Abstract
Objective:
There is accumulating evidence that complement activation is important in ANCA vasculitis pathogenesis. Our objective was to investigate complement activation in MPO-ANCA and PR3-ANCA vasculitis after determining optimal methods for measuring activated complement factors in circulation.
Methods:
Subjects included 98 patients with ANCA vasculitis (45 MPO-ANCA, 53 PR3-ANCA) and 35 healthy controls. Plasma was obtained from blood collected in EDTA tubes including no or 100 μg/mL futhan. Bb, C3a, C5a, sC5b-9, properdin, and C4d levels were measured by ELISA. Group comparisons were made using Wilcoxon two-sample test. Paired data were analyzed with paired signed-rank test.
Results:
Compared to healthy controls, C3a (p<0.0001), C5a (p=0.0004), and sC5b-9 (p=0.0007) levels were higher in active MPO-ANCA vasculitis; Bb (p=0.001), C3a (p<0.0001), and sC5b-9 (p=0.003) were also higher in remission. Compared to healthy controls, C3a (p<0.0001), C5a (p=0.002), sC5b-9 (p=0.0001), and C4d (p=0.005) levels were higher in active PR3-ANCA vasculitis; C3a (p<0.0001) and C4d (p=0.007) were also higher in remission. While the groups of patients with active disease and remission did not differ significantly in any complement analyte for either ANCA serotype, significant differences were detected in sC5b-9 comparing paired active and remission disease samples within patients. C5a was significantly lower among patients in long-term remission off therapy. For Bb, C5a, and sC5b-9, median levels and individual values were considerably higher for control and patient samples processed without futhan compared to with futhan.
Conclusions:
Complement activation occurs in both MPO-ANCA and PR3-ANCA vasculitis. The complement activation profile differs by disease activity and possibly ANCA serotype. Futhan reduces in vitro complement activation and provides a more accurate measurement.
Keywords: ANCA vasculitis, MPO-ANCA, PR3-ANCA, complement activation, futhan
INTRODUCTION
Emerging evidence supports a pivotal role of complement activation in the pathogenesis of antineutrophil cytoplasmic antibody (ANCA) vasculitis. This evidence represents a substantial shift in the understanding of complement’s participation in ANCA vasculitis. The complement system was long thought not to have a significant role in ANCA vasculitis pathogenesis because there is typically little immunoglobulin or complement deposition at sites of tissue injury.1 Studies in murine models of vasculitis, however, have established that activation of complement is critical in disease pathogenesis.2
In an experimental mouse model of ANCA vasculitis, intravenous injection of mouse anti-myeloperoxidase (MPO) IgG antibody induces a pauci-immune necrotizing and crescentic glomerulonephritis (NCGN) and vasculitis similar to human ANCA disease.3 Transfer of anti-MPO IgG to mice deficient in C4, a component of the classical and lectin complement pathways, produced a pauci-immune NCGN similar to wild-type mice. Mice deficient in alternative complement pathway component Factor B, however, were protected from developing disease.3,4 Mice deficient in C5, which is required for terminal activation in all three complement pathways, and C5a receptor (C5aR) also did not develop disease.4,5 Blockade of the C5aR with a small molecule antagonist also ameliorated disease severity, suggesting C5a-C5aR engagement is critical for disease induction.6 Complement-mediated injury appears to be independent of the membrane attack complex because mice deficient in C6 developed disease no different from wild-type mice.6 The results from these key studies established that complement activation, specifically through the alternative pathway, is crucial in the development of anti-MPO-mediated vasculitis in mice.
Alternative complement pathway activation also appears important in human ANCA vasculitis. In a cohort of patients with perinuclear ANCA (P-ANCA) and MPO-ANCA vasculitis, plasma levels of complement activation fragments Bb, C3a, C5a, and soluble C5b-9 (sC5b-9) were significantly higher and properdin significantly lower in patients with active disease compared to remission.7 Plasma Bb levels also correlated with Birmingham Vasculitis Activity Score (BVAS) and proportion of cellular crescents on kidney biopsy.7 Moreover, urinary levels of Bb, C3a, C5a, and sC5b-9 were significantly higher in active disease compared to remission.8,9 Bb deposition was also detectable in the glomeruli and correlated with cellular crescents, tubular atrophy, interstitial infiltration, and interstitial fibrosis.8 It is important to note that these prior studies involved patients exclusively with MPO-ANCA vasculitis and glomerulonephritis. Complement activation has yet to be fully characterized in patients with proteinase 3 (PR3)-ANCA vasculitis or without renal disease.
