Abstract
Objective
To examine cause-specific mortality beyond cardiovascular (CV) diseases in patients with gout compared to the general population.
Methods
We included all residents of Skåne (Sweden) aged 18+ in the year 2002. Using the Skåne Healthcare Register, we identified subjects with new diagnosis of gout (2003–2013) and matched each person with gout with 10 comparators free of gout by age and sex. Using information on the underlying cause of death from the Causes of Death Register (until 31st Dec 2014), we estimated hazard ratios (HR) of mortality for specific causes of death in a multi-state Cox model, adjusting for potential confounders.
Results
Among 832,258 persons, 19,497 had a new diagnosis of gout (32% women) and were matched with 194,947 comparators. Persons with gout had higher prevalence of chronic kidney disease, metabolic and CV comorbidities. Gout was associated with 17% increased hazard of all-cause mortality (HR 1.17, 95% confidence interval 1.14–1.21) overall, and 23% (HR 1.23, 1.17–1.30) in women and 15% (HR 1.15, 1.10–1.19) in men. In terms of cause-specific mortality, the strongest associations were seen for the relation of gout to risk of death due to renal disease (HR of 1.78, 1.34–2.35), diseases of digestive system (HR 1.56, 1.34–1.83), CV diseases (HR 1.27, 1.22–1.33), infections (HR 1.20, 1.06–1.35), dementia (HR 0.83, 0.72–0.97).
Conclusions
Several non-CV causes of mortality are increased in persons with gout, highlighting the need for improved management of comorbidities.
Keywords: gout, mortality, cardiovascular, epidemiology
INTRODUCTION
Gout is the most common inflammatory arthritis, with 8.3 million affected adults in the US (1). It is often associated with comorbidities, especially metabolic syndrome, cardiovascular (CV) diseases, and chronic kidney disease (CKD), while there is conflicting evidence regarding the association between gout and dementia (2–6).
The increased risk of renal and CV diseases is thought to be due to hyperuricemia contributing to low-grade systemic inflammation, increased oxidative stress and activation of the renin-angiotensin system, which primarily results in vasculopathy (7, 8). The association between gout and dementia is not so clear. On the one hand, the hydrophilic antioxidant properties of serum urate may be neuroprotective; on the other hand, the vascular disease associated with hyperuricemia may contribute to cognitive decline (9, 10).
Historically, the burden of comorbidities in gout has translated into higher mortality rates (11). This excess mortality among gout patients has been previously attributed mainly to CV causes (12–19). Whether women have similar risks to men is unclear as most studies have included mostly or exclusively men, and thus precise estimates in women are lacking (13, 14, 17–19).
This burden of excess mortality may reasonably be expected to decrease in the 21th century in line with decreasing rates of overall mortality, including deaths attributable to CV diseases, over the past decade in the general population, and decreasing premature mortality related to rheumatoid arthritis (20, 21). However, the premature mortality gap in gout seems to have remained stable over the last 16 years (22). Whether this finding of a stable premature mortality gap remains true for the 21st century, and what comorbidities may be contributing to the excess burden of mortality beyond CV diseases in persons with gout requires clarification.
We therefore evaluated the risk of overall mortality in gout and cause-specific contributions to mortality beyond CV diseases, including renal disease, dementia, infection, diabetes, diseases of the digestive system, lung diseases, and neoplasm in a large population-based cohort.
PATIENTS AND METHODS
Data sources
We used the Swedish Population Register to identify all residents of the Skåne region (~1 million inhabitants aged 18+ years), and the Skåne Healthcare Register, a mandatory register containing data regarding healthcare visits for all residents of Skåne between 1998 and 2014, to collect information about diagnosed diseases. The register contains information about all healthcare visits made in the region including, among others, the personal unique identification number, the date of visit, the healthcare provider, specialist, healthcare level (in-patients, specialist, primary). For the visits within public healthcare providers, we retrieved up to 8 diagnostic codes registered by the treating physicians for each visit according to the International Classification of Diseases 10th Revision (ICD-10). Data from the Causes of Death Register, containing information from death certificate, were used to identify the underlying cause of death for all deceased individuals between 2003 and 2014 (23). The Causes of Death Register is held by the National Board of Health and Welfare and follows recommendations from WHO for identification of the underlying cause of death (23). It has been shown to have high validity with respect to main diagnostic categories (24, 25). The diagnoses in the Cause of Death are also registered according to the ICD-10 system. Further, we collected socioeconomic data from the LISA (Longitudinal Integration Database for Health Insurance and Labour Market Studies, from the Swedish Longitudinell Integrationsdatabas för Sjukförsäkrings- och Arbetsmarknadsstudier) databases provided by Statistics Sweden.
