Abstract
The molecular epidemiology of Mycobacterium tuberculosis (M. tuberculosis, Mtb) is poorly documented in Ethiopia. The data that exists has not yet been collected in an overview metadata form. Thus, this review summarizes available literature on the genomic diversity, geospatial distribution and transmission patterns of Mtb lineages (L) and sublineages in Ethiopia. Spoligotyping and Mycobacterial Interspersed Repetitive Units-Variable Number Tandem Repeats (MIRU-VNTR) based articles were identified from MEDLINE via PubMed and Scopus. The last date of article search was done on 12th February 2019. Articles were selected following the PRISMA flow diagram. The proportion of (sub)lineages was summarized at national level and further disaggregated by region. Clustering and recent transmission index (RTI) were determined using metan command and random effect meta-analysis model. The meta-analysis was computed using Stata 14 (Stata Corp. College Station, TX, USA). Among 4371 clinical isolates, 99.5% were Mtb and 0.5% were M. bovis. Proportionally, L4, L3, L1 and L7 made up 62.3%, 21.7%, 7.9% and 3.4% of the total isolates, respectively. Among sublineages, L4.2. ETH/SIT149, L4.10/SIT53, L3. ETH1/SIT25 and L4.6/SIT37 were the leading clustered isolates accounting for 14.4%, 9.7%, 7.2% and 5.5%, respectively. Based on MIRU-VNTR, the rate of clustering was 41% and the secondary case rate from a single source case was estimated at 29%. Clustering and recent transmission index was higher in eastern and southwestern Ethiopia compared with the northwestern part of the country. High level of genetic diversity with a high rate of clustering was noted which collectively mirrored the phenomena of micro-epidemics and super-spreading. The largest set of clustered strains deserves special attention and further characterization using whole genome sequencing (WGS) to better understand the evolution, genomic diversity and transmission dynamics of Mtb.
Keywords: Molecular epidemiology, M. tuberculosis, Ethiopia, Review
1. Introduction
Tuberculosis (TB) is a complex and chronic infectious disease of the lung, the lymph node and other parts of the body. According to the 2018 global TB report, there were 10 million infections and 1.6 million deaths due to TB in 2017 [1].
In Ethiopia, the incidence of TB was 172/100,000 and TB/HIV coinfection rate was 12% [1]. However, rates as high as 26% TB/HIV coinfection prevalence had previously been reported by other studies [2,3]. The 117,705 TB cases notified in 2017 represented 68% of the estimated incidence [1]. Reports showed that, 32% and 30% of TB cases are extra-pulmonary TB (EPTB) and sputum smear negative pulmonary TB (PTB−), respectively [4–7]. The remaining 38% are sputum smear-positive pulmonary TB (PTB+) which are likely responsible for most of the transmission [8]. The multidrug resistance/rifampicin resistance (MDR/RR) rate was 2.18% and 21% among new and previously treated TB cases, respectively [9]. Moreover, the treatment success rate was 90% for new TB cases [1] and 59.2% for MDR/RR TB cases [10].
Previous host genomic [11–16], sociodemographic [17] and pathogen genomic [18,19] studies have attempted to define the determinants leading to PTB, EPTB and latent TB in different populations. However, results across different studies are heterogeneous and in conflict with each other.
Species of TB causing Mycobacteria were grouped as Mycobacterium tuberculosis complex (MTBC) [20] to delineate them from nontuberculosis Mycobacteria (NTM), M. leprae, M. ulcerans and environmental Mycobacteria [21–24]. However, Riojas et al. (2017) using next-generation sequencing (NGS), digital DNA-DNA hybridization (dDDH), and average nucleotide identity (ANI) data detected high degree of identity (dDDH: 91.2–99.2%, ANI: 99.21–99.92%) among the species of MTBC. Based on these data, these authors challenged the species nomenclature of the MTBC complex and recommend infrasubspecific designations using the term “variant” (var.) such as M. tuberculosis var. tuberculosis, M. tuberculosis var. africanum [25].
Mtb genotypes are geographically structured in to seven major lineages and several sublineages [26,27]. Lineage and sublineage classification is based on the work of Brudy et al. [26], Gagneux and Small [27] and Coll et al. [28]. The seven major lineages includes lineage 1 (L1: East African Indian/EAI/or Indo-Oceanic), L2 (East Asia, EAS, Beijing), L3 (Central Asia, CAS), L4 (Euro-American, EA), L5 and L6 (M. africanum I and II) and L7. Sublineages are spoligotyping families under each lineage.
Based on best available evidence, East Africa in particular Ethiopia, is the most likely evolutionary origin of the recent most common ancestor of Mtb [29] and Homo sapiens [30]. As such, Ethiopia hosts diverse specialist and generalist types of Mtb strains [31] that have established sympatric and allopatric association with their hosts [32,33]. These evolutionary trajectories together with changing social and economic determinants, are probably responsible for the current unique epidemiology of TB in Ethiopia (including a high proportion of lymph node tuberculosis, 32% of total vs 38% PTB+, and 30% PTB−).