Accumulating observations indicate that complement activation is not only important in ANCA vasculitis pathogenesis, but also other autoimmune conditions.10 As the role of complement activation is explored further in various disorders, standardization of plasma collection and processing methods is essential. Standardization is critical because complement activation products can be rapidly generated in vitro.11 An exhaustive study by Yang, et al. demonstrated that plasma collected with ethylenediaminetetraacetic acid (EDTA) is superior to serum and citrate plasma samples when a variety of pre-analytical variables were tested, including storage of blood samples prior to processing at room temperature or 4°C and stability during thawing of stored plasma samples.12 In the 3 healthy individuals examined, Yang et al. found substantial increases in measures of Bb, C3a, C5a, C5b-9, and C4d when serum or citrated plasma was thawed at 37°C. Conversely, even when thawed at 37⁰C, plasma collected with EDTA remained stable for up to 60 minutes except for measures of C4d.12 Other investigators have shown that cleavage of complement proteins occurs in vitro despite collecting blood with EDTA.13,14 Careful and systematic sample processing to avoid in vitro complement activation is therefore required for more accurate measurement. Futhan, a broad-specificity protease inhibitor, blocks in vitro complement activation.13,15 Futhan has been shown to inhibit the esterolytic activities of C1r, C1s, and factor B and hemolytic activities mediated by both the classical and alternative complement pathways.16-18 Futhan’s inhibitory activity is thought to be mediated by binding to catalytic sites of the serine proteases of the complement system.19,20 The addition of futhan in combination with EDTA can therefore reduce in vitro complement activation and improve analytical testing.21,22
The primary objective of our study was to investigate complement activation in both MPO-ANCA and PR3-ANCA vasculitis. A secondary objective was to determine the effects of futhan on accurately measuring complement activation in patients with ANCA vasculitis and healthy controls.
PATIENTS AND METHODS
Patient Cohort
Patients with ANCA vasculitis were diagnosed according to the Chapel Hill Consensus Conference definitions.23,24 Active disease was defined as a BVAS>3 with supporting clinical and/or laboratory features obtained through extensive chart review.25,26 Disease remission was defined as a BVAS=0 without disease activity within 3 months of sample procurement. Patients were considered in long-term remission off therapy (LTROT) if they had been therapy-free and without clinical signs of disease activity for ≥ 2 years. For patients treated with rituximab, remission off therapy status did not start until evidence of B-cell repopulation or > 1 year from last rituximab dose, whichever occurred sooner. A sample was excluded if the subject was dialysis-dependent, had undergone plasmapheresis, received rituximab, or had an infection within 3 months of procurement. Patients were excluded if they had eosinophilic granulomatosis with polyangiitis, drug-induced ANCA vasculitis, were ANCA negative by enzyme-linked immunosorbent assay (ELISA), received a kidney transplant, or had an overlapping autoimmune disease. Informed consent was obtained in accordance with the University of North Carolina’s Institutional Review Board guidelines.
Sample acquisition
Samples were collected from patients with MPO-ANCA and PR3-ANCA vasculitis as well as healthy controls from September 2009 to October 2017. Blood collected in EDTA tubes was placed on ice immediately and processed at 4°C within 30 minutes. Plasma was obtained from blood including no or 100 μg/mL of futhan (ApexBio Technology, Houston, TX) by centrifugation at 1,244 g for 15 minutes twice at 4°C. Samples were maintained on ice throughout processing and stored in aliquots at −80°C until use. For testing, aliquoted samples were rapidly thawed at 37°C and transferred immediately to ice. Repeat freeze and thaw cycles were completely avoided.
Quantification of plasma complement activation products
Plasma levels of circulating complement activation products were measured by ELISA and included properdin (Hycult Biotech, Plymouth Meeting, PA) and Bb, C3a, C5a, sC5b-9, and C4d (Quidel, San Diego, CA). All assays were performed according to the manufacturers’ instructions and included negative and positive controls. Each sample was tested in duplicate. ELISAs were analyzed with GraphPad Prism or Microsoft Excel using the appropriate curve-fitting equation. Each ELISA assay had the following quality control processes: (1) sample duplicates not within 20% of each other were excluded and retested; (2) r2 value and y-intercept had to fall within the recommended range for the standard curve; (3) high and low controls were required to be within the recommended range as set forth in the certificate of analysis; (4) values above the highest standard were retested at a higher dilution to fall on the standard curve. Samples and assays that did not meet quality control standards were repeated.
Statistical analyses
Descriptive statistics were used for demographic and clinical characteristics. Estimated glomerular filtration rate (eGFR) was calculated using the 4-variable Modification of Diet in Renal Disease equation.27 For patients with paired samples obtained during active disease and remission, only the active disease sample was used for analyses to comply with the assumption of independent samples. Data were not normally distributed, therefore, medians and IQRs are reported. Paired samples simultaneously processed with and without futhan were used to examine the effects of futhan. Samples processed with futhan were used for analyses because this improved the accurate measurement of complement activation products. The Wilcoxon two-sample test was used for two group comparisons. Bonferroni correction was applied for multiple comparisons of complement activation measures in healthy controls and patients with MPO-ANCA and PR3-ANCA vasculitis, and a p-value <0.0083 was considered statistically significant. Paired signed-rank test was used to assess differences in complement activation between paired active and remission samples and paired samples processed with and without futhan. A p-value of <0.05 was considered statistically significant. The association between complement activation and BVAS was measured with Spearman’s rank correlation. Analyses were done using SAS v9.4 statistical software (SAS Institute, Inc. Cary, NC, USA). Graphs were created using GraphPad Prism 7 (GraphPad Software, Inc. La Jolla, CA, USA).
RESULTS
Demographic and clinical data
We measured complement activation in 98 patients with ANCA vasculitis (45 MPO-ANCA, 53 PR3-ANCA) and 35 healthy controls (Table 1). Among the 98 patients with ANCA vasculitis, 43 (21 MPO-ANCA, 22 PR3-ANCA) had active disease and 55 (24 MPO-ANCA, 31 PR3-ANCA) were in remission. There were 10 patients (3 MPO-ANCA, 7 PR3-ANCA) with paired samples obtained during disease activity and remission, and 8 patients (3 MPO-ANCA, 5 PR3-ANCA) who achieved LTROT status.
