Mouse Proinflammatory Chemokine Panel and Mouse Inflammation Panel; Biolegend) were performed to monitor cytokine/chemokine expression. Briefly, tracheas were collected and the luminal side was washed five times with 100 µl of ice-cold PBS. Tracheal washes were centrifuged at 1,500 rpm for 5 min and the supernatant was collected. 25 μl of supernatant were processed following the manufacturer’s instructions. Samples were analysed by flow cytometry on LSRFortessa™ (BD Biosciences) and data were analysed using LEGENDPlex™ software (Biolegend).
Generation of IDC and in vitro culture with influenza HA
IDC were generated by intranasal administration of 40 µg of Poly (I:C) to mimic the replication intermediates present in cells infected with RNA viruses in the lungs57. After 2 days of administration lungs were harvested, IDC were sorted as mentioned in the previous section and 5x104 cells were plated per well into a round-bottom 96-well plate in 65 µl complete RPMI media alone or RPMI+ Inflexal V® (60 µl of media+ 5µl of vaccine solution) media. After 12 h, 25 µl of supernatant was used to perform Mouse Proinflammatory Chemokine Panel LEGENDPlex™ assay.
RQ-PCR
For cytokine expression in whole tissue, trachea were collected at the specified time after infection in 700µL of TRIzol™ Reagent (ThermoFisher Scientific), disrupted in lysing matrix D 1.4 mm ceramic sphere tubes using FastPrep®-24 tissue disruption (MP Biomedicals) and RNA was isolated using a RNAeasy Mini kit (Qiagen). For analysis of cytokine expression of IDC and epithelial cells, tracheas were collected at 0 and 3 d.p.i., single cell suspension obtained, IDC (CD45+ / CD11c+ / MHCII+ / CD11b+, Ly6chi) and epithelial cells (CD45- / EpCam+) sorted as mentioned before and RNA was isolated using a RNAeasy Mini kit (Qiagen). Due to the absence of IDC at 0 d.p.i., RNA was extracted from uninfected tracheal DC (CD45+ / CD11c+ / MHCII+). After RNA isolation, 0.5-1μg of cDNA was synthesised using a cDNA synthesis kit (Applied Biosystems) following the manufacturer’s recommendations. For the RQ-PCR reaction, a SYBR® Master Mix (Applied Biosystems) was used and samples were run on a 7900HT Fast Real-Time PCR System (Applied Biosystems). To measure the expression levels of the cytokines IFN-α, IFN-β, CCL2, IL-18, CCL5, CXCL9 and CXCL10 a sets of primers listed in Supplementary Table 2 were designed. The Pfaffl method58 was used to calculate the relative expression of the transcripts.
Viral Titres
Influenza titres from trachea homogenates were measured by a 50% tissue culture infective dose (TCID50) assay. Briefly, tracheas were aseptically removed from mice, weighed and disrupted in 1ml of ice-cold sterile PBS. The determination of TCID50 was carried out using 96-well plates containing confluent Madine-Darby canine kidney (MDCK) cell monolayers. The MDCK cells were incubated with serial 3-fold dilutions of influenza virus culture supernatant in infection medium (Minimum Essential Medium Eagle+ GlutaMAX ™-I [Gibco] with antibiotics and 1µg/mL of TPCK-Treated Trypsin [Sigma Aldrich] and without FCS) at 37°C. After 1 h, the monolayer was rinsed with PBS, overlaid with infection medium, and incubated at 37°C for 4 days. To identify influenza virus-positive wells, the monolayers where stained with Crystal Violet (Sigma Aldrich) in 70% methanol. Titres were expressed as the dilution of tracheal extract at which 50% of the MDCK cultures revealed virus growth, as calculated by the Spearman and Karber method.
Surface Plasmon Resonance (SPR)
The experiments were carried out at 25°C on a ProteON XPR-36 instrument (Bio-Rad Laboratories) in PBS (GIBCO, Thermo Fisher Scientific) and 0.05% Tween-20 (Sigma). The indicated proteins were immobilised on a GLM sensor chip surface through amine coupling at 100 nM and a blank surface with no protein was created under identical coupling conditions for use as a reference. Analyte proteins (HA with different glycosylation pattern), were injected at a flow rate of 100 μl/min, at concentrations of 100, 75, 50, 25 and 12.5 nM in different sensor channels. The data were processed using Proteon Manager Software and double-referenced by subtraction of the blank surface and buffer-only injection. kon, koff, and KD were calculated by two-state fitting.
Statistics
For statistical analyses and data presentation, Prism 7 (GraphPad Software) was used. Group comparisons were assessed using the unpaired two-tailed Student’s t-test. For statistical analysis between survival curves the Mantel-Cox method was used.