The complete genome of Mtb is nearly 4.4 mega base pairs (Mbp) long and is subdivided into RNA coding sequences, repetitive DNA sequences, direct repeats (DR), PE/PPE family members, polymorphic G + C rich sequences (PGRS) and protein coding sequences [34]. Strains have over 99% genomic similarity; on average differing by about 1200 single nucleotide polymorphisms (SNP) [35]. Such clonality was maintained through absence of horizontal gene transfer, selective sweeps, purifying selection, background selection, and transmission bottlenecks [31]. Mtb molecular typing relies on the above-mentioned genomic regions and the principles of each typing techniques are reviewed elsewhere [36]. Briefly, spoligotyping is based on DR regions which contain 43 sets of repetitive spacer sequences interspersed with 34–41 base pair (bp) long nonrepetitive sequences [37]. The MIRU-VNTR with 24 loci is used for classification of isolates into (sub) lineages and is currently the minimum recommended technique for the analysis of transmission pattern. However, for high resolution molecular epidemiology and evolutionary analysis, working with whole genome SNPs is superior [38–43].
Contact screening has served as a tool to both study and control TB transmission control. A simplified TB transmission dynamics model contains five groups: susceptible, latently infected, infectious, non-in-fectious and recovered cases [44]. Over time, an individual might move from one state to another through spontaneous cure and relapse [44]. The “stone-in-the-pond” principle of contact tracing is the gold standard non-molecular method for the identification of the transmission chain in TB infection [45]. A symptom-based contact screening of 15, 527 people whose source cases were PTB+ was done in Amhara and Oromia region between 2013 and 2014. Of these, 6.1% had presumptive TB. All forms of TB and PTB+ were diagnosed in 2.5% and 0.76% of contacts, respectively [46]. A similar study also screened 272,441 close contacts of 47, 021 index cases. Of these, 5.1% and 0.8% had presumptive and active TB, respectively [47]. Another study compared the prevalence of TB among contacts of MDR-TB and drug-sensitive (DS) PTB+ index cases. The study screened 331 MDR-TB and 353 DS-TB contacts where the prevalence of TB among contacts was 2.7% and 4.0%, respectively [48].
Contact screening-based transmission analysis does not always link the true transmission chain [49,50]. Thus, currently, molecular typing is used to determine the chain and timing (cluster age) of transmission [43]. In Ethiopia, MIRU-VNTR and spoligotyping have been used for molecular typing and transmission analysis. Recently, a systematic review of spoligotyping based Mtb genetic diversity was reported [51]. However, this study excluded MIRU-VNTR based articles. Moreover, nationally representative pooled transmission dynamics data were not provided. Thus, this review aimed to summarize existing spoligotyping and MIRU-VNTR based articles: (1) to determine the diversity and phylogeography of (sub) lineages, (2) to identify the dominant spoligotyping international types (SIT) so as to bring them into sharper focus for further study and (3) to characterize the transmission pattern.
2. Methods
2.1. Eligibility criteria
For the analysis of the genomic diversity, phylogeography and transmission patterns, both spoligotyping and MIRU-VNTR based articles were included. However, for clustering and RTI analysis, only MIRU-VNTR based articles were considered. Articles from Ethiopia that characterized Mtb (sub) lineages published in English language irrespective of publication year, demography, clinical and TB types were included.
2.2. Information sources and search strategy
MEDLINE through PubMed and Scopus were the source of articles and last date of article search was 12th February 2019. Additionally, the reference list of some reviews was used to retrieve further literature. The search was done using medical subject headings (MeSH terms) and Boolean operators such as ‘molecular typing’, or ‘molecular epidemiology’, and ‘M. tuberculosis’ and ‘Ethiopia’ (supplementary file1, S1).
2.3. Study selection
All of the identified articles were exported to EndNote library. After removing the duplicate, screening was done by title followed by reading the abstract and then by reviewing the full work. Articles that did not fulfill the inclusion criteria were excluded. Articles were independently assessed for inclusion by two authors of this paper (DM, EN). Disagreements regarding the inclusion or exclusion of articles were resolved by discussion among authors.
2.4. Data collection process and data items
Data from the included articles were extracted independently by two authors using data extraction sheet. Disagreements on the extracted data were resolved by discussion. The extraction included: author and year of publication, study period, molecular techniques, sample size, type and history of TB, sex and age classification, HIV status, study area, types and number of (sub) lineage, SIT, cluster size, number of isolates within the cluster and non-clustered isolates.
2.5. Risk of bias in individual studies
Methodological quality of included articles was appraised in duplicate (by DM & AD) using customized check lists of Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases (STROMEID) [52] and the Joanna Briggs Institute (JBI) [53]. The customized tool has ten domains and each domain scores either one or zero point. Discordance on rating was resolved by the third author (FB). The quality of each article was rated and judged.
2.6. Summary measures and synthesis of results
The extracted data were summarized using frequency and percentage. The national Mtb population genomic structure was divided into (sub) lineages and SIT and then summarized using tables. The geographic distribution of sub (lineages) was mapped using ArcGIS 10.3 (ArcGIS Desktop, ESRI 2011. Redlands, Canada). The spatial data used for the maps were taken from Map library which is a public domain that can be accessed at www.maplibrary.org. Clustering and RTI were analyzed using Stata 14 (Stata Corp. College Station, TX, USA).
Tuberculosis cases were said to be clustered when strains isolated from different patients showed similar genotyping patterns [54]. Recent transmission index is the average number of secondary cases of the disease from a single primary/source/case [55]. The RTI from individual articles was calculated using RTI (n-1) method described elsewhere [56]; where RTI= (nc-c)/N, ‘N’ is the total number of cases, ‘nc’ is the number of isolates within the cluster and ‘c’ is the number of clusters or source cases.