Table 1.
Main demographic and clinical characteristics.
| Characteristic | Healthy Control |
MPO-ANCA Vasculitis |
PR3-ANCA Vasculitis |
Paired Samples |
|---|---|---|---|---|
| N=35 | N=45 | N=53 | N=14 | |
| Male (N, %) | 19 (54%) | 21 (47%) | 32 (60%) | 5 (36%) |
| Female (N, %) | 16 (46%) | 24 (53%) | 21 (40%) | 9 (64%) |
| Race/ethnicity (N, %) | ||||
| Caucasian | 31 (88.5%) | 35 (78%) | 45 (85%) | 12 (86%) |
| African American | 1 (3%) | 7 (15%) | 4 (7.5%) | 1 (7%) |
| Asian/Hispanic/Other | 3 (8.5%) | 3 (7%) | 4 (7.5%) | 1 (7%) |
| Age at time of sample (years) (Median, IQR) | 51 (36, 59) | 64 (58, 74) | 56 (42, 68) | 53 (45, 61) |
| Age at disease diagnosis (years) (Median, IQR) | 59 (50, 70) | 49 (36, 61) | 48 (38, 58) | |
| Active (N, %) | 21 (47%) | 22 (42%) | ||
| Remission (N, %) | 24 (53%) | 31 (58%) | ||
| Disease subtype (N, %) | ||||
| Renal-limited | 19 (42%) | 4 (7%) | 3 (21.5%) | |
| Microscopic polyangiitis | 21 (47%) | 19 (36%) | 4 (28.5%) | |
| Granulomatosis with polyangiitis | 5 (11%) | 30 (57%) | 7 (50%) | |
| Disease manifestations at diagnosis (N, %) | ||||
| Upper respiratory | 14 (31%) | 35 (66%) | 10 (71%) | |
| Lower respiratory | 10 (22%) | 39 (74%) | 10 (71%) | |
| Kidney | 42 (93%) | 44 (83%) | 12 (86%) | |
| Dermatologic | 5 (11%) | 16 (30%) | 3 (21%) | |
| Musculoskeletal | 12 (27%) | 29 (55%) | 5 (36%) | |
| Gastrointestinal | 0 (0%) | 2 (4%) | 0 (0%) | |
| Neurologic | 2 (4%) | 11 (21%) | 3 (21%) | |
| eGFR (mL/min/1.73m2) (Median, IQR) | ||||
| Active | 24.57 (15.46, 40.35) | 58.53 (34.54, 79.03) | 72.12 (56.22, 80.49) | |
| Remission | 54.12 (36.31, 69.32) | 65.89 (50.27, 81.60)* | 67.59 (53.41, 79.16) |
1 PR3-ANCA sample did not have data available for eGFR calculation. ANCA, antineutrophil cytoplasmic antibody; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MPO, myeloperoxidase; PR3, proteinase 3.
There were no significant differences in gender or race/ethnicity between healthy controls and patients with MPO-ANCA and PR3-ANCA vasculitis (supplementary Table S1). Patients with MPO-ANCA vasculitis were older at diagnosis and time of sample acquisition compared to those with PR3-ANCA vasculitis, but this observation is likely of little impact since we found that complement activation does not correlate with age (supplementary Figure S1A-F). As expected, a higher proportion of PR3-ANCA vasculitis had granulomatosis with polyangiitis (GPA) with more upper and lower respiratory tract involvement compared to those with MPO-ANCA vasculitis (supplementary Table S1).28-31 While the proportions of patients with kidney involvement were comparable, those with MPO-ANCA vasculitis had renal-limited disease more commonly and a significantly lower eGFR during active disease compared to those with PR3-ANCA vasculitis (supplementary Table S1).
Effect of futhan on accurately measuring complement activation in vitro
The addition of futhan improved the accurate measurement of complement activation. Using a paired signed-rank test, levels of Bb, C5a, and sC5b-9 significantly differed between samples processed with and without futhan (all p<0.005). Median levels of Bb, C5a, and sC5b-9 were higher in samples processed without futhan compared to paired samples with futhan (supplementary Table S2 and Figure S2A-C). For Bb, measurements were notably higher for 100% of samples processed without futhan compared to the paired sample with futhan. In addition, the value without futhan was >20% of that with futhan for 60% of samples. For C5a and sC5b-9, measurements were also notably higher for 61% and 67% of samples processed without futhan compared to the paired sample with futhan, respectively. Given the considerable elevation in values for samples processed without futhan, we used samples processed with futhan for analyses. For completeness, we performed analyses on samples processed without futhan (supplementary Table S2 and Figure S3A-F).