The study has been approved by the Ethical Review Board in Lund (Dnr 2011/432 and Dnr 2014/276).
Participants
We included all persons resident in Skåne between 1998 and 2002 and aged 18 years or older at the end of the year 2002.
The exposure of interest was a new diagnosis of gout. We excluded all persons with at least one gout diagnosis code (ICD-10 code M10) recorded by a physician between 1998 and 2002. Then we identified persons with a new diagnosis of gout recorded by a physician between 01/01/2003 and 12/31/2013. The date of the new diagnosis of gout was the index date, i.e., the start of follow-up. From among the population at risk (i.e., from among persons without a gout diagnosis yet), we randomly selected up to ten comparators free of gout matched by age and sex to each person with gout. The comparators received the same index date as their respective gout match.
Outcome definition
The outcomes were all-cause mortality and cause-specific mortality. We classified the specific causes of death based on the ICD-10 classification of diseases into CV disease, renal disease, dementia, infection, diabetes, disease of the digestive system, lung disease, neoplasm and other (Appendix Table 1).
Confounders
Potential confounders were selected based on previous studies on risk factors of gout and mortality and included demographic factors and specific comorbidities. Age and sex were assessed at the index date. Year of the entry into the study, marital status, income, education, and whether the person was born outside of Sweden were assessed in the year of the index date. Comorbidities were assessed within 5 years preceding the index date through diagnostic codes registered in the Skåne Healthcare Register: hypertension, ischemic heart disease, heart failure, cerebrovascular disease, diabetes mellitus, CKD, dementia, diseases of the digestive system, lung diseases, and neoplasm (Appendix Table 2).
Follow-up
The follow-up started at the index date. The end of the follow-up was at the date of death, relocation outside Skåne region or end of the study (December 31st, 2014), whichever occurred first.
Statistical analysis
We used Cox proportional hazards regression models to estimate the association between new gout diagnosis and death from any cause. The relation of new gout diagnosis to cause-specific mortality was assessed using multi-state Cox proportional hazards models with robust standard errors. The hazard ratios (HR) for the 9 distinct causes of death were estimated through a stratified model, where each person was included 9 times, with the outcome assessed as either having occurred if the person died from the specific cause of death or absent if the person was alive or died from another cause. This model allows for valid estimation of epidemiological associations between exposure and outcome in the presence of competing risks (26, 27). All analyses were adjusted for potential confounders, and stratified by neoplasms and hypertension due to non-proportionality of hazards. We also performed analyses stratified by sex.
Sensitivity analyses
First, we adjusted our primary analysis model for additional potential confounders, diagnosed tobacco use, obesity, dyslipidemia or alcohol-related disorders. Second, to further examine the possible impact of competing causes of death on our results, we used the Fine and Gray competing risk regression model. The Fine and Gray analysis answers a slightly different question from that of our main competing risk analysis, which is that of a real-life hazard of dying from a specific cause and not of an etiologic association between gout and cause-specific mortality (26).
To further evaluate the association between gout and dementia related death, we have performed an additional adjusted analysis where the causes of death of interest were: vascular dementia (ICD-10 code F01), neurodegenerative diseases (Alzheimer’s disease, G30 or Parkinson’s disease, G20) other dementias, or other cause of death.
Additionally, to account for potential misclassification of gout status due to possible limitations of the register data, we performed a probabilistic quantitative bias analysis. We assumed possible non-differential misclassification, with a range of positive predictive values (PPV) of the gout diagnosis from 50 to 90% and negative predictive values (NPV) from 98.5 to 99.75% (Appendix). Lastly, to assess the potential impact of unmeasured confounding we computed E-values (28).
RESULTS
Cohort selection
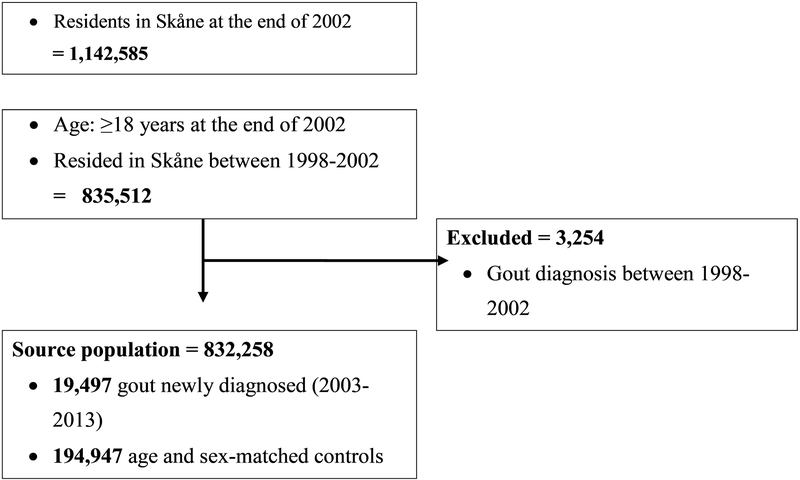
From our source population of more than 800 thousand subjects, there were 19,497 subjects with newly diagnosed gout from 2003–2013. These subjects with gout were matched by age and sex with 194,947 subjects free of gout – two persons with gout had <10 comparators and 1 person with gout had no comparator (Figure 1).
