Abstract
Rationale:
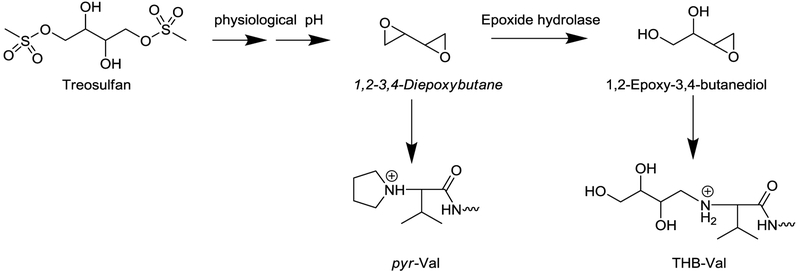
Treosulfan is a substance that is being studied as part of the conditioning regimen given prior to allogeneic stem cell transplantation in patients with hematological malignancies. It is known to decompose into 1,2:3,4-diepoxybutane (DEB) under physiologic conditions. In this study, we investigate whether N-terminal valine adducts can be utilized to monitor differences in DEB formation of patients receiving treosulfan as part of the conditioning regimen for transplantation.
Method:
Blood samples were collected from a group of 14 transplant recipients and analyzed for N,N-(2,3-dihydroxy-1,4-butadiyl)-valine (pyr-Val) and 2,3,4-trihydroxybutyl-valine (THB-Val) adducts as biomarkers for drug uptake and metabolism before and 6 days after treosulfan treatment.
Results:
A new direct injection LC/MS/MS method was developed and validated prior to clinical analysis. The assay precision was determined by 3 replicate analyses on 3 individual days using control globin spiked with known amounts of pyr-Val and THB-Val. The intra- and inter-day precision coefficient of variance (CV) and accuracy were <10% and 15% respectively. In clinical specimens the means ± SD of pyr-Val and THB-Val background were 0.29 ± 0.10 pmol/g HB and 5.17 ± 1.7 pmol/g HB, respectively.
CONCLUSIONS:
These values are similar to those found previously. Treosulfan treatment leads to a significant increase of pyr-Val and THB-Val adducts in each patient (Student’s t-test p<0.0001). The mean ± SD amounts of adduct formed were 245.3 ± 89.6 and 210 ± 78.5 pmol/g globin for pyr-Val and THB-Val, respectively. Importantly, these results show that this direct injection method can quantitate both background and treosulfan-induced pyr-Val and THB-Val N-terminal valine globin adducts in humans.
1. Introduction
Treosulfan is being studied as part of the conditioning regimen given to patients with hematological malignancies prior to allogeneic stem cell transplantation. In the body under physiological pH, treosulfan loses both dimethylsulfone groups and decomposes to 1,2:3,4-diepoxybutane (DEB), a well-known alkylating agent (Figure 1).1,2 DEB is known to alkylate DNA and proteins, and thereby exhibit its pharmacological and toxic activity.3 Formation of DNA-DNA and DNA–protein cross-links alter DNA translation and transcription.4,5 Incomplete DNA repair of these DNA lesions leads to single and double stand breaks, leading to cytotoxicity.
Figure 1:
Scheme of treosulfan metabolism.
DEB can also be formed from exposure to 1,3-butadiene (BD), and alkylation of DNA and hemoglobin (HB) has been utilized to monitor butadiene exposure and metabolism.6 The most common DEB-derived protein adduct reported is the N,N-(2,3-dihydroxy-1,4-butadiyl)-valine (pyr-Val) adduct at the N-terminal valine of alpha-chain of HB. DEB clearance is mediated by epoxide hydrolase which converts the epoxide ring to diol leading to 1,2-epoxy-butane-3,4-diol (EB-diol). EB-diol maintains alkylating activity and forms the corresponding 1,2,3-trihydroxybutyl-valine (THB-Val) adduct that is an indicator of detoxification metabolism. Human and rodent studies have shown that hemoglobin adducts are a suitable tool to study metabolism of BD, including DEB.7,8
The first studies on BD-derived protein adducts in vivo were performed in rodents using [14C]butadiene.9 Subsequently, Osterman-Golkar et al. developed a modified Edman procedure to selectively cleave the alkylated N-terminal valine of the hemoglobin and quantitation by gas-chromatography mass spectrometry.10 This procedure has been widely utilized to quantitate 1,2-butene-derived 1,2-hydroxybuene-valine (HB-Val) and THB-Val.10–12 More recently, Törnquist and colleagues pioneered the FIRE procedure™, a modification of the Endman procedure that allows quantitation by liquid-chromatography.13,14 Despite great sensitivity and specificity, the Edman degradation-based methods, are unsuitable for analysis of ring-closed cycle adducts, such as pyr-Val that is formed from treosulfan. The cyclization is characterized by tertiary amine at the N-terminus prohibiting Edman chemistry. To overcome this limitation, Boysen et al. established a method based on trypsin digestion and detection of the N-terminal 1–7 mer of rodent and human globin after immune affinity enrichment.8,15,16
All the above described methods have been applied for monitoring environmental and occupational exposures to provide intimate knowledge about the formation and fate of reactive toxicants and their metabolites in rodent and humans.6 These data allowed modeling and extrapolation of exposure response from animals to humans and to establish key events for the genotoxic mode of action of BD and its most toxic metabolite DEB.17,18
We report herein the application of N-terminal valine adducts as biomarkers for uptake and metabolism of treosulfan in patients receiving treosulfan as part of the conditioning regimen for allogeneic transplantation. N-terminal valine adducts derived from treosulfan treatment are expected to be much greater than adducts derived from environmental and occupational exposures because: (a) treatment doses are much higher and (b) conversion of Treosulfan to reactive DEB does not require enzyme mediated metabolism. Because of the much higher dose of the reactive agents and expected amounts of adducts, a simplified method was utilized for this study. The simplified method consists of trypsin digestion followed by direct injection and quantitation of alkylated peptides. A priori, the goal was to monitor and quantify pyr-Val and THB-Val after treosulfan treatment for determining if a difference in adduct amounts, if present, correlates with the overall outcome. Therefore, blood specimens from a group of 14 transplant recipients were analyzed for pyr-Val and THB-Val adducts as biomarkers for treosulfan uptake, metabolism and elimination.