Complement activation in ANCA vasculitis
We found that complement activation occurs in both MPO-ANCA and PR3-ANCA vasculitis (Table 2, Figure 1A-F). Compared to healthy controls, patients with active MPO-ANCA vasculitis had significantly higher plasma levels of C3a (29.54 vs. 67.77 ng/mL, p<0.0001), C5a (5.50 vs. 9.47 ng/mL, p=0.0004), and sC5b-9 (114.90 vs. 166.99 ng/mL, p=0.0007). Compared to healthy controls, patients with MPO-ANCA vasculitis in remission also had higher plasma levels of Bb (0.62 vs. 0.74 μg/mL, p=0.001), C3a (29.54 vs. 71.58 ng/mL, p<0.0001), and sC5b-9 (114.90 vs. 149.94 ng/mL, p=0.003). Compared to healthy controls, patients with active PR3-ANCA vasculitis had significantly higher plasma levels of C3a (29.54 vs. 75.56 ng/mL, p<0.0001), C5a (5.50 vs. 9.74 ng/mL, p=0.002), and sC5b-9 (114.90 vs. 152.59 ng/mL, p=0.0001). Compared to healthy controls, patients with PR3-ANCA vasculitis in remission also had higher plasma levels of C3a (29.54 vs. 52.80 ng/mL, p<0.0001). Interestingly, compared to healthy controls, patients with PR3-ANCA vasculitis had higher plasma levels of C4d during active disease (0.94 vs. 1.87 μg/mL, p=0.005) and remission (2.02 μg/mL, p=0.007). The active disease group and remission group did not differ significantly for any complement analyte among patients with either ANCA serotype. No significant differences in properdin were detected between any groups.
Table 2.
Measuring complement activation with futhan in healthy controls and MPO-ANCA and PR3-ANCA vasculitis.
| Median (IQR) Number |
Healthy Control |
MPO-ANCA Active |
MPO-ANCA Remission |
PR3-ANCA Active |
PR3-ANCA Remission |
|---|---|---|---|---|---|
|
Bb (μg/mL) |
0.62 (0.49, 0.65) N=26 |
0.66 (0.48, 0.79) N=14 |
0.74‡ (0.64, 0.90) N=21 |
0.58 (0.50, 0.79) N=19 |
0.65 (0.58, 0.85) N=25 |
|
Properdin (μg/mL) |
14.62 (13.16, 17.30) N=22 |
15.83 (13.37, 21.89) N=14 |
17.40 (13.75, 20.59) N=21 |
15.71 (14.26, 19.60) N=19 |
14.89 (13.00, 18.16) N=25 |
|
C3a (ng/mL) |
29.54 (23.81, 38.22) N=26 |
67.77‡ (42.60, 93.49) N=14 |
71.58‡ (45.84, 107.60) N=21 |
75.56‡ (50.84, 126.20) N=19 |
52.80‡ (44.47, 67.83) N=25 |
|
C5a (ng/mL) |
5.50 (4.05, 7.53) N=26 |
9.47‡ (8.41, 12.95) N=13 |
8.07 (5.67, 9.42) N=21 |
9.74‡ (6.42, 15.52) N=18 |
7.14 (5.29, 8.50) N=25 |
|
sC5b-9 (ng/mL) |
114.90 (101.50, 135.30) N=25 |
166.99‡ (131.44, 217.96) N=14 |
149.94‡ (128.62, 181.51) N=21 |
152.59‡ (137.52, 266.42) N=19 |
143.06 (119.13, 173.58) N=25 |
|
C4d (μg/mL) |
0.94 (0.49, 1.55) N=19 |
2.06 (1.40, 3.24) N=14 |
1.56 (0.68, 2.66) N=18 |
1.87‡ (1.41, 3.77) N=18 |
2.02‡ (1.17, 2.91) N=18 |
ANCA, antineutrophil cytoplasmic antibody; IQR, interquartile range; MPO, myeloperoxidase; PR3, proteinase 3.
Significantly different from healthy control.
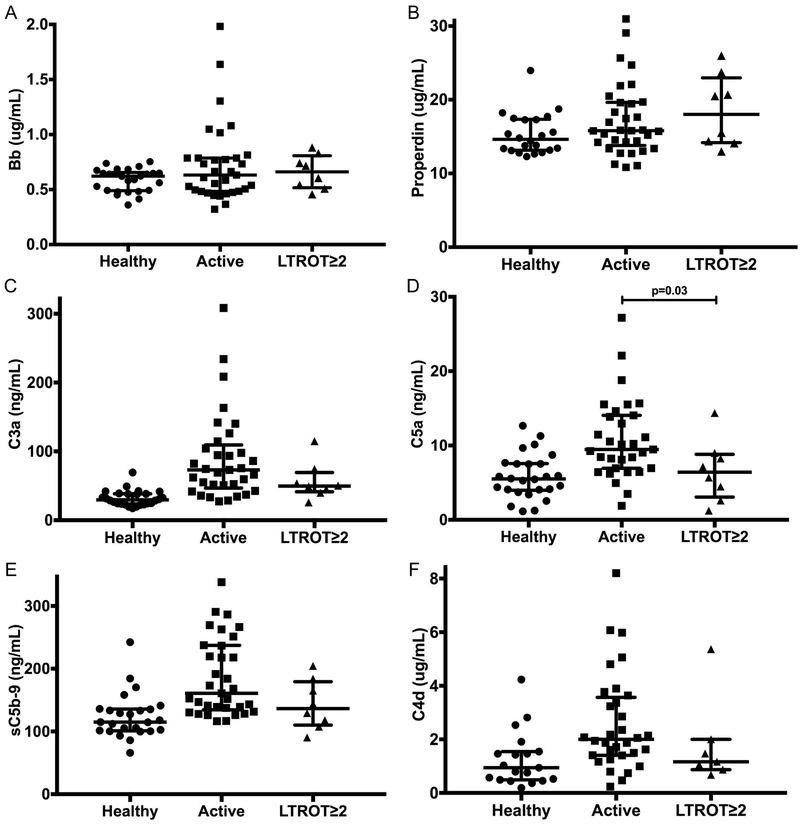
Figure 1A-F.