Figure 1.
Flow diagram of study participants.
Patient Characteristics
Both groups had a mean age of 70 years and 68% were male, with other demographic features very similar between the exposed and unexposed. All evaluated comorbidities had higher prevalence among the gout subjects than their matched comparators with the exception of dementia (Table 1). The age at gout diagnosis was higher for females (74.7±13.7 years) than for males (67.9±14.1). Baseline characteristics of participants stratified by sex are presented in Appendix Table 3.
Table 1.
Baseline characteristics of participants.
| Variables | Subjects free of gout n = 194,947 | Newly diagnosed gout n = 19,497 |
|---|---|---|
| Demographics | ||
| Age, years, mean (SD) | 70.1 (14.3) | 70.1 (14.3) |
| Male sex, n (%) | 132,217 (68) | 13,224 (68) |
| Annual income, USD, mean (SD) | 20,570 (13,860) | 19,800 (12,870) |
| Education, n (%) | ||
| ≤ 9 years | 78,327 (41) | 8,575 (45) |
| 10–12 years | 72,572 (38) | 7,515 (39) |
| 13–14 years | 16,608 (9) | 1,393 (7) |
| ≥15 years | 23,518 (12) | 1,698 (9) |
| Married, n (%) | 133,827 (69) | 13,666 (70) |
| Born outside Sweden, n (%) | 24,232 (12) | 2,328 (12) |
| Tobacco use*, n (%) | 1,825 (1) | 279 (1) |
| Comorbidities**, n (%) | ||
| Alcohol-related disorders | 3,036 (2) | 552 (3) |
| Hypertension | 55,636 (29) | 10,386 (53) |
| Ischemic heart disease | 26,967 (14) | 5,071 (26) |
| Heart failure | 13,854 (7) | 4,205 (22) |
| Cerebrovascular disease | 14,368 (7) | 2,076 (11) |
| Diabetes mellitus | 20,233 (10) | 3,818 (20) |
| Dyslipidemia | 16,261 (8) | 3,234 (17) |
| Obesity* | 1,279 (1) | 542 (3) |
| Chronic kidney disease | 2,183 (1) | 1,153 (6) |
| Dementia | 7,651 (4) | 580 (3) |
| Diseases of digestive system | 48,411 (25) | 6,261 (32) |
| Lung diseases | 15,726 (8) | 2,687 (14) |
| Neoplasm | 30,169 (15) | 3,387 (17) |
Tobacco use and obesity were reported based on ICD-10 (International Classification of Diseases 10th Revision) codes registered at consultations to a physician during 5 years prior to the index date.
Comorbidities were based on ICD-10 codes reported on consultations during 5 years prior to the index date. SD: standard deviation.
All-Cause Mortality
There were 46,268 deaths among non-gout subjects and 5,881 among people with gout during a median follow-up time of 4.5 and 4.2 years, respectively. All-cause mortality rate per 1,000 person-years was 47.3 for non-gout subjects and 63.6 for gout subjects, with an adjusted hazards ratio (HR) of 1.17, meaning a 17% higher hazard of mortality among persons with gout than those without gout. The mortality increment related to gout was larger among women than men, with respective adjusted HR of 1.23 (95% confidence interval (CI) of 1.17 to 1.30) and 1.15 (95% CI 1.10–1.19), likely reflecting the lower background risk of mortality among women generally.
Cause-Specific Mortality
Overall, CV diseases were the main cause of death for both gout and non-gout subjects, at 49.5% and 41.3%, respectively. The proportion of death due to renal diseases was higher among gout subjects while the proportion of dementia-related deaths among comparators was more than twice that among gout persons (Table 2).
Table 2.