2. Materials and Methods
Materials.
Trypsin (biotin agarose, from bovine pancreas) was purchased from Sigma-Aldrich (St. Louis, MO). All reagents and solvents used were ACS grade or higher. Centrifugal filter devices (Ultrafree-MC, 0.1 μm) were from Millipore (Billerica, MA). The non-alkylated ((VLSPADK) peptide standards used for synthesis of N-terminal valine standards were purchased from RS Synthesis (Louisville, KY).
Synthesis of standard peptides.
The synthesis of analytical standard (AST) peptides was performed by direct reaction of the non-alkylated (VLSPADK) peptide with the reagents of interest in 0.1M NH4HCO3 buffer for 72 hours at 37 °C at the optimal molar ratio of 1:50 and pH 6.5 as reported previously.19 Full scan MS and MS/MS experiments of the products were used to confirm their peptide sequence and site of alkylation. Alkylated peptides were subsequently purified by high performance liquid chromatography (HPLC collection and quantitated).20
Human Subjects.
Fourteen patients were studied for biomarkers of treosulfan metabolites, DEB and EB-diol. Patients were given a conditioning regimen consisting of fludarabine 30 mg/m2 on days −6 to −2 and treosulfan 12 g/m2 on days −6 to −4 days prior to transplantation. Patients with an unrelated donor or no prior chemotherapy were also given antithymocyte globulin (ATG Fresenius 5 mg/kg on days −3 to −1). Allogeneic stem cells were infused on day 0. Cyclosporine and a short course of methotrexate or mycophenolate mofetil were used for prevention of graft-versus-host disease (GVHD). A standard regimen of antibiotic prophylaxis was used.21,22
Sample collection and processing.
The study was approved by the institutional review board and all patients gave informed consent in writing prior to participation. Two tubes of 10 mL blood from each patient were collected into EDTA vacutainer tubes containing 5.4 mg K2E (Becton Dickinson and Co.), one before the first dose of treosulfan (day 0) and then six days post treatment (day 6) or 5 days after treatment initiation before transplantation. Red blood cells were isolated by centrifugation and washed twice with phosphate buffered solution and stored at −80 °C.23 Amounts of pyr-Val and THB-Val were measured based on a simplified procedure similar to the one described by Boysen et al., 2004.8 Globin was isolated from red blood cells according to Mowrer et al.23 Globin (2–2.5 mg) were dissolved in 1.5 mL of 0.1M NH4HCO3 and 2 pmol of internal standard [13C5]N,N-(2,3-dihydroxy-1,4-butadyil)-valine (1–11) peptide was added. Samples were digested for 18 hours at 37 °C with 100 μL of trypsin-biotin-agarose suspension. Each sample was transferred to a centrifugal filter device and filters were rinsed with water for a final volume of 380–430 μL. Samples were brought to dryness in a speed vac and stored at −80 °C until analyses. Peptide standards were accurately quantified as described by Bordeerat et al., 2009.20
Tandem Mass Spectrometry Analysis.