Approximating likelihood approach was followed. In addition, to preserve the deviations, Freeman-Tukey double arcsine rooted transformation was done [57]. To estimate the transformed pooled prevalence, Dersimonian and Laird method was used [58]. Together, using the metan command in Stata, study estimate (ES) as prevalence was computed using Freeman-Tukey double arcsine root transformation with 95% confidence interval with the approach of Dersimonian and Laird method.
2.7. Risk of bias across studies
Statistical heterogeneity estimate among the articles was judged using I2 statistic and P-value of above and below 0.05. The I2 value of < 25%, 25–50% and > 50% was taken as low, moderate and high degree of heterogeneity, respectively [59]. Moreover, publication bias was assessed using funnel plot asymmetry. To assess the influence of an individual study on pooled prevalence, sensitivity analysis was performed.
3. Results
3.1. Study selection
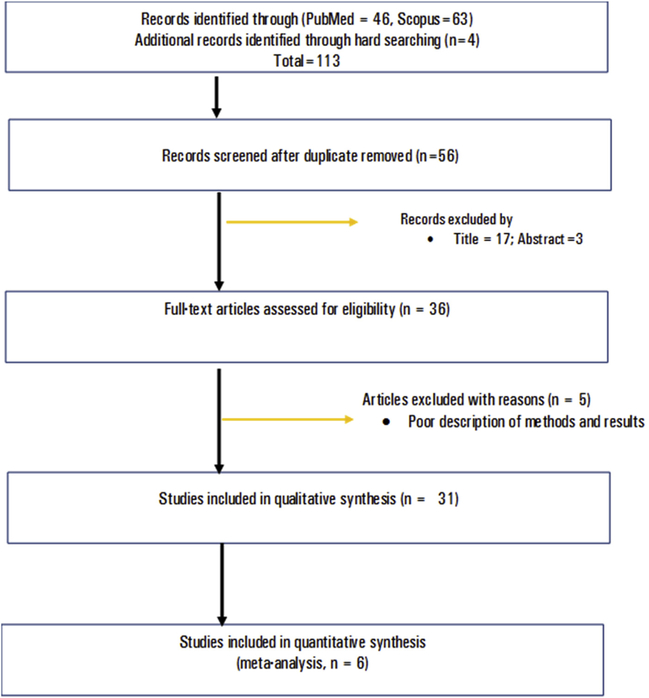
A total of 113 articles were retrieved from MEDLINE, Scopus and reference lists of reviews. After removing 57 duplicate articles; 56 articles were screened by title, abstract and full work as per the inclusion criteria. Finally, 31 articles fulfilled the eligibility criteria and were enrolled in the review. The article selection was carried out following the PRISMA flow diagram (Fig. 1).
Fig. 1.
Flow diagram showing selection process of articles, 2005–2018.
3.2. Study characteristics
Of the total thirty-one articles, twenty-four used SIT and the rest seven used combined SIT and MIRU-VNTR techniques. The study period lay between 2005 and 2018 (14 years). Twenty articles focused on PTB, six articles on TB lymphadenitis (TBLN) cases and the rest five included both PTB and TBLN cases (with a few non TBLN extra-pulmonary TB cases). Based on the available data presented in Table 1, 71.5%, 83%, 53%, 74.6% and 23.5% were PTB, new TB cases, male, within the age range of 15–44 years and HIV positive, respectively (see Table 1).
Table 1:
Profile of included articles, Ethiopia, 2005 2018.
| Author, year | Typing | Study Period | Region | Sample Size (PTB/TBLN) | Types (N/R) | Sex (M/F) | Age* | HIV (Y/N/U) |
|---|---|---|---|---|---|---|---|---|
| Agonafir, 2010 [60] | SIT | 2005–2006 | Ethiopia | 114/0 | 48/66 | 70/44 | 5/82/20 | ND |
| Ali 2016 [61] | SIT + 24 MIT | 2013 | O/SNNP/H/DD | 127/0 | 105/4/ | 64/45 | 0/99/10 | ND |
| Ameni, 2013 [62] | SIT | ND | Fiche | 141/0 | ND | 74/72 | ND | ND |
| Bedewi, 2017 [63] | SIT | 2012–2013 | Woliso/Atat | 281/0 | 271/10 | 151/130 | 0/171/110 | ND |
| Belay, 2014 [64] | SIT | 2009–2010 | Afar | 105/0 | 81/24 | 69/36 | 0/105 | 37/58/10 |
| Biadglegne 2015 [65] | SIT+24 MIT | 2012 | Amhara | 0/226 | 183/11 | 73/121 | 21/156/27 | ND |
| Chemeda 2018 [66] | SIT | 2014 | AA | 143/0 | ND | 55/88 | 0/125/18 | 143/0/0 |
| Debebe 2014 [67] | SIT | 2010–2011 | Bahir Dar | 118/0 | 116/2 | 66/52 | 6/93/19 | 12/106/0 |
| Deribew 2012 [68] | SIT | 2009 | Jimma | 17/0 | 5//4 | 7//10 | NI | ND |
| Diriba 2013 [69] | SIT | 2009–2012 | Ethiopia | 183/0 | 3/180 | 100/83 | 0/169/14 | ND |
| Disassa 2015 [70] | SIT | 2012–2013 | BG | 33/0 | ND | 31//22 | 0/48/5 | ND |
| Firdessa 2013 [71] | SIT+24 MIT, SNP | 2006–2010 | Ethiopia | 622/328 | N/N | 331/246//138/168 | 128/355/87//120/148/21 | ND |
| Garedew 2013 [72] | SIT | 2010–2011 | Debre Birhan | 0/98 | 89/9 | ND | 16//66//16 | ND |