Causes of death by exposure status.
| Causes of death | Subjects free of gout (N = 194,947) | Newly diagnosed gout (N = 19,497) |
|---|---|---|
| Cardiovascular diseases, n (%) | 19,095 (41.3) | 2,910 (49.5) |
| Renal disease, n (%) | 290 (0.6) | 96 (1.6) |
| Dementia, n (%) | 3,895 (8.4) | 221 (3.8) |
| Infections, n (%) | 2,611 (5.7) | 327 (5.6) |
| Diabetes mellitus, n (%) | 1,001 (2.2) | 201 (3.4) |
| Diseases of the digestive system, n (%) | 1,310 (2.8) | 211 (3.6) |
| Lung diseases, n (%) | 1,706 (3.7) | 208 (3.5) |
| Neoplasm, n (%) | 10,448 (22.6) | 1,069 (18.2) |
| Other, n (%) | 5,912 (12.8) | 638 (10.8) |
The estimates for cause-specific mortality were in magnitude closer to 1 after adjustment for comorbidities as compared to those adjusted for age and sex only (Table 2). An elevated hazard of CV-related mortality in gout was confirmed, with a HR of 1.27 (95% CI of 1.22 to 1.33). Further, we found increased hazard of death due to renal diseases, with a HR of 1.78 (95% CI 1.34–2.35), diseases of the digestive system (see Appendix 1 for list of conditions) (HR 1.56, 1.34–1.83) and infections (HR 1.20, 1.06–1.35) in persons with gout as compared to those without. Gout was associated with lower hazard of dementia-related death, with a HR of 0.83 (95% CI 0.72–0.97) (Table 3).
Table 3.
Cause-specific mortality overall and stratified by sex, among newly diagnosed gout subjects compared to persons free of gout.
| Cause-specific mortality | Adjusted Hazards Ratio [95% confidence interval] | |||
|---|---|---|---|---|
| Overall1 | Overall2 | Men2 | Women2 | |
| Cardiovascular diseases | 1.76 [1.69–1.84] | 1.27 [1.22–1.33] | 1.25 [1.18–1.32] | 1.34 [1.24–1.44] |
| Renal diseases | 3.84 [3.05–4.84] | 1.78 [1.34–2.35] | 1.38 [0.97–1.97] | 4.19 [2.81–6.23] |
| Dementia | 0.67 [0.59–0.77] | 0.83 [0.72–0.97] | 0.85 [0.70–1.03] | 0.80 [0.65–0.99] |
| Infections | 1.48 [1.32–1.66] | 1.20 [1.06–1.35] | 1.05 [0.90–1.23] | 1.51 [1.25–1.83] |
| Diabetes mellitus | 2.28 [1.96–2.65] | 1.05 [0.89–1.24] | 1.10 [0.90–1.34] | 0.98 [0.74–1.32] |
| Diseases of the digestive system | 1.82 [1.58–2.11] | 1.56 [1.34–1.83] | 1.73 [1.43–2.10] | 1.36 [1.04–1.78] |
| Lung diseases | 1.38 [1.19–1.59] | 0.90 [0.77–1.05] | 0.86 [0.71–1.05] | 1.27 [1.00–1.61] |
| Neoplasms | 1.14 [1.07–1.21] | 1.05 [0.99–1.13] | 1.05 [0.97–1.14] | 1.08 [0.95–1.22] |
| Other3 | 1.24 [1.14–1.35] | 1.15 [1.05–1.25] | 1.14 [1.02–1.28] | 1.18 [1.02–1.36] |
Adjusted for age, sex and year of entry into the study.
Adjusted for age, sex, year of entry into the study, marital status, income, education, birth outside Sweden, and comorbidities diagnosed within 5 years preceding index date: hypertension, ischemic heart disease, heart failure, cerebrovascular disease, diabetes mellitus, chronic kidney disease, dementia, diseases of the digestive system (please see Appendix for a detailed list of causes), lung diseases, and neoplasm.
Most common underlying causes of death in the “other” category: ill-defined and unknown cause of mortality, age-related physical debility, exposure to unspecified factor, unspecified fall and other disorders of urinary system.
In the stratified analyses, the effect of gout on cause-specific mortality was similar between the sexes. However, we found some indication that the association between gout and death from infections and renal disease may be more pronounced among women than men. However, there were relatively few deaths from renal diseases in this cohort and these results need to be interpreted with caution (Table 3).
Sensitivity analyses
We found similar magnitude of association between gout and death related to specific dementia types, with adjusted estimated of 0.86 (95% CI 0.71–1.04) for unspecified dementia, 0.72 (95% CI 0.51–1.01) for vascular dementia and 0.83 (95% CI 0.62–1.10) for neurodegenerative diseases. For other causes, similar yet slightly lower results were obtained in the models adjusted for additional comorbidities and with the Fine & Gray method (Appendix Table 4). Slightly lower estimates from the competing risks model were expected and mostly due to the majority of deaths occurring due to CV causes.