Supplementary Material
Acknowledgments
We would like to thank David Jarrossay for the provision of technical support, and Mariagrazia Uguccioni for critical discussion of the manuscript. We thank James Paulson (The Scripps Research Institute, La Jolla, CA) for initial providing of KO mice. We thank Davide Corti (Humabs) for providing the antibody FI6. We thank Core G of the Consortium for Functional Glycomics (Sally Orr) for mouse phenotyping.
This work was supported by the Swiss National Foundation (SNF) grants, R’equipt (145038), Ambizione (148183) and the grant 176124 to S.F.G., the European Commission Marie Curie Reintegration Grant (612742), and the SystemsX.ch for a grant to D.U.P. (2013/124). This work was partly supported by CRIP (Center for Research on Influenza Pathogenesis), and NIAID funded Center of Excellence on Influenza Research and Pathogenesis (CEIRS, contract number HHSN272201400008C).
Footnotes
Data Availability
All data of this study are available from the corresponding authors upon request.
Authors Contributions
M.P.-S. and S.F.G. conceived the project, designed experiments, analysed and interpreted the results. M.P.-S. performed most of the experiments. L.P. designed, performed and analysed in vitro SIGN-R1-HA interaction experiments with help from S.F.G. and M.P.-S.. T.V. helped to perform confocal microscopy experiments. I.L. helped to study the in vitro chemokine production of IDC. R.D. and D.U.P. analysed confocal microscopy data. G.W. and A.G.S. generated the recombinant influenza virus. Y.F., N.C., F.S., M.C.C, and O.N. advised on the experiments, interpreted results, helped to develop protocols and contributed with reagents. S.F.G. and M.P.-S. wrote the manuscript with the help of N.C., L.P. and O.N.. S.F.G. directed the study.
Competing Interests
The authors declare no competing financial interests.
References
- 1.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty PC. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991;174:875–80. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulendran B, Maddur MS. Innate Immune Sensing and Response to Influenza. Life Science Journal. 2014;6:23–71. doi: 10.1007/82_2014_405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rello J, Pop-Vicas A. Clinical review: Primary influenza viral pneumonia. Crit Care. 2009;13:235. doi: 10.1186/cc8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wikstrom ME, Stumbles PA. Mouse respiratory tract dendritic cell subsets and the immunological fate of inhaled antigens. Immunol Cell Biol. 2007;85:182–188. doi: 10.1038/sj.icb.7100039. [DOI] [PubMed] [Google Scholar]
- 5.GeurtsvanKessel CH, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–34. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza Virus–induced Dendritic Cell Maturation Is Associated with the Induction of Strong T Cell Immunity to a Coadministered, Normally Nonimmunogenic Protein. J Exp Med. 2003;198:133–144. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geurtsvankessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1 doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 8.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro J, Lepenies B. Myeloid C-Type Lectin Receptors in Viral Recognition and Antiviral Immunity. Viruses. 2017;9:59. doi: 10.3390/v9030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang Y, et al. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc Natl Acad Sci. 2004;101:215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez SF, et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol. 2010;11:427–434. doi: 10.1038/ni.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor PR, et al. The role of SIGNR1 and the beta-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J Immunol. 2004;172:1157–62. doi: 10.4049/jimmunol.172.2.1157. [DOI] [PubMed] [Google Scholar]
- 14.Parent SA, et al. Molecular characterization of the murine SIGNR1 gene encoding a C-type lectin homologous to human DC-SIGN and DC-SIGNR. Gene. 2002;293:33–46. doi: 10.1016/s0378-1119(02)00722-9. [DOI] [PubMed] [Google Scholar]
- 15.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auffray C, Sieweke MH, Geissmann F. Blood Monocytes: Development, Heterogeneity, and Relationship with Dendritic Cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 17.McKean D, et al. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984;81:3180–4. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corti D, et al. A Neutralizing Antibody Selected from Plasma Cells That Binds to Group 1 and Group 2 Influenza A Hemagglutinins. Science (80-. ) 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 19.Khan WH, S RVln, Broor S, Parveen S. Glycosylation studies of G protein of group B human respiratory syncytial virus (hRSV) in eukaryotic system. Int J Curr Microbiol App Sci. 2014;3:107–113. [Google Scholar]