For quantitation, lyophilized samples were dissolved in 50 μl 15 mM ammonium formate-0.1% formic acid. The quantitative analysis of the adducted N-terminal Val (1–7) peptides by LC–MS/MS was performed with a UPLC (Agilent 1290 Infinity LC) coupled to a triple quad mass analyzer (Agilent 2490). An Agilent HPLC Poroshell 120 C18 column (2.7μm 4.6 × 150 mm) was operated with a linear gradient of 5% 15 mM ammonium formate-0.1% formic acid for 1 min and then linearly increased to 40% acetonitrile in 12 min, at a flow rate of 0.4 mL/min. Between injections the column was washed at 90% acetonitrile for 5 min before re-equilibration for 8 minutes. The retention times of analytes were determined with synthesized peptide standards. The peptides were detected in multiple reaction monitoring (MRM) mode, monitoring the transition of the doubly charged precursor ions to the a1-and y6-fragments listed in Table 1. The LC/MS/MS conditions were as follows: column temperature 60°C, fragmentor 380 V, sheath gas temperature 400°C, sheath gas flow 12 L/min, gas temperature 350°C, gas flow 15 L/min, nebulizer 40 psi. Sample injection volume was 20 μl. Amounts of adducts were calculated based on the corresponding ion transition peak area and the standard curve. Standard curves were constructed daily and repeatedly showed linear response from 2 to 500 fmol/injection.
Table 1:
Ion transitions used for selected reaction monitoring
| Adduct | Ion transition |
|---|---|
| pyr-Val | 408.5 → 158.2 408.5 → 630.2a |
| [13C5]pyr-Val | 411.2 → 162.2 |
| THB-Val | 417.2 → 176.1 417.2 → 630.2a |
| [13C5]THB-Val | 419.5 → 181.2 |
double charged precursor ion to y6-fragment
3. Results
3.1. Patient Characteristics and outcomes.
The current study included 14 patients, 9 male and 5 female, median age of 63 years (range, 24–68 years). The underlying diagnoses were acute myeloid leukemia (n=6), myelodysplastic syndrome (n=3), non-Hodgkin lymphoma (n=4) and Hodgkin lymphoma (n=1). Four patients had a prior autologous transplantation and one had a prior allogeneic transplantation. The donor was a human leukocyte antigen (HLA)- matched sibling (n=5) or matched unrelated donor (n=9).
Eleven patients engrafted within a median of 13 days (range, 10–19). Three patients died prior to engraftment due to multi-organ toxicities (Patient #3,4,5). Six patients had acute GVHD (4 grade I, 1 grade II and 1 grade IV) and 2 had chronic GVHD (1 mild, 1 severe). At the median follow-up of 32 months, 4 patients are alive. Ten patients died of relapse (n=5), organ toxicities (n=3) or GVHD (n=2). Due to small sample size, we could not correlate outcomes with treosulfan pharmacokinetics.
3.2. Method validation.
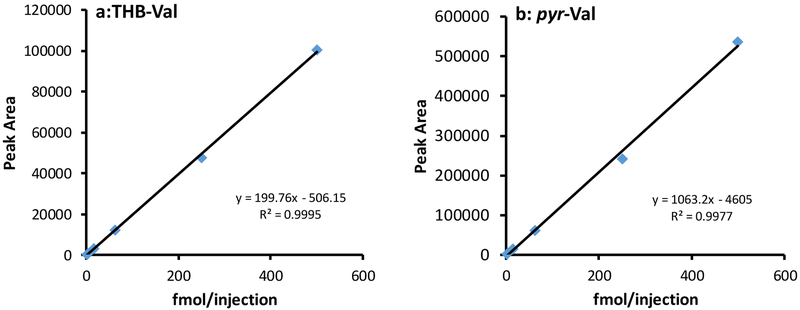
Before application to clinical specimens, the simplified method was evaluated using alkylated peptide standards and globin reacted in vitro with 1,2-epoxybutene, DEB and EB-diol. EB-derived HB-Val adduct peptide standards were included in the method evaluation to be comparable to previous BD exposure studies. Standard curves for pyr-Val and THB-Val were generated by linear regression analysis of the peak areas versus the analyte concentration in water (Figure 2). The R2 value for each analyte was >0.98, demonstrating a linear range for adduct detection. The assay precision was determined by 3 replicate analyses on 3 individual days using control globin spiked with known amounts of HB-val, pyr-Val and THB-Val. The intra- and inter-day precisions (coefficient of variance CV) and accuracy were <10% and 15% respectively (Table 2A). In addition, aliquots from patients #04, #06 and #08, from blood samples collected of day 6 after treosulfan initiation, were analyzed in quintuplicate and the variability within samples was <7% (Table 2B). The limit of detection was determined using globin spiked with minute amounts of adduct peptide standards. Amounts of 0.01, 0.02 and 0.4 pmol/g globin of HB-Val, pyr-Val and THB-Val, respectively, were detected with a signal-to-noise ratio of >5, based on 1 mg globin sample.
Figure 2:
Standard curves for pyr-Val and THB-Val adducts.