Accounting for a wide range of plausible non-differential misclassification of gout diagnoses, the effect estimates in the probabilistic bias analysis were within 0–15% of the original effect estimates (Appendix Table 5) reinforcing the internal validity of our results. Lastly, e-values ranged from 1.4 to 2.3, meaning that for the lower bound of the confidence intervals to reach 1, the association of a residual confounder(s) with both gout and mortality would need to be ≥1.4–2.3 (depending on the specific cause of death). Such strong residual confounders are unlikely to be unaccounted for given that many known confounders were adjusted for.
DISCUSSION
This is one of the few studies to assess cause-specific mortality beyond CV diseases among gout patients and to include a high number of women with gout – a subgroup of gout patients often not included in previous studies. We could confirm the increased risk of death from CV diseases in line with historical reports (12–19). Interestingly, gout was also associated with higher risk of death due to renal diseases, diseases of the digestive system and infections. These findings underscore the importance of comorbidities other than CV in management of gout. We also found 17% lower hazard of dementia-related death which calls for further attention towards the association between hyperuricemia and dementia.
This study assessing mortality due to gout has included the largest number of women (over 6,000) to date and confirms the finding from previous studies that gout also increases mortality among females. This effect may appear more pronounced than that in men due to the lower background risk of mortality in women generally, although we cannot rule out a possible real difference since women with gout are older and seem to be sicker than men (Appendix Table 3). Previous studies have included mostly Asian populations and reported consistently higher all-cause hazards in women (13, 14, 19, 29). Novel in our study is the sex-specific estimates that are more precise than before, and adding cause-specific results.
Of the few studies that have assessed specific causes of death beyond CV diseases in gout subjects, most have evaluated Asian cohorts and have restricted additional causes to renal diseases (14, 19). Considering the particularities of such populations, including genetics (as the HLA-B5801-related risk for allopurinol hypersensitivity syndrome) and possible differences in dietary patterns and life style, the extrapolation of those results to other populations may not be appropriate (19, 30, 31).
To our knowledge, our study is the first to evaluate the relation between gout and the risk of dementia-related death. Most available studies have focused on the relation between serum urate levels and cognitive decline or dementia (incident or established). The findings have been contradictory, with some studies having identified an inverse association between hyperuricemia and dementia (3, 4, 32), others reporting increased risk (33–35), and two systematic reviews summarizing that the data are inconclusive (36, 37). There are pathophysiological and methodological explanations for the different results. On the one hand, urate has antioxidant properties, eliminating reactive oxygen specimens and peroxynitrite and preventing iron-mediated ascorbate oxidation (38). On the other hand, some studies reported that urate may be pro-oxidant depending on the microenvironment and there is extensive evidence that hyperuricemia induces vasculopathy (39). Therefore, urate may play complex and different roles in the central nervous system depending on its concentration, microenvironment, and other factors. It is possible that hyperuricemia could increase the risk of vascular dementia and be protective against neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases. However, the results from our sensitivity analysis do not suggest such differential effect. We note, that these should be interpreted with caution, as the majority of dementia-related deaths were registered as “unspecified dementia”. Apart from this issue of a possible urate paradox, the classification of those neurological diseases is difficult and the combination of two etiologies in the same patient is not rare, especially vascular dementia with one of the neurodegenerative disorders. Further, a new form of dementia has been recently identified (“LATE – limbic-predominant age-related TDP-43 encephalopathy”), highlighting additional difficulty in examining specific types of dementia in this type of registry database. This complexity is lost when using the underlying cause of death, where only one diagnosis can be registered.
In addition, this is one of the few studies to assess the risk of death related to renal disease among gout subjects, although the relation of gout and renal diseases has been extensively explored. The presence of gout may negatively affect the kidneys through many mechanisms: hyperuricemia-induced vascular and tubulointerstitial damage, frequently associated comorbidities such as hypertension and diabetes, frequent use of diuretics and the common use of nonsteroidal anti-inflammatory drugs to treat the acute gout attacks (40). With the increasing body of evidence suggesting a protective renal effect of urate-lowering therapies, the results of our study of higher risk of death related to renal diseases among gout subjects reinforce the need for better gout care, especially related to CKD (41). Another consequence of gout may be increased mortality from diseases of the gastrointestinal system. We were unable to analyze specific subtypes of gastrointestinal system diseases due to small numbers, and the most common condition was listed as “other diseases of digestive system”. The group was heterogeneous, but the most common specified causes of death related to diseases of the gastrointestinal system were gastroduodenal ulcers (gastric, duodenal and peptic ulcers combined), paralytic ileus and intestinal obstruction without hernia, vascular disorders of intestine and alcoholic liver disease suggesting that they may be reflective of consequences of drug side effects (especially NSAIDs, prednisone for upper gastrointestinal bleeding), metabolic comorbidities (obesity, dyslipidemia), excessive alcohol ingestion by some patients, and theoretical pro-inflammatory effects of urate in the gastrointestinal tract (42). The relationship between gout and infections is not clear and has been seldom studied. In one report, it has been hypothesized that persons with gout may have enhanced resistance to infections due to a pro-inflammatory state related to hyperuricemia (43). However, the authors have not confirmed this in a population-base register study from UK (43). Moreover, another UK population-based cohort study has identified an increased likelihood of septic arthritis among gout subjects (44). More generally, it is reasonable to hypothesize that this risk may be partly explained by treatment of flares with systemic glucocorticoids.