- 20.Yang CF, et al. Human metapneumovirus G protein is highly conserved within but not between genetic lineages. Arch Virol. 2013;158:1245–1252. doi: 10.1007/s00705-013-1622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregoire C, et al. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophy Acta - Mol Cell Res. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J Interf Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia T, et al. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–53. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano H, et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute TH1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herold S, et al. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol. 2006;177:1817–24. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- 27.Pattison MJ, MacKenzie KF, Elcombe SE, Arthur JSC. IFNβ autocrine feedback is required to sustain TLR induced production of MCP-1 in macrophages. FEBS Lett. 2013;587:1496–1503. doi: 10.1016/j.febslet.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatziandreou N, et al. Macrophage Death following Influenza Vaccination Initiates the Inflammatory Response that Promotes Dendritic Cell Function in the Draining Lymph Node. Cell Rep. 2017;18:2427–2440. doi: 10.1016/j.celrep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Tate MD, Hertzog PJ. P109 The role of the Type I interferon receptor during influenza virus infection. Cytokine. 2012;59:554–555. [Google Scholar]
- 30.Helft J, et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122:4037–47. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geijtenbeek TBH, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanne A, et al. A murine DC-SIGN homologue contributes to early host defense against Mycobacterium tuberculosis. J Exp Med. 2009;206:2205–20. doi: 10.1084/jem.20090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 34.Taub DD, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–14. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hickman HD, et al. CXCR3 Chemokine Receptor Enables Local CD8+ T Cell Migration for the Destruction of Virus-Infected Cells. Immunity. 2015;42:524–537. doi: 10.1016/j.immuni.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biron CA, Byron KS, Sullivan JL. Severe Herpesvirus Infections in an Adolescent without Natural Killer Cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 37.Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136:1435–41. [PubMed] [Google Scholar]
- 38.Jost S, Altfeld M. Control of Human Viral Infections by Natural Killer Cells. Annu Rev Immunol. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 39.He X-S, et al. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest. 2004;114:1812–9. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge MQ, et al. NK Cells Regulate CD8+ T Cell Priming and Dendritic Cell Migration during Influenza A Infection by IFN- and Perforin-Dependent Mechanisms. J Immunol. 2012;189:2099–2109. doi: 10.4049/jimmunol.1103474. [DOI] [PubMed] [Google Scholar]
- 41.Hwang I, et al. Activation Mechanisms of Natural Killer Cells during Influenza Virus Infection. PLoS One. 2012;7:e51858. doi: 10.1371/journal.pone.0051858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verbist KC, Rose DL, Cole CJ, Field MB, Klonowski KD. IL-15 Participates in the Respiratory Innate Immune Response to Influenza Virus Infection. PLoS One. 2012;7:e37539. doi: 10.1371/journal.pone.0037539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaix J, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–31. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haeberlein S, Sebald H, Bogdan C, Schleicher U. IL-18, but not IL-15, contributes to the IL-12-dependent induction of NK-cell effector functions by Leishmania infantum in vivo. Eur J Immunol. 2010;40:1708–1717. doi: 10.1002/eji.200939988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson TC, Beck MA, Kuziel WA, Henderson F, Maeda N. Contrasting Effects of CCR5 and CCR2 Deficiency in the Pulmonary Inflammatory Response to Influenza A Virus TL - 156. Am J Pathol. 2000;156:1951–1959. doi: 10.1016/S0002-9440(10)65068-7. VN- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rimmelzwaan GF, Baars MM, de Lijster P, Fouchier RA, Osterhaus AD. Inhibition of influenza virus replication by nitric oxide. J Virol. 1999;73:8880–3. doi: 10.1128/jvi.73.10.8880-8883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo SH, Webster RG. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J Virol. 2002;76:1071–6. doi: 10.1128/JVI.76.3.1071-1076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ Monocyte-Derived Dendritic Cells and Exudate Macrophages Produce Influenza-Induced Pulmonary Immune Pathology and Mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 49.Lin S-J, et al. The pathological effects of CCR2+ inflammatory monocytes are amplified by an IFNAR1-triggered chemokine feedback loop in highly pathogenic influenza infection. J Biomed Sci. 2014;21:99. doi: 10.1186/s12929-014-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pamer EG. Tipping the balance in favor of protective immunity during influenza virus infection. Proc Natl Acad Sci. 2009;106:4961–4962. doi: 10.1073/pnas.0901574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindquist RL, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 52.Boring L, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Müller U, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 54.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palomino-Segura M, Virgilio T, Morone D, Pizzagalli DU, Gonzalez SF. Imaging Cell Interaction in Tracheal Mucosa During Influenza Virus Infection Using Two-photon Intravital Microscopy. J Vis Exp. 2008:1–12. doi: 10.3791/58355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez SF, Jayasekera JP, Carroll MC. Complement and natural antibody are required in the long-term memory response to influenza virus. Vaccine. 2008;26:I86–93. doi: 10.1016/j.vaccine.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 57.Harris P, et al. Double-stranded RNA induces molecular and inflammatory signatures that are directly relevant to COPD. Mucosal Immunol. 2013;6:474–484. doi: 10.1038/mi.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.