Measuring complement activation with futhan in healthy controls and MPO-ANCA and PR3-ANCA vasculitis. Bb (A), properdin (B), C3a (C), C5a (D), sC5b-9 (E), C4d (F). Horizontal lines represent median values and interquartile ranges. Data were analyzed using the Wilcoxon two-sample test. After Bonferroni correction, a p-value <0.0083 is considered statistically significant. MPO, myeloperoxidase; PR3, proteinase 3.
Among patients with active ANCA vasculitis, there were no differences in any complement analytes when comparing those with new-onset versus relapsed disease. Moreover, none of the complement analytes correlated with BVAS score. We did find, however, that treatment exposure affected measures of complement activation among patients with active ANCA vasculitis. Patients with active disease and on any treatment, including corticosteroids and/or a steroid-sparing immunosuppressant, had significantly lower Bb (0.48 vs. 0.73 μg/mL, p=0.02) and C5a (6.94 vs. 10.54 ng/mL, p=0.04) plasma levels compared to patients with active disease off treatment (supplementary Figure S4A-B). There were no significant differences in any complement activation analyte among patients in remission on or off treatment.
Analyses of paired samples
There were 10 patients (3 MPO-ANCA, 7 PR3-ANCA) with paired samples from active and remission disease states (Table 1, Figure 2A-F). Compared to the overall population, this cohort had milder renal insufficiency with a median eGFR of 72.12 mL/min/1.73m2 during active disease. Plasma levels of sC5b-9 were lower during remission compared to active disease (139.5 vs. 179.9 ng/mL, p=0.01). We also observed decreases in median levels of C3a, C5a, and C4d during remission compared to active disease, but these were not statistically significant (supplementary Table S3). Although not statistically significant, the complement activation analytes trended in the same direction as our primary analyses within this small number of individuals with longitudinal data.
Figure 2A-F.
Changes in complement activation in patients with ANCA vasculitis and paired samples from active and remission disease states. Changes in complement activation in patients with ANCA vasculitis and paired samples from active and remission disease states. Bb (A), properdin (B), C3a (C), C5a (D), sC5b-9 (E), C4d (F). Samples processed with futhan. Data were analyzed using the paired signed-rank test. A p-value <0.05 is considered statistically significant.
Long-term remission off therapy
Our cohort included 8 patients (3 MPO-ANCA, 5 PR3-ANCA) who achieved LTROT for ≥ 2 years (Figure 3A-F). Compared to patients with active ANCA vasculitis, those in LTROT had significantly lower plasma levels of C5a (9.47 vs. 6.40 ng/mL, p=0.03). We also observed decreases in median levels of C3a, sC5b-9, and C4d between patients with active disease and in LTROT, but these were not statistically significant (supplementary Table S4). Although not statistically significant, the complement activation analytes trended in the same direction as our primary analyses, even with a small sample size.
Figure 3A-F.
Measuring complement activation in patients with ANCA vasculitis in long-term remission off therapy ≥ 2 years (LTROT≥2). Bb (A), properdin (B), C3a (C), C5a (D), sC5b-9 (E), C4d (F). Horizontal lines represent median values and interquartile ranges. Wilcoxon two-sample test was used to compare patients with active disease to those with LTROT status. A p-value <0.05 is considered statistically significant. MPO, myeloperoxidase; PR3, proteinase 3.
DISCUSSION
The complement system comprises over 30 plasma proteins and can be activated via the classical, alternative, and lectin pathways. All three pathways converge at complement component C3 with subsequent formation of the membrane attack complex.32,33 While classical pathway involvement has long been recognized in some systemic autoimmune diseases, there is now increasing evidence that the alternative pathway is also involved.10,34 In ANCA vasculitis, complement was initially considered not to have a role in disease pathogenesis. Studies involving an MPO-ANCA murine model, however, revealed that the alternative pathway is crucial for disease development. Conversely, neither intact classical or lectin pathways nor formation of the terminal membrane attack complex appear necessary for disease pathogenesis. In a human cohort of MPO-ANCA vasculitis, alternative pathway activation fragments correlated with disease activity and were demonstrated at sites of tissue injury.7 Prior to our study, the role of complement activation in PR3-ANCA vasculitis had yet to be characterized.