We did not find an association between gout and cancer-related mortality. Although the model adjusted for age and sex only suggests increased cancer-related mortality in persons with gout, this association becomes much weaker after adjustment for comorbidities, suggesting important confounding by such factors. A recent study with 700 crystal-proven gout patients has found a larger association, but reported standardized mortality ratios only adjusted for age and sex, and thus there is still residual confounding unaccounted for; as well, that study only had 15 cancer-related deaths, limiting their precision (45). A recent meta-analysis evaluated the relation between gout and cancer incidence only, not cancer mortality; thus the data from that meta-analysis are not directly comparable to our results (46).
We recognize some limitations of this report. The common criticism of register data is a possibility of misclassification of diagnosis. As the data used in this report did not allow for identification of individuals, we were not able to perform validation of gout diagnosis against medical records. However, in our previous study the validation of gout diagnosis against the use of allopurinol in the Skåne Healthcare Register was performed (47). In addition, in order to address the issue of potential misclassification, we have used state-of-the-art methods of quantification of bias. Even with our liberal assumptions of a high degree of misclassification (especially underdiagnosis), we found results very similar to those from the primary analysis. Further, there was potential for residual confounding due to lack of exact data on body mass index and to confounding from unmeasured risk factors, including baseline serum urate and glomerular filtration rate, and medication use, alcohol consumption and dietary habits preceding the diagnosis. Further, the assessment of comorbidities was done using the data from healthcare register, which implies that only comorbidities that a physician records were identified. Tobacco use and obesity are particularly not reliably coded, but comprehensive adjustment for associated comorbidities is expected to minimize the bias resulting from the undercoding of these factors. We also recognize that the age at gout diagnosis in our cohort is higher than reported in other studies (48), but it is consistent with reports from the Swedish or UK population (47, 49, 50). Also, our definition of gout depends on healthcare seeking behavior. Thus, the results may not be generalizable to persons diagnosed with gout at younger ages and to those who do not seek healthcare due to gout. In addition, we did not evaluate the effect of gout treatment on mortality in patients with gout. Although we recognize that this is an important issue, it was beyond the scope of the current work.
There are also important strengths. The large sample size of our population-based cohort and thus minimal risk of selection bias allows for precise and valid estimation of the effects. The register data capture most cases of gout that consult public healthcare, regardless of age, sex, disease duration, gout severity. The Causes of Death Register has been reported to have high accuracy with respect to main disease groups and thus we used such groupings to define causes of death in the present report (24, 25). Also, the evaluation of newly diagnosed gout, as opposed to including subjects with longstanding disease, was important to minimize survival bias. Another strength was that after performing sensitivity analyses to address misclassification of exposure and unmeasured confounding the conclusions remained similar. The comparison of the primary findings with the Fine and Grey estimates reinforced the low likelihood of competing causes of death having a significant impact on our results. In other words, our estimated association between gout and dementia-related deaths, for instance, were not a consequence of premature CV mortality.
In summary, with this population-based study, we provided cause-specific mortality data for men and women with gout as compared to the general population without gout. The novel finding of ~80% increased hazard of renal-related mortality among gout patients highlights the need for improved understanding and management of both gout and renal disease. The finding of 17% lower dementia-related mortality calls for the need to better clarify the relation between hyperuricemia and dementia, while the ~50% higher mortality from diseases of the digestive system and the 20% higher mortality from infections may be at least partly explained by the frequent use of NSAIDs and steroids – drugs that become less necessary with appropriate treatment of hyperuricemia in gout. Finally, our findings suggest important comorbidities beyond CV that may lead to premature mortality in persons with gout, highlighting the need for better management of comorbidities.
Supplementary Material
ACKNOWLEDGMENTS AND AFFILIATIONS
The authors are thankful to Professor Martin Englund, from the Lund University, for providing access to the register data.
Funding: Dr. Neogi’s work was supported by National Institutes of Health grants P60AR47785 and K24AR070892. Funders had no role in conception of study, study design, data analysis, drafting the manuscript, interpretation or decision to submit the manuscript for publication.
The authors declare no support from commercial sources for this work.
Footnotes
Conflict of interest:
The authors declare that they have no relevant financial interests.