Table 2:
Precision, Accuracy and Reproducibility for detection of pyr-Val and THB-Val in human globin spiked with authentic standards.
| A: Spiked control HB [fmol/sample] | ||||
|---|---|---|---|---|
| HB spiked* | mean | SD | CV | |
| Day 1 | HB-Val | 9.25 | 1.23 | 13% |
| THB-Val | 138.23 | 19.93 | 14% | |
| pyr-Val | 11.72 | 2.35 | 20% | |
| Day 2 | HB-Val | 16.52 | 1.47 | 9% |
| THB-Val | 225.29 | 14.19 | 6% | |
| pyr-Val | 20.90 | 1.44 | 7% | |
| Day 3 | HB-Val | 12.33 | 0.36 | 3% |
| THB-Val | 203.58 | 9.62 | 5% | |
| pyr-Val | 23.94 | 1.28 | 5% | |
| Overall | HB-Val | 12.70 | 1.02 | 8% |
| THB-Val | 189.03 | 14.58 | 8% | |
| pyr-Val | 18.86 | 1.69 | 11% | |
| B: Patient globin analyzed in quintuplet [pmol/g globin] | ||||
| Patient ID | mean | SD | CV | |
| #04 | THB-Val | 2919.31 | 171.3 | 6% |
| pyr-Val | 758.09 | 26.0 | 3% | |
| #06 | THB-Val | 1157.83 | 57.47 | 5% |
| pyr-Val | 514.29 | 34.2 | 7% | |
| #08 | THB-Val | 2104.93 | 100.9 | 5% |
| pyr-Val | 882.39 | 26.0 | 3% | |
Globin samples (2mg) were spiked with 10, 200 and 20 fmol/sample HB-Val, pyr-Val and THB-Val, respectively, and analyzed by LC/MS/MS.
3.3. Application to treosulfan-treated patients
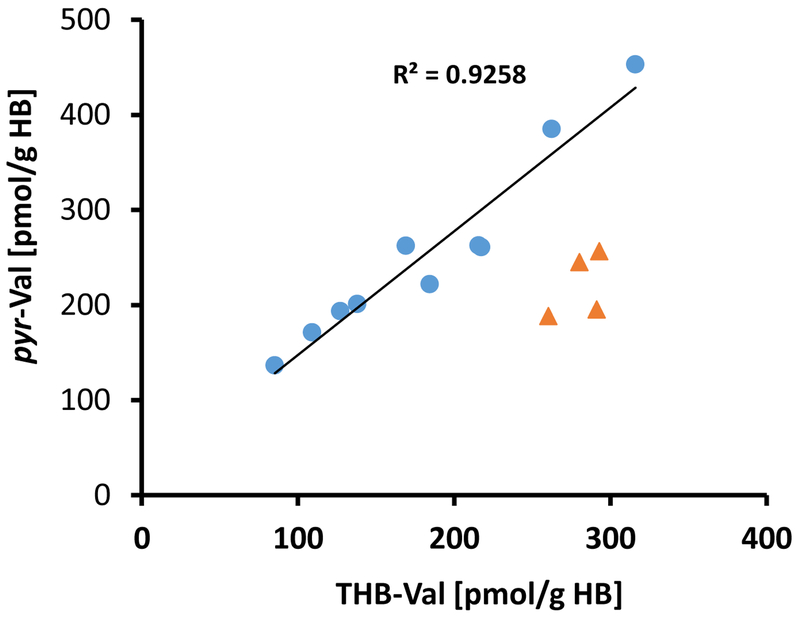
Treosulfan treatment lead to a significant increase of pyr-Val and THB-Val adducts in each patient (Student t-test p<0.0001) (Table 3, Figure 3). The mean ± SD amounts of adduct formed were 245 ± 89.6 and 210.63 ± 78.5 pmol/g globin for pyr-Val and THB-Val, respectively (Table 3). The scatter plot of pyr-Val versus THB-Val shows a strong correlation in 10 of the 14 patients with a Pearson correlation coefficient of R2=0.920 (Figure 4). HB-Val adducts were not expected to be from treosulfan and their amounts were below the limit of detection.
Table 3:
Amount of N-terminal valine adducts (pmol/g globin) before and after 6 days of Treosulfan treatment.