Our results establish that complement activation occurs in both MPO-ANCA and PR3-ANCA vasculitis. Our data also suggest that the complement activation profile differs by disease activity and possibly by ANCA serotype. In active MPO-ANCA vasculitis, C3a, C5a, and sC5b-9 levels were higher compared to healthy controls. Bb, C3a, and sC5b-9 levels were also higher in remission compared to healthy controls but did not differ significantly compared to active disease. In active PR3-ANCA vasculitis, C3a, C5a, sC5b-9, and C4d levels were higher compared to healthy controls. C3a and C4d levels were also higher in remission compared to healthy controls but did not differ significantly compared to active disease. While patients with MPO-ANCA vasculitis did not have elevated plasma levels of C4d that reached statistical significance, it will be important to confirm this finding in larger numbers of patients especially with paired active and remission samples. Elevated C4d levels suggest the classical or lectin complement pathways may be involved in disease pathogenesis, possibly as a secondary augmentation of complement activation.35
Potential differences in complement activation would be consistent with known pathophysiologic and phenotypic differences between MPO-ANCA and PR3-ANCA vasculitis. MPO-ANCA and PR3-ANCA are associated with different genetic predispositions, pathophysiologic mechanisms, cytokine profiles, organ predilection, and treatment response.29,31,36 MPO-ANCA and PR3-ANCA are also predictive of long-term prognosis and relapse risk.28 Complement activation may be another feature that differs between MPO-ANCA and PR3-ANCA vasculitis.
Prior studies have demonstrated that MPO may have regulatory effects on complement. MPO released from ANCA-stimulated neutrophils isolated from ANCA vasculitis patients can bind a modified form of C-reactive protein (mCRP).37 mCRP can inhibit the alternative complement pathway by binding complement factor H (CFH), and it can activate the classical complement pathway by binding C1q.38,39 MPO binding to mCRP blocks mCRP binding to CFH and may therefore contribute to alternative complement pathway activation in ANCA vasculitis. MPO binding mCRP also blocks binding to C1q and may therefore result in less complement activation through the classical pathway. In contrast, PR3 showed no binding to mCRP.37 Another recent study demonstrated that CFH co-localized with MPO in neutrophil extracellular traps induced in serum from patients with MPO-ANCA vasculitis and that CFH and MPO were closely adjacent in kidney biopsies.40,41 The binding of MPO to CFH impaired CFH binding to C3b and the breakdown of the alternative pathway C3 convertase. Since CFH is a major regulator of the alternative complement pathway, its inhibition by MPO binding may amplify the alternative complement pathway activation in ANCA vasculitis.40 Additional mechanistic studies are needed to determine whether the influence of MPO on CFH function and CRP also occurs in PR3-ANCA vasculitis.
Compared to the prior study by Gou et. al., we did not observe significant differences in complement activation based on disease activity in our overall cohort; however, we did discern notable trends between active and remission samples from the same individual.7 There are several potential explanations. First, the two cohorts differed clinically in several respects. In the prior study, patients were newly diagnosed and samples obtained prior to treatment.7,8 In comparison, our cohort included patients with new and relapsed disease who may have received immunosuppressive treatment (except rituximab or plasma exchange) at the time of sample procurement. There is evidence that corticosteroids quickly reduce complement activation.42-46 In our cohort, 26% of patients with active disease were on corticosteroids and 48% on steroid-sparing immunosuppressants when the sample was obtained. We did find that patients with active ANCA vasculitis on any treatment had significantly lower levels of Bb and C5a compared to those off treatment. Treatment exposure in our patients with active disease may therefore diminish differences in complement activation when compared to those with quiescent disease. We also excluded patients with concomitant infection, dialysis, and plasmapheresis, all of which can contribute to complement activation.47-49 Additionally, the prior cohort consisted exclusively of MPO-ANCA patients and had 12% more ENT, 26% more pulmonary, and 18% more gastrointestinal involvement than our patients with MPO-ANCA vasculitis.7,8 It is possible that complement activation does not contribute equally to all disease manifestations. Of particular interest would be evaluating whether complement activation correlates with glomerulonephritis. All patients in the prior MPO-ANCA vasculitis cohort had renal involvement and biopsy-proven pauci-immune necrotizing and crescentic glomerulonephritis.7,8 All but 3 of our patients had renal involvement, so our numbers were too small to evaluate for differences in complement activation based on renal disease. Further study is need to determine whether the profile of complement activation differs between patients with and without renal disease.
We also adopted different processing methods. We centrifuged samples twice, potentially removing any cellular contaminants that could contribute to complement activation in vitro. We added futhan and found that values of Bb, C5a, and sC5b-9 were considerably higher in samples processed without futhan compared to its paired sample with futhan. We used samples with futhan to ensure any differences observed in complement activation were accurately reflective of the patient’s status at time of sample procurement and not attributable to in vitro complement activation. Last, we also used a different properdin ELISA assay (Hycult Biotech, Plymouth Meeting, PA).
Although our results further substantiate the contribution of complement activation in ANCA vasculitis pathogenesis, the clinical implications remain uncertain. Specifically, it is unknown whether normalization of complement activation analytes and immunologically inactive disease are necessary for clinical disease remission or are predictors of lower relapse risk. Among paired samples, sC5b-9 levels were significantly lower in remission than active disease. Soluble C5b-9 may be the most sensitive complement activation marker in assessing disease activity, as in systemic lupus erythematosus.50,51 Of potential significance is the median time difference between paired active and remission samples in our cohort was only 263 days, and half of the patients had paired samples ≤ 7 months apart, raising the question whether patients need more time to truly achieve disease remission. We may also be underestimating differences in complement activation between active disease and remission since these patients had milder renal insufficiency. Among our patients who achieved LTROT, C5a levels were significantly lower compared to patients with active ANCA vasculitis. Although not statistically significant, we did observe increases in properdin levels among patients who achieved LTROT compared to patients with active ANCA vasculitis. A similar trend occurred among paired samples, with higher properdin levels in remission than in active disease. Properdin levels were previously shown to correlate with disease activity, with increased levels during remission compared to active disease.7 These initial analyses merit further study in larger longitudinal cohorts to determine if any complement activation analytes may be markers of stable disease remission or associated with lower relapse risk.