This work has been orally presented at the American College of Rheumatology Annual Meeting 2017 and at the G-CAN (Gout, Hyperuricemia and Crystal-Associated Disease Network) Annual Meeting 2017.
Contributor Information
Ana Beatriz Vargas-Santos, Internal Medicine Department, Rheumatology Unit, State University of Rio de Janeiro, Avenida Marechal Rondon, 381, ground floor, Rio de Janeiro, RJ 20950-003, Brazil.
Tuhina Neogi, Clinical Epidemiology Research and Training Unit, Boston University School of Medicine, X building, Suite 200, 650 Albany Street, Boston, MA 02118, USA.
Geraldo da Rocha Castelar-Pinheiro, Internal Medicine Department, Rheumatology Unit, State University of Rio de Janeiro, Avenida Marechal Rondon, 381, ground floor, Rio de Janeiro, RJ 20950-003, Brazil.
Meliha C. Kapetanovic, Department of Clinical Sciences, Section of Rheumatology Lund University and Skåne University Hospital, Lund University, Lund, Sweden.
Aleksandra Turkiewicz, Clinical Sciences, Lund, Orthopedics, Clinical Epidemiology Unit, Lund University, Lund, Sweden, Remissgatan 4, 221 85 Lund, Sweden.
REFERENCES
- 1.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125(7):679–87 e1. [DOI] [PubMed] [Google Scholar]
- 3.Hong JY, Lan TY, Tang GJ, Tang CH, Chen TJ, Lin HY. Gout and the risk of dementia: a nationwide population-based cohort study. Arthritis Res Ther. 2015;17:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu N, Dubreuil M, Zhang Y, Neogi T, Rai SK, Ascherio A, et al. Gout and the risk of Alzheimer’s disease: a population-based, BMI-matched cohort study. Ann Rheum Dis. 2016;75(3):547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latourte A, Soumare A, Bardin T, Perez-Ruiz F, Debette S, Richette P. Uric acid and incident dementia over 12 years of follow-up: a population-based cohort study. Ann Rheum Dis. 2017. [DOI] [PubMed] [Google Scholar]
- 6.Singh JA. Role of serum urate in neurocognitive function and dementia: new evidence contradicts old thinking. Ann Rheum Dis. 2017. [DOI] [PubMed] [Google Scholar]
- 7.Sharaf El Din UAA, Salem MM, Abdulazim DO. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J Adv Res. 2017;8(5):537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richette P, Perez-Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, et al. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. 2014;10(11):654–61. [DOI] [PubMed] [Google Scholar]
- 9.Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53(5):613–25. [DOI] [PubMed] [Google Scholar]
- 10.Vannorsdall TD, Jinnah HA, Gordon B, Kraut M, Schretlen DJ. Cerebral ischemia mediates the effect of serum uric acid on cognitive function. Stroke. 2008;39(12):3418–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarson LE, Chandratre P, Hider SL, Belcher J, Heneghan C, Roddy E, et al. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22(3):335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Ruiz F, Martinez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. 2014;73(1):177–82. [DOI] [PubMed] [Google Scholar]
- 14.Teng GG, Ang LW, Saag KG, Yu MC, Yuan JM, Koh WP. Mortality due to coronary heart disease and kidney disease among middle-aged and elderly men and women with gout in the Singapore Chinese Health Study. Ann Rheum Dis. 2012;71(6):924–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo CF, See LC, Luo SF, Ko YS, Lin YS, Hwang JS, et al. Gout: an independent risk factor for all-cause and cardiovascular mortality. Rheumatology (Oxford). 2010;49(1):141–6. [DOI] [PubMed] [Google Scholar]
- 16.Cohen SD, Kimmel PL, Neff R, Agodoa L, Abbott KC. Association of incident gout and mortality in dialysis patients. J Am Soc Nephrol. 2008;19(11):2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH, Group MR. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168(10):1104–10. [DOI] [PubMed] [Google Scholar]
- 18.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894–900. [DOI] [PubMed] [Google Scholar]
- 19.Kuo CF, Yu KH, See LC, Chou IJ, Tseng WY, Chang HC, et al. Elevated risk of mortality among gout patients: a comparison with the national population in Taiwan. Joint Bone Spine. 2011;78(6):577–80. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Lu N, Peloquin C, Dubreuil M, Neogi T, Avina-Zubieta JA, et al. Improved survival in rheumatoid arthritis: a general population-based cohort study. Ann Rheum Dis. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher MC, Rai SK, Lu N, Zhang Y, Choi HK. The unclosing premature mortality gap in gout: a general population-based study. Ann Rheum Dis. 2017. [DOI] [PubMed] [Google Scholar]
- 23.Brooke HL, Talback M, Hornblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dödsorsaksstatistik - Historik, produktionsmetoder och tillförlitlighet - Komplement till rapporten Dödsorsaker 2008:[38 p.]. Available from: http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18019/2010-4-33.pdf.