| Subject ID |
Day 0 | Day 6 | ||||
|---|---|---|---|---|---|---|
| pyr-Val | THB-Val |
pyr-Val THB-Val |
pyr-Val | THB-Val |
pyr-Val THB-Val |
|
| #01 | 0.41 | 4.61 | 0.09 | 262.4 | 169.0 | 1.55 |
| #02 | 0.15 | 4.58 | 0.03 | 193.7 | 127.2 | 1.52 |
| #03 | 0.25 | 1.72 | 0.14 | 201.3 | 138.0 | 1.46 |
| #04 | 0.21 | 4.03 | 0.05 | 385.3 | 262.4 | 1.47 |
| #05 | 0.14 | 4.38 | 0.03 | 453.1 | 316.0 | 1.43 |
| #06 | 0.23 | 5.22 | 0.04 | 171.5 | 109.0 | 1.57 |
| #08 | 0.42 | 4.08 | 0.10 | 260.9 | 217.3 | 1.20 |
| #10 | 0.25 | 5.24 | 0.05 | 136.6 | 85.1 | 1.61 |
| #13 | 0.41 | 7.95 | 0.05 | 262.7 | 215.7 | 1.22 |
| #14 | 0.58 | 5.73 | 0.10 | 221.9 | 184.4 | 1.20 |
| #07* | 0.32 | 6.65 | 0.05 | 188.4 | 260.4 | 0.72 |
| #09* | 0.22 | 5.58 | 0.04 | 256.3 | 292.9 | 0.88 |
| #11* | NA | NA | 195.2 | 291.4 | 0.67 | |
| #12* | 0.25 | 7.38 | 0.03 | 244.9 | 280.2 | 0.87 |
| mean | 0.29 | 5.17 | 245.31 | 210.63 | ||
| SD | 0.10 | 1.7 | 89.6 | 78.5 | ||
Globin was isolate and analyzed by LC/MS/MS. Day 0 samples were taken before the first dose of treosulfan and day 6 samples were taken after 6 days of treatment.
Samples were not included in correlation analysis because their pyr-Val/THB-Val radios at day 6 were significantly different form that of the others (Student t-test, mean±SD 1.39±.15 vs 0.77±.10, p<0.0001).
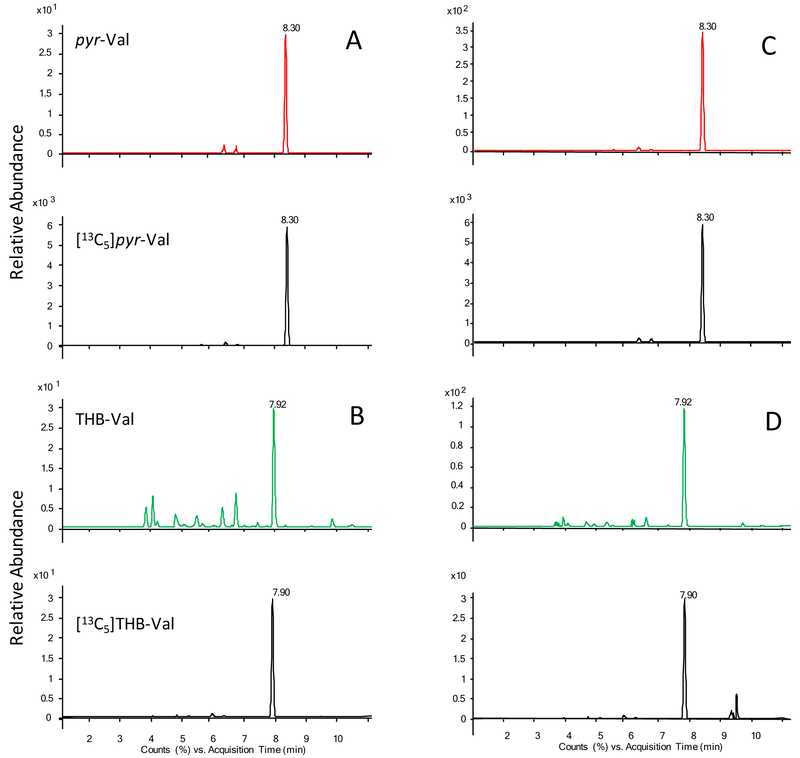
Figure 3:
Representative extracted ion-chromatogram from pyr-Val and THB-val from a patient before (A & B) and after (C & D) treosulfan therapy. Shown are the relative abundance of the ion intensities over time. Note the different scales of the y-axes for sample before and after treosulfan treatment.
Figure 4:
Correlation between pyr-Val and THB-Val formation after 6 days of treosulfan therapy. The mean pyr-Val/THB-Val ratio was 1.22 with a correlation efficient of 1.35 and R2 = 0.920. Patients #07, #09, #11 and #13 (orange triangles) were excluded from the correlation analysis because they appear to have different metabolism producing significantly different pyr-Val/THB-Val (Student t-test, mean±SD 1.39±.15 vs. mean±SD 0.77±.10, p<0.0001).
4. Discussion
Treosulfan is usually given at a fixed dose. Adjusting treatment doses according to pharmacokinetic parameters may increase efficacy and limit toxicity.
4.1. Method validation.
Before application to clinical specimens, the simplified procedure was evaluated using (i) alkylated peptide standards and (ii) globin reacted in vitro with 1,2-epoxybutene, DEB and EB-diol and (iii) repetitive analysis of human globin (Table 2). The accuracy and precision of the simplified procedure was expected based on previous methods measuring native and modified peptides by tandem mass spectrometry.5,11,13 The Poroshell column has unique longitudinal diffusion characteristics where small molecules, such as peptides, enter the porous silica particles and interact with the C¬18 within the particle and are retained.24 In contrast, larger impurities such as undigested proteins bypass the particle on the outside and avoid interaction with the C-18 surfaces and therefore are less retained.24 Additionally, utilizing the 4.6 mm ID column allows for larger sample loading, which in turn enables injection of 1 mg of tryptic digestion, while maintaining separation. Using heat-assisted electrospray ionization overcomes the loss of sensitivity seen in ESI mass spectrometry. Overall, combining these technical features produced a methodology with sufficient sensitivity for analyses of clinical specimens.