| Garedew 2013 [73] | SIT | 2010–2011 | Debre Birhan | 99/0 | 80/19 | 43/56 | 0/77/22 | ND |
| Gebrezgabiher 2015 [74] | SIT | 2012–2013 | Gedeo | 76/21 | ND | ND | ND | ND |
| Getahun 2015 [75] | SIT | 2010–2011 | Ethiopia | 96/0 | ND | 51/45 | 0/70/26 | ND |
| Gumi 2012 [76] | SIT | 2008–2010 | Guji & Liben | 164/9 | ND | ND | ND | ND |
| Korma 2015 [77] | SIT | 2012–2013 | AA | 53/116 | 167/33 | 94/106 | 21/161/18 | 62/123/15 |
| Maru 2015 [78] | SIT | 2012–2013 | Dessie | 144/0 | 128/16 | 80/64 | 10/119/15 | 25/119/0 |
| Mekonnen 2018 [79] | SIT+24 MIT | 2016–2017 | O/S/H/DD | 160/0 | 114/46 | 128/32 | 16/124/20 | 17/143/0 |
| Mihret 2012 [80] | SIT | 2009–2010 | AA | 192/0 | 192/0 | 109/83 | ND | 26/136/30 |
| Molina-Moya 2018 [81] | SIT | ND | SNNP | 91/0 | ND | ND | ND | ND |
| Nuru 2015 [82] | SIT | 2012–2014 | Bahir Dar | 54/114 | 142/26 | 80/88 | 0/134/34 | ND |
| Nuru 2017 [83] | SIT | 2012–2014 | Bahir Dar | 0/70 | ND | 52/18 | 0/41/29 | ND |
| Tadesse 2017 [84] | SIT+15MIT, SNP | 2013–2015 | Jimma | 0/304 | ND | 143/161 | 33/246/25 | 24/232/48 |
| Tessema 2013 [85] | SIT+24 MIT | 2009 | Amhara | 260/0 | 201/43 | 142/102 | 7/203/34 | 62/182/0 |
| Tilahun 2018 [86] | SIT | 2014–2015 | Ambo | 105/0 | 105/0 | 43/43 | 0/90/15 | 8/42/36 |
| Workalemahu 2013 [87] | SIT | 2011 | Jimma | 121/0 | 121/0 | 69/52 | 121/0/0 | 15/106 |
| Yimer 2013 [88,89] | SIT+24MIT | 2008–2010 | Amhara | 240/0 | 240/0 | 131/109 | 0/240/0 | 51/180 |
| Zewdie 2016 [90] | SIT | 2013 | AA | 0/206 | 62/12 | 94/112 | 0/119/13 | ND |
| Ratio | 3739/1492 | 2453/505 | 2488/2188 | 504/3241/598 | 482/1427/139 | |||
| % | 71.5/28.5 | 83/17 | 53/47 | 11.6/74.6/13.8 | 23.5/69.7/6.8 |
Age(< 15/15–44/≥45); SIT: Spoligotyping international type; MIT: MIRU-VNTR international type; SNNP: Southern nations, nationalities and people’s; BG: Benishangul Gumuz; O/SNNP/H/DD: Oromia/SNNP/Hareri/Dire Dawa; O/S/H/DD: Oromia/Somali/Hareri/Dire Dawa; M/F: Male/Female, PTB: Pulmonary tuberculosis; TBLN: tuberculous lymphadenitis; ND: No data found; AA: Addis Ababa; N/R: New/retreated; P/N/U: Positive/Negative/Unknown.
3.3. Risk of bias within studies
The articles were appraised based on their molecular methods and associated outcomes. Thus, all the 31 articles obtained a score of five and above out of 10 points. Hence, overall risk of bias judgment is considered low and the articles were judged as of good quality (Table 2).
Table 2:
Risk of bias appraisal summary of included articles, Ethiopia, 2005–2018.
| Author, Year | OQS |
|---|---|
| Agonafir, 2010 [60] | 7 |
| Ali, 2016 [61] | 6 |
| Ameni, 2013 [62] | 6 |
| Bedewi, 2017 [63] | 6 |
| Belay, 2014 [64] | 6 |
| Chemeda, 2018 [66] | 5 |
| Debebe, 2013 [67] | 5 |
| Deribew, 2012 [68] | 5 |
| Diriba, 2013 [69] | 6 |
| Disassa, 2015 [70] | 5 |
| Biadglegne, 2015 [65] | 7 |
| Firdessa, 2013 [71] | 8 |
| Garedew, 2013 [72] | 5 |
| Garedew, 2013 [73] | 5 |
| Gebrezgabiher, 2015 [74] | 5 |
| Getahun, 2015 [75] | 6 |
| Gumi, 2012-PTB [76] | 6 |
| Korma, 2015 [77] | 8 |
| Maru, 2015 [78] | 6 |
| Mekonnen, 2018 [79] | 10 |
| Mihret, 2012 [80] | 6 |
| Molina-Moya, 2017 [81] | 5 |
| Nuru, 2015 [82] | 6 |
| Nuru, 2017 [83] | 6 |
| Tadesse, 2017 [84] | 7 |
| Tessema 2013 [85] | 7 |
| Tilahun, 2018 [86] | 7 |
| Workalemahu, 2013 [87] | 5 |
| Yimer, 2013 [88] | 8 |
| Yimer, 2015 [89] | 8 |
| Zewdie, 2016 [90] | 6 |
OQS: Overall quality score.