- 25.Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Int J Epidemiol. 2000;29(3):495–502. [PubMed] [Google Scholar]
- 26.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–430. [DOI] [PubMed] [Google Scholar]
- 27.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 29.De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis. 2010;69(6):1162–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko TM, Tsai CY, Chen SY, Chen KS, Yu KH, Chu CS, et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. 2015;351:h4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong D, Tan-Koi WC, Teng GG, Finkelstein E, Sung C. Cost-effectiveness analysis of genotyping for HLA-B*5801 and an enhanced safety program in gout patients starting allopurinol in Singapore. Pharmacogenomics. 2015;16(16):1781–93. [DOI] [PubMed] [Google Scholar]
- 32.Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain. 2009;132(Pt 2):377–82. [DOI] [PubMed] [Google Scholar]
- 33.Afsar B, Elsurer R, Covic A, Johnson RJ, Kanbay M. Relationship between uric acid and subtle cognitive dysfunction in chronic kidney disease. Am J Nephrol. 2011;34(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cicero AF, Desideri G, Grossi G, Urso R, Rosticci M, D’Addato S, et al. Serum uric acid and impaired cognitive function in a cohort of healthy young elderly: data from the Brisighella Study. Intern Emerg Med. 2015;10(1):25–31. [DOI] [PubMed] [Google Scholar]
- 35.Perna L, Mons U, Schottker B, Brenner H. Association of cognitive function and serum uric acid: Are cardiovascular diseases a mediator among women? Exp Gerontol. 2016;81:37–41. [DOI] [PubMed] [Google Scholar]
- 36.Khan AA, Quinn TJ, Hewitt J, Fan Y, Dawson J. Serum uric acid level and association with cognitive impairment and dementia: systematic review and meta-analysis. Age (Dordr). 2016;38(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Guo X, Huang R, Chen Y, Zheng Z, Shang H. Serum uric acid levels in patients with Alzheimer’s disease: a meta-analysis. PLoS One. 2014;9(4):e94084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue L, Liu Y, Xue H, Xue J, Sun K, Wu L, et al. Low uric acid is a risk factor in mild cognitive impairment. Neuropsychiatr Dis Treat. 2017;13:2363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latourte A, Bardin T, Richette P. Uric acid and cognitive decline: a double-edge sword? Curr Opin Rheumatol. 2018;30(2):183–7. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RJ. Why focus on uric acid? Curr Med Res Opin. 2015;31 Suppl 2:3–7. [DOI] [PubMed] [Google Scholar]
- 41.Vargas-Santos AB, Neogi T. Management of Gout and Hyperuricemia in CKD. Am J Kidney Dis. 2017;70(3):422–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crane JK, Mongiardo KM. Pro-inflammatory effects of uric acid in the gastrointestinal tract. Immunol Invest. 2014;43(3):255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spaetgens B, de Vries F, Driessen JHM, Leufkens HG, Souverein PC, Boonen A, et al. Risk of infections in patients with gout: a population-based cohort study. Sci Rep. 2017;7(1):1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim SY, Lu N, Choi HK. Septic arthritis in gout patients: a population-based cohort study. Rheumatology (Oxford). 2015;54(11):2095–9. [DOI] [PubMed] [Google Scholar]
- 45.Disveld IJM, Zoakman S, Jansen T, Rongen GA, Kienhorst LBE, Janssens H, et al. Crystal-proven gout patients have an increased mortality due to cardiovascular diseases, cancer, and infectious diseases especially when having tophi and/or high serum uric acid levels: a prospective cohort study. Clin Rheumatol. 2019. [DOI] [PubMed] [Google Scholar]
- 46.Xie Y, Xu P, Liu K, Lin S, Wang M, Tian T, et al. Hyperuricemia and gout are associated with cancer incidence and mortality: A meta-analysis based on cohort studies. J Cell Physiol. 2019;234(8):14364–76. [DOI] [PubMed] [Google Scholar]
- 47.Kapetanovic MC, Hameed M, Turkiewicz A, Neogi T, Saxne T, Jacobsson L, et al. Prevalence and incidence of gout in southern Sweden from the socioeconomic perspective. RMD Open. 2016;2(2):e000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11(11):649–62. [DOI] [PubMed] [Google Scholar]
- 49.Dehlin M, Drivelegka P, Sigurdardottir V, Svard A, Jacobsson LT. Incidence and prevalence of gout in Western Sweden. Arthritis Res Ther. 2016;18:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74(4):661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.