CONCLUSION
Our study demonstrates that complement activation not only occurs in MPO-ANCA vasculitis, but also PR3-ANCA vasculitis. The complement activation profile differs by disease activity and may differ by ANCA serotype. Additional study is needed to further understand the respective roles of complement activation in MPO-ANCA and PR3-ANCA vasculitis pathogenesis, in particular the potential involvement of the classical pathway. More longitudinal analysis is needed to evaluate whether measures of complement activation are useful biomarkers of treatment response, relapse risk, and/or stable remission.
Our study also indicates that the addition of futhan reduces complement activation in vitro and improves the accurate measurement of complement activation analytes. Given the potential clinical value of measuring complement activation, standardization of plasma collection and processing methods is warranted. We recommend adding futhan when processing samples to assess complement activation analytes.
Supplementary Material
KEY MESSAGES.
What is already known about this subject?
Alternative complement pathway activation is pathogenically important in a mouse model of MPO-ANCA vasculitis and plasma levels of complement activation fragments were found to correlate with disease activity in a cohort of patients with MPO-ANCA vasculitis.
What does this study add?
We found complement activation occurs in patients with both MPO-ANCA and PR3-ANCA vasculitis. The complement activation profile differs by disease activity and possibly by ANCA serotype.
How might this impact on clinical practice or future developments?
Futhan should be systematically added for optimal and accurate measurement of activated complement factors in circulation.
Complement activation analytes, especially C5a and sC5b-9, may be sensitive markers of disease activity and treatment response and/or predictors of relapse risk.
Acknowledgements
We thank Srijana Bhattarai Chhetri for help with sample processing and Lauren N. Blazek for assistance with clinical chart review.
Funding These studies were supported by NIH/NIDDK Program Project Grant 5P01DK058335–19. Eveline Y. Wu, MD, MSCR was also supported by a UNC Junior Faculty Development Award, an institutional start-up fund, and a generous donation from the Kioti Tractor Company and the Kim family.
Footnotes
Conflicts of Interest All named authors have no conflicts to declare
REFERENCES
- 1.Charles Jennette J, Xiao H, Hu P. Complement in ANCA-associated vasculitis. Semin Nephrol 2013;33:557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennette JC, Xiao H, Falk R, et al. Experimental models of vasculitis and glomerulonephritis induced by antineutrophil cytoplasmic autoantibodies. Contrib Nephrol 2011;169:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002;110:955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao H, Schreiber A, Heeringa P, et al. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol 2007;170:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schreiber A, Xiao H, Jennette JC, et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 2009;20:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao H, Dairaghi DJ, Powers JP, et al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol 2014;25:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gou SJ, Yuan J, Chen M, et al. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int 2013;83:129–37. [DOI] [PubMed] [Google Scholar]
- 8.Gou SJ, Yuan J, Wang C, et al. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol 2013;8:1884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing GQ, Chen M, Liu G, et al. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol 2009;29:282–91. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun 2010;34:J276–86. [DOI] [PubMed] [Google Scholar]
- 11.Mollnes TE, Garred P, Bergseth G. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clin Exp Immunol 1988;73:484–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S, McGookey M, Wang Y, et al. Effect of blood sampling, processing, and storage on the measurement of complement activation biomarkers. Am J Clin Pathol 2015;143:558–65. [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer PH, Kawahara MS, Hugli TE. Possible mechanism for in vitro complement activation in blood and plasma samples: futhan/EDTA controls in vitro complement activation. Clin Chem 1999;45:1190–9. [PubMed] [Google Scholar]
- 14.Watkins J, Wild G, Smith S. Nafamostat to stabilise plasma samples taken for complement measurements. Lancet 1989;1:896–7. [DOI] [PubMed] [Google Scholar]
- 15.Bergseth G, Ludviksen JK, Kirschfink M, et al. An international serum standard for application in assays to detect human complement activation products. Mol Immunol 2013;56:232–9. [DOI] [PubMed] [Google Scholar]
- 16.Fujii S, Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta 1981;661:342–5. [DOI] [PubMed] [Google Scholar]
- 17.Aoyama T, Ino Y, Ozeki M, et al. Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments. Jpn J Pharmacol 1984;35:203–27. [DOI] [PubMed] [Google Scholar]
- 18.Inagi R, Miyata T, Maeda K, et al. FUT-175 as a potent inhibitor of C5/C3 convertase activity for production of C5a and C3a. Immunol Lett 1991;27:49–52. [DOI] [PubMed] [Google Scholar]
- 19.Ikari N, Sakai Y, Hitomi Y, et al. New synthetic inhibitor to the alternative complement pathway. Immunology 1983;49:685–91. [PMC free article] [PubMed] [Google Scholar]