3.4. M. tuberculosis genomic diversity in Ethiopia
From the total 4371 clinical isolates (S2A), 99.5% were M. tuberculosis sensu stricto, and M. bovis made up the remaining 0.5%. The L4/EA, L3/CAS, L1/EAI and L7 shared 62.3%, 21.7%, 7.9% and 3.4%, respectively and the rest 3.6% of isolates were unclassified at lineage level (Table 3).
Table 3:
Genomic diversity of Mycobacterium tuberculosis in Ethiopia, 2005–2018 (N = 4371).
| Lineages, % | Sublineages, Number (%) | Sublineage prevalence among | ||
|---|---|---|---|---|
| PTB, n (%) | TBLN, n (%) | |||
| L1/EAI,7.9 | EAI | 73 (1.7) | 43 (59) | 30 (41) |
| MANU | 202 (4.6) | 177 (87.6) | 25 (12.4) | |
| Family 32/33/34/36 | 69 (1.6) | 64 (92.8) | 5 (7.2) | |
| L2/SEA,0.3 | Beijing/SIT1 | 13 (0.3) | 10 (76.9) | 3 (23.1) |
| L3/CAS, 21.7 | L3. ETH1/SIT25 | 316 (7.2) | 225 (71.2) | 91 (28.8) |
| Delhi/CAS | 407 (9.3) | 251 (61.7) | 156 (38.3) | |
| L3.1.1/SIT21, SIT1675 | 150 (3.4) | 132 (88) | 18 (12) | |
| CAS | 77 (1.8) | 63 (81.8) | 14 (18.2) | |
| L4/EA, 62.3 | H | 447 (10.2) | 277 (62) | 170 (38) |
| LAM | 187 (4.3) | 136 (72.7) | 51 (27.3) | |
| T | 573 (13.1) | 409 (71.4) | 164 (28.6) | |
| L4.6/SIT37 | 240 (5.5) | 166 (69.2) | 74 (30.8) | |
| L4.2. ETH1/SIT149 | 638 (14.6) | 532 (83.4) | 106 (16.6) | |
| L4.10/SIT53 | 423 (9.7) | 320 (75.7) | 103 (24.3) | |
| L4.1.1/X | 73 (1.8) | 59 (80.8) | 14 (19.2) | |
| L4.4.1.1/S, SIT34 | 29 (0.7) | 6 (20.7) | 23 (79.3) | |
| Other L4 | 121 (2.8) | 72 (59.5) | 49 (40.5) | |
| L7,3.4 | L7 | 151 (3.4) | 85 (56.3) | 66 (43.7) |
| M.bovis, 0.5 | M. bovis | 22 (0.5) | 10 (45.5) | 12 (54.5) |
| Unclassified, 3.6 | Unclassified | 160 (3.7) | 115 (71.9) | 45 (28.1) |
| Total | 4371 (100) | 72.1 | 27.9 | |
EAI: East African Indian, SEA: Southeast Asia, CAS: Central Asia; EA: Euro-American; H: Haarlem, LAM: Latin American and Mediterranean, T: Tuscany, ETH: Ethiopia, S: Sicily, PTB: Pulmonary tuberculosis, TBLN: Tuberculous lymphadenitis. SIT: Spoligotyping international type.
Extending down to sublineages, Tuscany (T) constituted 42.8% followed by Delhi/CAS 16.5% and Haarlem (H) 10.2%. Of specific genotypes and SITs, L4.2. ETH1/SIT149, L4.10/SIT53, L3. ETH1/SIT25 and L4.6/SIT37 were the leading clustered isolates accounting for 14.6%, 9.7%,7.2% and 5.5% of the total isolates, respectively (Tables 3 and S2A). Taken together, the data from S2A and Table 3 support the conclusion that, population genetic structure of Mtb in Ethiopia is very diverse.
3.5. Ethiopian M. tuberculosis phylogeography
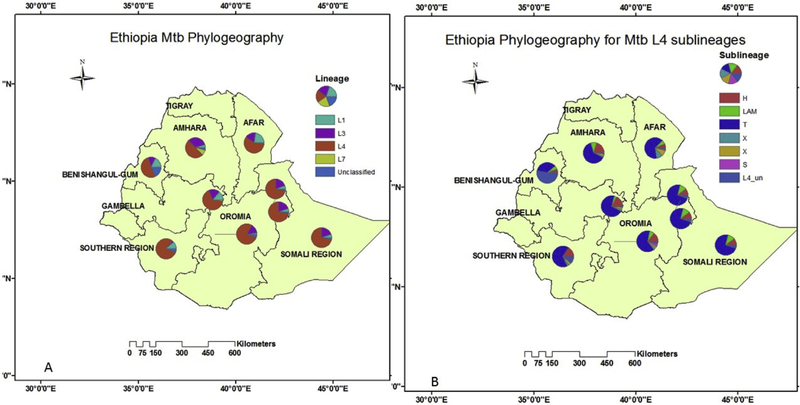
After removing low frequency isolates (L2 and M. bovis) and regionally unclassified study [75], 4222 isolates were mapped to their place of isolation (S2B). Some studies included multi-regional data. Thus, the area was categorized as Amhara, Afar, Southern Nations Nationalities and Peoples (SNNP), Benishangul Gumuz (BG), central Ethiopia (which included Addis Ababa, Fiche, Woliso, Atat, Butajira), south eastern Ethiopia which included (Dire Dawa, Somali, Harari, Guji, Liben, Filtu) and, other Oromia. Figs. 2 and S2B shows some regional variation in terms of both (sub) lineages. For instance, L1 was proportionally higher in Afar and L3 and L7 were relatively more prevalent in Amhara. L4 was relatively higher in Oromia (77%), SNNP (80%), central Ethiopia (67%) and SEE (72%). Likewise, the L4 sublineage of H is slightly higher in Amhara region; L4 sublineage of S is more frequent in Oromia. On the other hand, the L4 sublineage of T which is the largest family of isolates is lower in Benishangul Gumuz (Fig. 2A and B, S2B).