4.2. Application to treosulfan-treated patients
Treosulfan is a member of the alkylating family of pharmaceuticals that, under physiological conditions, spontaneously decomposes to DEB, a very reactive alkylating agent. As expected Treosulfan treatment produces to a significant increase and fairly consistent amount of pyr-Val. This was expected since treosulfan instantly decomposes to the reactive metabolites capable of adduct formation. The lack of inter-individual variability demonstrates that the treatment produces a constant internal dose. This is most likely because treosulfan spontaneously converts to DEB and instantly binds to hemoglobin without requiring enzymatic activation similar to what has been observed in in vitro reactions.12,25 Amounts of THB-Val were similar when compared to pyr-Val, suggesting a constant capacity of humans to detoxify the treosulfan metabolite DEB. The observation of THB-Val adducts being approximately equal to the amounts of pyr-Val demonstrates high efficiency and activity of epoxide hydrolase in these patients. Four patients (#07,#09,#11 and #12) seem to have even greater epoxide hydrolase activity than the majority (n=10) of patients studied, leading to a relatively lower pyr-Val/THB Val ratio compared to the other patients. This difference in metabolic phenotype was not observed on day 0, suggesting that background exposures were insufficient to produce specific metabolic responses. Whether this unique metabolic phenotype is derived from genetic background, environmental factors or lifestyle needs to be determined. EB-diol is a less reactive and toxic metabolite and it remains uncertain whether or not the differences in metabolism in these patients are clinically relevant.26
Surprisingly, the simplified method reliably detected pre-treatment background amounts for pyr-Val and THB-Val (before treatment). The pyr-Val background value of 0.4 ± 0.02 pmol/g HB is slightly higher than reported previously by our group,16 but falls within the reported range found in the literature (0–95 pmol/g HB)11. The mean ± SD THB-Val background was 5.17 ± 1.7 pmol/g HB and was in good agreement with previously reported background.16,27 Together these results suggest that environmental exposure to BD is similar to exposures reported for other populations. At the beginning, we were not expecting detection of background amounts, but these have been found routinely by us and others (unpublished data) as a result of improved sensitivity of LC/MS/MS systems11.
Importantly, these results show that this simplified method can quantitate environmental background and treosulfan-induced pyr-Val and THB-Val N-terminal valine globin adducts in humans. Utilization of the Poroshell column technology enables adduct analysis without the need of adduct enrichment such by the FIRE assay, immunoaffinity purification or modified Edman degradation and gas chromatography.16,19,28–32 The simplicity of the applied method suggests that it can be expanded to unbiased adduct profiling, using the next generation of more sensitive and specific mass spectrometers.33–35
Acknowledgements
Support has been provided in part by NIH/NIEHS 1R21ES019684 and the Arkansas Bioscience Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
Abbreviations
- BD
1,3-butadiene
- DEB
1,2:3,4-diepoxybutane
- EB-diol
1,2-epoxy-3,4-butanediol
- GVHD
graft-versus-host disease
- HB-Val
N-(2-hydroxy-3-buten-1-yl)-valine
- HB
hemoglobin
- HPLC
high performance liquid chromatography
- LC/MS/MS
Liquid Chromatography-Tandem Mass Spectrometry
- MRM
multiple reaction monitoring
- pyr-Val
N,N-(2,3-dihydroxy-1,4-butadiyl)-valine
- THB-Val
2,3,4-trihydroxybutyl-valine
References
- 1.Treosulphan Ashby J., myleran and butadiene: similarities and differences. Mutat Res. 1993;300(2):77–78. http://www.ncbi.nlm.nih.gov/pubmed/7685496. [DOI] [PubMed] [Google Scholar]
- 2.Zhu S, Zeiger E. Mutagenicity of the human carcinogen treosulphan, and its hydrolysis product, dl-1,2:3,4-diepoxybutane in mammalian cells. Environ Mol Mutagen. 1993;21(1):95–99. http://www.ncbi.nlm.nih.gov/pubmed/8419160. [DOI] [PubMed] [Google Scholar]
