Figure 3.
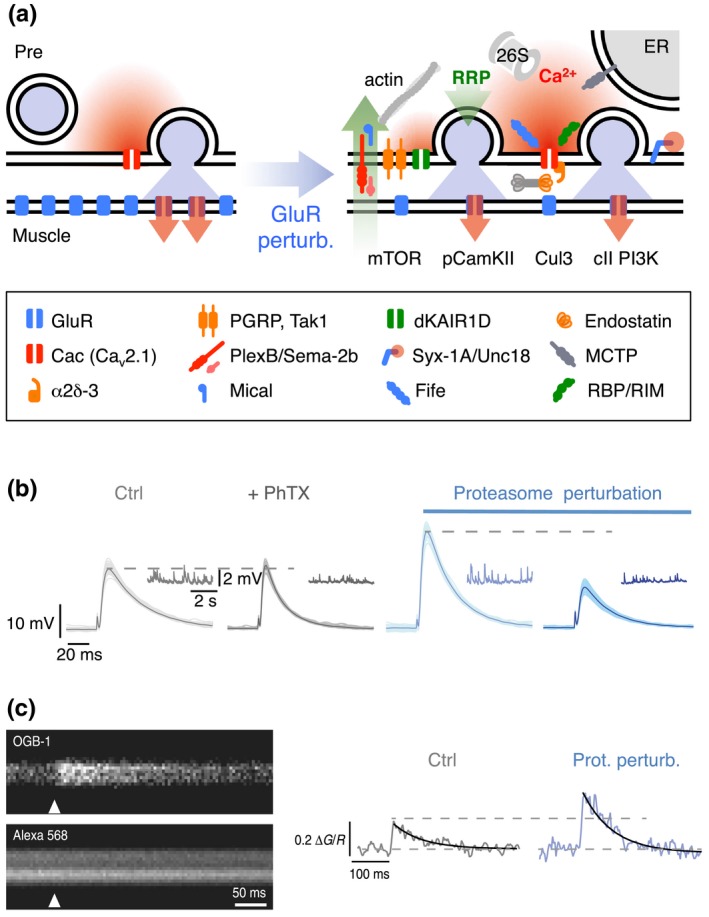
Molecular mechanisms underlying PHP. (a) Cartoon of a synapse under control conditions (left) and after GluR perturbation (right). Glutamate receptor perturbation enhances presynaptic Ca2+ influx (red) (Frank et al., 2006; Müller & Davis, 2012) and RRP size (green) (Weyhersmüller et al., 2011). Cav2.1 (cacophony, cac; Frank et al., 2006; Müller & Davis, 2012), α2δ‐3 (Wang, Jones, et al., 2016), endostatin/multiplexin (Wang et al., 2014), and rim‐binding protein (rbp) (Müller et al., 2015) have been implicated in homeostatic regulation of presynaptic Ca2+ influx. The following genes have been implicated in RRP size regulation under baseline conditions and/or during PHP: The presynaptic proteasome (“26S”) (Wentzel et al., 2018), fife (Bruckner et al., 2017), mctp (Genç et al., 2017), mical (Orr et al., 2017), pgrp, tak1 (Harris et al., 2015, 2018), dKaiR1D (Kiragasi et al., 2017), α2δ‐3 (Wang, Jones, et al., 2016), plexB/sema2b (Orr et al., 2017), syntaxin-1A (syx-1A), unc18 (rop) (Ortega et al., 2018), rbp (Müller et al., 2015), and rim (Müller et al., 2012). Retrograde PHP signaling involves multiplexin/endostatin (Wang et al., 2014) and Sema‐2B/Plexin B (Orr et al., 2017). PHP requires postsynaptic mTOR signaling (Goel et al., 2017; Penney et al., 2012), class II PI3 kinase function (Hauswirth et al., 2018), and reduced pCaMKII levels (Goel et al., 2017; Li, Goel, Chen, et al., 2018; Newman et al., 2017). Note that the cartoon only summarizes recent genes implicated in PHP. More molecular PHP mechanisms are reviewed in (Davis & Müller, 2015; Delvendahl & Müller, 2019; Wondolowski & Dickman, 2013; Frank, 2014a). (b) At wild‐type NMJs (gray), application of the glutamate receptor antagonist philanthotoxin‐433 (“PhTX”) decreases miniature EPSP amplitudes (inset) and enhances presynaptic release, thereby maintaining AP‐evoked EPSP amplitudes at control levels. Acute or sustained proteasome perturbation (blue) enhances presynaptic release in the absence of glutamate receptor inhibition and blocks PHP. Reprinted and adapted from (Wentzel et al., 2018) with permission under a Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/). (c) Presynaptic Ca2+ imaging (motor neuron boutons were loaded with the nonmembrane permeable Ca2+ indicator Oregon‐Green‐BAPTA‐1, “OGB‐1,” and the reference dye Alexa 568) revealed that presynaptic proteasome perturbation (elavc155‐Gal4 > UAS‐DTS) results in increased amplitudes of presynaptic Ca2+ transients upon single AP stimulation. These data suggest that presynaptic proteasomal degradation has the capacity to regulate Ca2+ influx. Reprinted and adapted from (Wentzel et al., 2018) with permission under a Creative Commons Attribution 4.0 License (https://creativecommons.org/licenses/by/4.0/)