Fig. 2.
Geographic distribution of M. tuberculosis lineage (A) and Euro-American sublineages (B) in Ethiopia, 2005–2018 (N = 4222).
EA: Euro-American, LAM: Latin American Mediterranean, CAS: Central Asia; EAI: East African Indian Lineage; *: Somali region data include part of Haromaya, Dire-Dawa, Harari, Somali, Guji (Negele) and Liben (Filtu); Addis Ababa represent the central Ethiopia phylogeography.
3.6. Transmission patterns
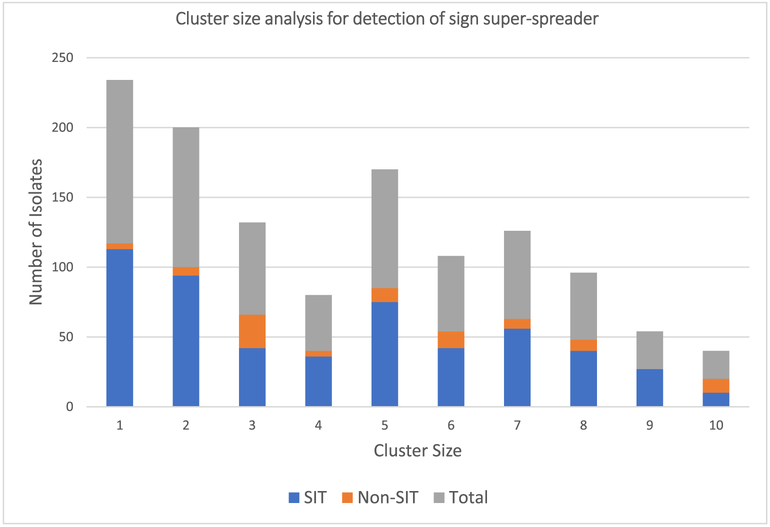
Out of the total of 4371 clinical isolates captured for transmission pattern analysis, 3291 (75.3%) isolates were grouped in to 146 different SIT clusters. The maximum cluster size contained 638 isolates. A total of 113 non-clustered SIT isolates were reported. Thus, 259 different SITs were identified in the present review. The remaining 967 isolates were non-SIT and were found as clustered or unique (S2A). To inspect for possible presence of super-spreaders who are infectious cases that produce more secondary cases than similar other index cases; we analyzed the data as per Ypma et al. [8]. Hence, similar topology was found suggesting the presence of super-spreader events (Fig. 3).
Fig. 3.
The number of isolates with unique and clusters having ≤10 isolates, Ethiopia, 2005–2018.
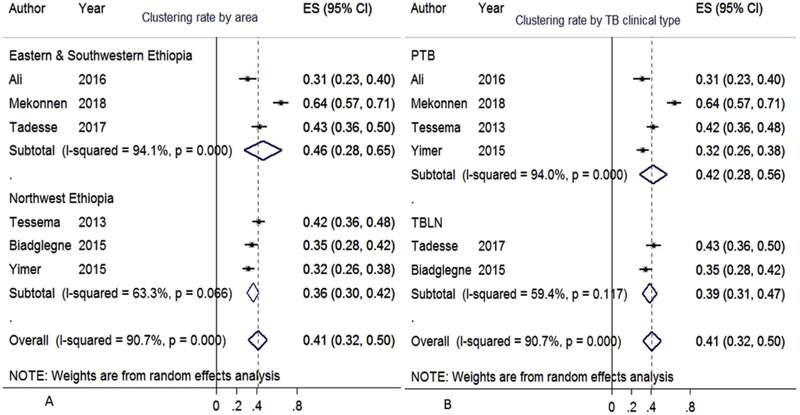
Six articles contained MIRU-VNTR based clustering and RTI data [56,61,79,84,85,89]. Their data collection period span six years (2013–2018). Clinical type and region wise sub-group meta-analysis was performed. The overall pooled clustering rate was 41% (95%CI:32–50%) (Fig. 4). The proportion of pooled clustering rate was 46% in eastern and southwestern Ethiopia and 36% in the northwestern part of the country (Fig. 4A). Fig. 4B depicts the rate of clustering among PTB and TBLN cases at 42% (95%CI: 28–56) and 39% (95%CI: 31–47), respectively. S2C depicts the stepwise cluster and RTI standard error rooted transformations (pooled prevalence) for the six included articles.
Fig. 4.
Pooled clustering rate of M. tuberculosis, Ethiopia, 2005–2018.
The box from the forest plot indicated weight of articles from random effect analysis. The crossed line is the 95% CI, the solid vertical line is zero values to x-axis. Random effect cluster meta-analysis showed individual and pooled estimates stratified by area (A) and by TB clinical type (B).