- 3.Galaup A, Paci A. Pharmacology of dimethanesulfonate alkylating agents: busulfan and treosulfan. Expert Opin Drug Metab Toxicol. 2013;9(3):333–347. doi: 10.1517/17425255.2013.737319 [DOI] [PubMed] [Google Scholar]
- 4.Wen Y, Zhang P-P, An J, et al. Diepoxybutane induces the formation of DNA-DNA rather than DNA-protein cross-links, and single-strand breaks and alkali-labile sites in human hepatocyte L02 cells. Mutat Res. 2011;716(1–2):84–91. doi: 10.1016/j.mrfmmm.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 5.Møller P, Knudsen LE, Loft S, Wallin H. The comet assay as a rapid test in biomonitoring occupational exposure to DNA-damaging agents and effect of confounding factors. Cancer Epidemiol Biomarkers Prev. 2000;9(10):1005–1015. http://www.ncbi.nlm.nih.gov/pubmed/11045781. [PubMed] [Google Scholar]
- 6.Swenberg JA, Fryar-Tita E, Jeong Y-C, et al. Biomarkers in Toxicology and Risk Assessment: Informing Critical Dose–Response Relationships. Chem Res Toxicol. 2008;21(1):253–265. doi: 10.1021/tx700408t [DOI] [PubMed] [Google Scholar]
- 7.Boysen G, Georgieva NI, Upton PB, Walker VE, Swenberg JA. N-terminal globin adducts as biomarkers for formation of butadiene derived epoxides. Chem Biol Interact. 2007;166(1–3):84–92. doi: 10.1016/j.cbi.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 8.Boysen G, Georgieva NI, Upton PB, et al. Analysis of Diepoxide-Specific Cyclic N -Terminal Globin Adducts in Mice and Rats after Inhalation Exposure to 1, 3-Butadiene. Cancer Res. 2004;64:8517–8520. doi: 10.1158/0008-5472.CAN-04-3184 [DOI] [PubMed] [Google Scholar]
- 9.Sun JD, Dahl AR, Bond JA, Birnbaum LS, Henderson RF. Characterization of hemoglobin adduct formation in mice and rats after administration of [14C]butadiene or [14C]isoprene. Toxicol Appl Pharmacol. 1989;100(1):86–95. http://www.ncbi.nlm.nih.gov/pubmed/2763304. [DOI] [PubMed] [Google Scholar]
- 10.Osterman-Golkar S, Ehrenberg L. Covalent binding of reactive intermediates to hemoglobin as an approach for determining the metabolic activation of chemicals--ethylene. Drug Metab Rev. 1982;13(4):647–660. doi: 10.3109/03602538209011090 [DOI] [PubMed] [Google Scholar]
- 11.Rubino FM, Pitton M, Di Fabio D, Colombi A. Toward an “omic” physiopathology of reactive chemicals: thirty years of mass spectrometric study of the protein adducts with endogenous and xenobiotic compounds. Mass Spectrom Rev. 2009;28(5):725–784. doi: 10.1002/mas.20207 [DOI] [PubMed] [Google Scholar]
- 12.Rydberg P, Magnusson AL, Zorcec V, Granath F, Törnqvist M. Adducts to N-terminal valines in hemoglobin from butadiene metabolites. Chem Biol Interact. 1996;101(0009–2797):193–205. file:///C:/Boysen/literature/papers/ChemBioInter_101_193.pdf. [DOI] [PubMed] [Google Scholar]
- 13.von Stedingk H, Rydberg P, Törnqvist M. A new modified Edman procedure for analysis of N-terminal valine adducts in hemoglobin by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(27):2483–2490. doi: 10.1016/j.jchromb.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 14.von Stedingk H, Vikström AC, Rydberg P, et al. Analysis of hemoglobin adducts from acrylamide, glycidamide, and ethylene oxide in paired mother/cord blood samples from Denmark. Chem Res Toxicol. 2011;24(11):1957–1965. doi: 10.1021/tx200284u [DOI] [PubMed] [Google Scholar]
- 15.Boysen G, Georgieva NI, Upton PB, Walker VE, Swenberg JA. N-terminal globin adducts as biomarkers for formation of butadiene derived epoxides. Chem Biol Interact. 2007;166(1–3):84–92. doi: 10.1016/j.cbi.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 16.Boysen G, Georgieva NI, Bordeerat NK, et al. Formation of 1,2: 3,4-diepoxybutane-specific hemoglobin adducts in 1,3-butadiene exposed workers. Toxicol Sci. 2012;125(1):30–40. doi: 10.1093/toxsci/kfr272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preston RJ. Cancer risk assessment for 1,3-butadiene: data integration opportunities. Chem Biol Interact. 2007;166(1–3):150–155. doi: 10.1016/j.cbi.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 18.Swenberg JA, Bordeerat NK, Boysen G, et al. 1,3-Butadiene: Biomarkers and application to risk assessment. Chem Biol Interact. 2011;192(1–2):150–154. doi: 10.1016/j.cbi.2010.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goel S, Evans-Johnson JA, Georgieva NI, Boysen G. Exposure profiling of reactive compounds in complex mixtures. Toxicology. 2013;313(2–3):145–150. doi: 10.1016/j.tox.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bordeerat NK, Georgieva NI, Klapper DG, et al. Accurate quantitation of standard peptides used for quantitative proteomics. Proteomics. 2009;9(15):3939–3944. doi: 10.1002/pmic.200900043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danylesko I, Shimoni A, Nagler A. Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike. Bone Marrow Transplant. 2012;47(1):5–14. doi: 10.1038/bmt.2011.88 [DOI] [PubMed] [Google Scholar]