- 20.Issekutz AC, Roland DM, Patrick RA. The effect of FUT-175 (Nafamstat Mesilate) on C3a, C4a and C5a generation in vitro and inflammatory reactions in vivo. Int J Immunopharmacol 1990;12:1–9. [DOI] [PubMed] [Google Scholar]
- 21.Kirschfink M, Mollnes TE. Modern complement analysis. Clin Diagn Lab Immunol 2003;10:982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollnes TE, Jokiranta TS, Truedsson L, et al. Complement analysis in the 21st century. Mol Immunol 2007;44:3838–49. [DOI] [PubMed] [Google Scholar]
- 23.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol 2013;17:603–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Qjm 1994;87:671–8. [PubMed] [Google Scholar]
- 26.Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009;68:1827–32. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54. [DOI] [PubMed] [Google Scholar]
- 28.Lionaki S, Blyth ER, Hogan SL, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum 2012;64:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jennette JC, Nachman PH. ANCA Glomerulonephritis and Vasculitis. Clin J Am Soc Nephrol 2017;12:1680–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solans-Laque R, Fraile G, Rodriguez-Carballeira M, et al. Clinical characteristics and outcome of Spanish patients with ANCA-associated vasculitides: Impact of the vasculitis type, ANCA specificity, and treatment on mortality and morbidity. Medicine (Baltimore) 2017;96:e6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornec D, Cornec-Le Gall E, Fervenza FC, et al. ANCA-associated vasculitis - clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016;12:570–9. [DOI] [PubMed] [Google Scholar]
- 32.Walport MJ. Complement. First of two parts. N Engl J Med 2001;344:1058–66. [DOI] [PubMed] [Google Scholar]
- 33.Walport MJ. Complement. Second of two parts. N Engl J Med 2001;344:1140–4. [DOI] [PubMed] [Google Scholar]
- 34.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol 2006;176:1305–10. [DOI] [PubMed] [Google Scholar]
- 35.Harboe M, Ulvund G, Vien L, et al. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol 2004;138:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berti A, Warner R, Johnson K, et al. Brief Report: Circulating Cytokine Profiles and Antineutrophil Cytoplasmic Antibody Specificity in Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol 2018;70:1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu PC, Li ZY, Yang XW, et al. Myeloperoxidase influences the complement regulatory function of modified C-reactive protein. Innate Immun 2014;20:440–8. [DOI] [PubMed] [Google Scholar]
- 38.Mihlan M, Stippa S, Jozsi M, et al. Monomeric CRP contributes to complement control in fluid phase and on cellular surfaces and increases phagocytosis by recruiting factor H. Cell Death Differ 2009;16:1630–40. [DOI] [PubMed] [Google Scholar]
- 39.Mihlan M, Blom AM, Kupreishvili K, et al. Monomeric C-reactive protein modulates classic complement activation on necrotic cells. Faseb j 2011;25:4198–210. [DOI] [PubMed] [Google Scholar]
- 40.Chen SF, Wang FM, Li ZY, et al. Myeloperoxidase influences the complement regulatory activity of complement factor H. Rheumatology (Oxford) 2018;57:2213–24. [DOI] [PubMed] [Google Scholar]
- 41.Heeringa P, Alan Little M. Releasing the complement brakes: is myeloperoxidase the missing link between factor H and C5a in anti-neutrophil cytoplasmic antibody vasculitis? Rheumatology (Oxford) 2018;57:2070–71. [DOI] [PubMed] [Google Scholar]
- 42.Weiler JM, Packard BD. Methylprednisolone inhibits the alternative and amplification pathways of complement. Infect Immun 1982;38:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Packard BD, Weiler JM. Steroids inhibit activation of the alternative-amplification pathway of complement. Infect Immun 1983;40:1011–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandslund I, Peters ND, Ejstrup L. Steroids reduce complement activation in rheumatoid arthritis. Int J Tissue React 1985;7:161–5. [PubMed] [Google Scholar]
- 45.Dernek S, Tunerir B, Sevin B, et al. The effects of methylprednisolone on complement, immunoglobulins and pulmonary neutrophil sequestration during cardiopulmonary bypass. Cardiovasc Surg 1999;7:414–8. [DOI] [PubMed] [Google Scholar]
- 46.Mikawa K, Ikegaki J, Maekawa N, et al. Perioperative effect of methylprednisolone given during lung surgery on plasma concentrations of C3a and C5a. Scand J Thorac Cardiovasc Surg 1990;24:229–33. [DOI] [PubMed] [Google Scholar]
- 47.Aljama P, Bird PA, Ward MK, et al. Haemodialysis-induced leucopenia and activation of complement: effects of different membranes. Proc Eur Dial Transplant Assoc 1978;15:144–53. [PubMed] [Google Scholar]
- 48.Dodd NJ, Vergani D, Turney JH, et al. Complement activation and C1q binding activity in haemodialysis. Proc Eur Dial Transplant Assoc 1981;18:300–4. [PubMed] [Google Scholar]
- 49.McLeod BC, Viernes A, Sassetti RJ. Complement activation by plasma separator membranes. Transfusion 1983;23:143–7. [DOI] [PubMed] [Google Scholar]
- 50.Gawryl MS, Chudwin DS, Langlois PF, et al. The terminal complement complex, C5b-9, a marker of disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 1988;31:188–95. [DOI] [PubMed] [Google Scholar]
- 51.Chiu YY, Nisihara RM, Wurzner R, et al. SC5b-9 is the most sensitive marker in assessing disease activity in Brazilian SLE patients. J Investig Allergol Clin Immunol 1998;8:239–44. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.