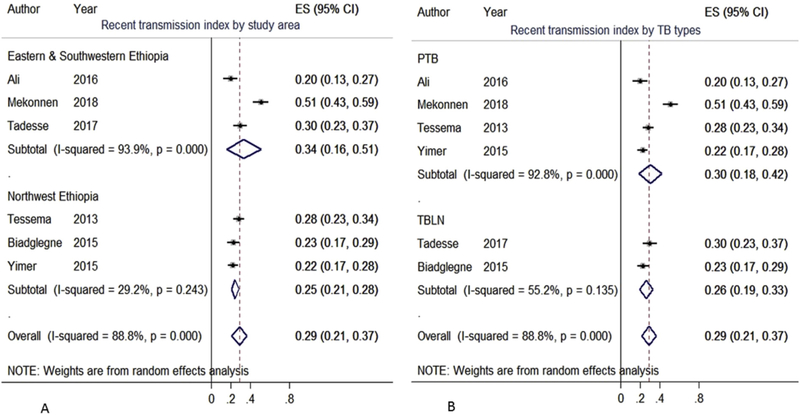
The nationwide pooled RTI was 29% (95%CI: 21–37) (Fig. 5). Further, TB clinical type and region wise sub-group meta-analysis was also performed. Hence, the pooled RTI was higher in eastern and southwestern Ethiopia, 34%, than in northwestern part of the country, 25% (Fig. 5A). As shown in Fig. 5B, the pooled RTI was also higher among PTB (30%) than among TBLN (26%) cases.
Fig. 5.
Pooled recent transmission index of M. tuberculosis, Ethiopia, 2005–2018.
The box from the forest plot indicated weight of articles from random effect analysis. The crossed line is the 95% CI, the solid vertical line is zero values to x-axis. Random effect RTI meta-analysis showed individual and pooled estimates stratified by area (A) and TB clinical type (B).
3.7. Risk of bias across studies
The P-value and I2 statistics showed heterogeneity across studies. To sort out the cause, publication bias was assessed. Part A and B of S3 shows the funnel plot which demonstrate publication bias. Sensitivity analysis also showed no influence of individual study on pooled estimate (S3C). Thus, methodological (demography, study setting, study population) and clinical (TB types, history, co-morbidity) heterogeneity might be responsible for heterogeneity across studies. Moreover, the number of included articles were small and this might also contribute to the heterogeneity. This collectively mirrored the low validity of the clustering and RTI estimates. Thus, readers are advised to interpret the clustering and RTI estimates with heterogeneity in mind.
4. Discussion
The review summarized phylogenetic and phylodynamic characteristics of 4371 clinical isolates from thirty-one articles. Based on the pooled data investigated, the majority of cases were newly diagnosedPTB, male and within the age range of 15–44 years. The TB/HIV co-infection rate was 23.5%, similar to the recent review by Tesfaye et al. (2018) of 25.6% [3]. M. tuberculosis was responsible for 99.5% of TB in Ethiopia and M. bovis accounted for only 0.5% of cases.
Analysis of the national Mtb strain diversity shows the presence of all Mtb lineages except for L5 and L6 (M. africanum) in varying proportions in Ethiopia; from 62.3% for L4 to 0.3% for the L2. Fig. 2A and B displays the slight regional variations with regard to both (sub) lineages. This is in line with a systematic review of spoligotyping based articles in Ethiopia [51]. Phylogeography is shaped by the interplay between multiple interacting factors such as geography, demography, human migration [26], climate [91], genetic drift [92] and host-pa-thogen interaction [93]. However, which factors are the key drivers of Mtb strain regional variation in Ethiopia is not addressed and could be the subject of future research. Furthermore, geographic mosaic of human-Mtb coevolutionary model should also be deciphered in the future to better understand the phylogeographic nature of Mtb.
Out of 1939 shared-types (STs) identified by Brudy et al., 2006 [26], 259 (13.4%) SITs were identified in this nationwide review. Comas et al. (2015) concluded that the population structure of Mtb in Ethiopia is complex and diverse [29]. Moreover, homoplasy, recurrent mutation and recombination as a likely cause of diversity is excluded by another Comas et al. (2013) study [94]. Rather, consecutive entrance of multiple genotypes through human migration and trade was proposed as explanation for such high level of genetic diversity. Ethiopia has long history of contact and admixture with African and Eurasian population groups [95]. According to Comas et al., L3. ETH1 might have been introduced into Ethiopia through trade routes across the Indian Ocean. Moreover, a two-way north-south and south-north internal migration has been going on over centuries with a possible role in its further spread and diversity [29].
Of the total 4371 isolates analyzed for transmission patterns, 3291 (75.3%) were grouped into 146 SIT clusters with cluster size ranges of 2–638 isolates per cluster (S2A). Specifically, L4.2. ETH1/SIT149, L4.10/SIT53, L3. ETH1/SIT25 and L4.6/SIT37 were isolates that had the largest cluster sizes (Table 3). Clustered strains were characterized by higher intracellular growth rate and hypo inflammatory phenotype which could facilitate higher transmission [27,96]. These dominant SITs expected to be specialist strains to Ethiopia having sympatric association within the local community. The spread of TB is a branching process [8] and the presence of high heterogeneity and skewness in the number of secondary cases per infectious individual (Fig. 3) indicates the presence of super-spreaders [97]. Sympatric host-pathogen association is important for establishing longstanding carrier states and shedding of the pathogen for lengthy periods of time [98]. Super-shedding reflects the interaction of the host with the pathogen, whereas super-spreading reflects the interaction of the host with other hosts [99].