- 22.Shimoni A, Shem-Tov N, Volchek Y, Danylesko I, Yerushalmi R, Nagler A. Allo-SCT for AML and MDS with treosulfan compared with BU-based regimens: reduced toxicity vs reduced intensity. Bone Marrow Transplant. 2012;47(10):1274–1282. doi: 10.1038/bmt.2012.4 [DOI] [PubMed] [Google Scholar]
- 23.Mowrer J, Törnqvist M, Jensen S, Ehrenberg L. Modified edman degradation applied to hemoglobin for monitoring occupational exposure to alkylating-agents. Toxicol Environ Chem. 1986;11(3):215–231. isi:A1986D090400005. [Google Scholar]
- 24.Gritti F, Guiochon G. Repeatability of the efficiency of columns packed with sub-3μm core-shell particles: Part III. 2.7μm Poroshell 120 EC-C18 particles in 4.6mm and 2.1mm × 100mm column formats. J Chromatogr A. 2012;1252:56–66. doi: 10.1016/j.chroma.2012.05.080 [DOI] [PubMed] [Google Scholar]
- 25.Basile A, Ferranti P, Pocsfalvi G, et al. A novel approach for identification and measurement of hemoglobin adducts with 1,2,3,4-diepoxybutane by liquid chromatography/electrospray ionisation mass spectrometry and matrix-assisted laser desorption/ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom. 2001;15(0951–4198):527–540. [DOI] [PubMed] [Google Scholar]
- 26.Turner CR, Losasso TJ, Muzzi DA, Weglinski MR. Brain relaxation and cerebrospinal fluid pressure during craniotomy for resection of supratentorial mass lesions. J Neurosurg Anesthesiol. 1996;8(2):126–132. http://www.ncbi.nlm.nih.gov/pubmed/8829559. [DOI] [PubMed] [Google Scholar]
- 27.van Sittert NJ, Megens HJJJ, Watson WP, Boogaard PJ. Biomarkers of Exposure to 1,3-Butadiene as a Basis for Cancer Risk Assessment. Toxicol Sci. 2000;56(1):189–202. http://toxsci.oxfordjournals.org/cgi/content/abstract/56/1/189. [DOI] [PubMed] [Google Scholar]
- 28.Carlsson H, Törnqvist M. An Adductomic Approach to Identify Electrophiles In Vivo. Basic Clin Pharmacol Toxicol. November 2016. doi: 10.1111/bcpt.12715 [DOI] [PubMed] [Google Scholar]
- 29.Carlsson H, Motwani HV, Osterman Golkar S, Törnqvist M. Characterization of a Hemoglobin Adduct from Ethyl Vinyl Ketone Detected in Human Blood Samples. Chem Res Toxicol. 2015;28(11):2120–2129. doi: 10.1021/acs.chemrestox.5b00287 [DOI] [PubMed] [Google Scholar]
- 30.Georgieva NI, Boysen G, Bordeerat N, Walker VE, Swenberg JA. Exposure-response of 1,2:3,4-diepoxybutane-specific N-terminal valine adducts in mice and rats after inhalation exposure to 1,3-butadiene. Toxicol Sci. 2010;115(2):322–329. doi: 10.1093/toxsci/kfq060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Sittert NJ, Boogaard PJ, Natarajan AT, Tates AD, Ehrenberg LG, Tornqvist MA. Formation of DNA adducts and induction of mutagenic effects in rats following 4 weeks inhalation exposure to ethylene oxide as a basis for cancer risk assessment. MutatRes. 2000;447(0027–5107 (Print)):27–48. [DOI] [PubMed] [Google Scholar]
- 32.Törnqvist M, Kautiainen A. Adducted proteins for identification of endogenous electrophiles. Environ Health Perspect. 1993;99:39–44. http://www.ncbi.nlm.nih.gov/pubmed/8319656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rappaport SM. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol. 2010;21(1):5–9. doi: 10.1038/jes.2010.50 [DOI] [PubMed] [Google Scholar]
- 34.Balbo S, Turesky RJ, Villalta PW. DNA adductomics. Chem Res Toxicol. 2014;27(3):356–366. doi: 10.1021/tx4004352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwa Yun B, Guo J, Bellamri M, Turesky RJ. DNA adducts: Formation, biological effects, and new biospecimens for mass spectrometric measurements in humans. Mass Spectrom Rev. June 2018. doi: 10.1002/mas.21570 [DOI] [PMC free article] [PubMed] [Google Scholar]