Based on MIRU-VNTR, the overall clustering and RTI was 41% and 29%, respectively. These two pooled prevalence rates were higher than the African average clustering rate of 30% and RTI of 20% [40]. Moreover, clustering and RTI were also higher in southwestern and eastern parts of Ethiopia than in the northwestern part of the country. This variation might be due to differences in the incidence of TB, host socio demographic status, study population and the strains [100]. Male gender and young adults were significantly more involved in clustering [101]. Variation in the strength of TB control and prevention packages such as the use of BCG (bacillus Calmette-Guerin) vaccine and isoniazid prophylaxis treatment (IPT), case finding and treatment strategy among regions might also affect rates of clustering [56]. Further, clustering is inversely related with HIV prevalence [102]. It would be interesting to investigate whether the HIV epidemic has impacted on clustering rates in Ethiopia, and whether the lower clustering rate in the northwest is thus related to the HIV epidemic, by comparing trends of HIV co-infection and clustering over time.
Of note is that, MIRU-VNTR or combined MIT and SIT could overestimate clustering and RTI compared to WGS based transmission analysis which uses five to twelve SNP difference as genetic relatedness [103].
Generalizability of these results is subject to certain limitations. First, the study did not include IS6110, large sequence polymorphism and SNP based articles. Second, articles were also retrieved from MEDLINE and Scopus only. Third, gray literature was not searched.
5. Concluding remarks
The Mtb population in Ethiopia is genetically diverse with lineages L4, L3, L1 and L7 making up 62.3%, 21.7%, 7.9% and 3.4% of strains, respectively. Among families, L4.2. ETH1/SIT149, L4.10/SIT53, L3. ETH1/SIT25 and L4.6/SIT37 were the leading clustered isolates. The dominance of a few genotypes argues in favor of the presence of superspreader phenomena. L3/CAS and L7 shared 33% and 8% of the Amhara regional Mtb population structure. L1/EAI was proportionally higher in Afar. L4/EA was relatively higher in Oromia, Southern Nations and Nationalities Region, central Ethiopia and south eastern Ethiopia. The overall pooled clustering and RTI rate was 41% and 29%, respectively. The proportion of pooled clustering and RTI rates was higher in eastern and southwestern Ethiopia than in northwestern region. Ethiopia is one of the epicenters of TB in Africa. Spoligotyping and MIRU-VNTR are prone to convergent evolution and have low resolution power for cluster analysis. Thus, to better understand the evolution and transmission pattern of Mtb in Ethiopia and ultimately implement suitable TB control strategies tailored to the Ethiopian context, WGS based information would be needed.
Supplementary Material
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
List of abbreviations
- ANI
average nucleotide identity
- BCG
bacille Calmette-Guerin
- CAS
Central Asia
- Dddh
digital DNA-DNA hybridization
- DR
Direct repeats
- DS-TB
drug-sensitive TB
- EA
Euro-American
- EAI
EastAfrican Indian
- EAS
East Asia
- EPTB
extra-pulmonary tuberculosis
- ES
study estimate
- ETH
Ethiopia
- IPT
Isoniazid prophylaxis treatment
- JBI
The Joanna Briggs Institute
- LAM
Latin American and Mediterranean
- M.tb
M. tuberculosis
- M/F
Male/Female
- MDR-TB
multidrug resistant TB
- MIRU-VNTR
Mycobacterial Interspersed Repetitive Units-Variable Number Tandem Repeats
- MIT
MIRU-VNTR international type
- MTBC
Mycobacterium tuberculosis complex
- NGS
next-generation sequencing
- NTM
nontuberculosis Mycobacteria
- PGRS
polymorphic G + C rich sequences
- PTB
Pulmonary tuberculosis
- PTB−
sputum smear negative pulmonary tuberculosis
- PTB+
sputum smear positive pulmonary tuberculosis
- RTI
Recent transmission index
- RR
rifampicin resistance
- S
Sicily
- SIT
Spoligotyping international type
- SNNP
Southern nations, nationalities and people’s
- SNPs
single nucleotide polymorphisms
- STROME-ID
Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases
- T
Tuscany
- TB
Tuberculosis
- TBLN
Tuberculous lymphadenitis
- WGS
whole genome sequence
- WHO
World Health Organization
Footnotes
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article and its supplementary files.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tube.2019.101858.
Conflicts of interest
The authors declare that they have no competing interests.
References
- [1].WHO. Global Tuberculosis report. Geneva Switzerland: World Health Oranization; 2018. [Google Scholar]
- [2].Mekonnen D, Derbie A, Desalegn E. TB/HIV co-infections and associated factors among patients on directly observed treatment short course in Northeastern Ethiopia: a 4 years retrospective study. BMC Res Notes 2015;8:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tesfaye B, Alebel A, Gebrie A, Zegeye A, Tesema C, BJPo Kassie. The twin epidemics: prevalence of TB/HIV co-infection and its associated factors in Ethiopia; A systematic review and meta-analysis. PLoS One 2018;13(10):e0203986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].WHO. Global Tuberculosis report. Geneva, Switzerland Geneva, Switzerland: WHO; 2017. Contract No.: WHO/HTM/TB/2017.23. [Google Scholar]
- [5].Mekonnen D, Derbie A, Mekonnen H, Zenebe Y. Profile and treatment outcomes of patients with tuberculosis in Northeastern Ethiopia: a cross sectional study. Afr Health Sci 2016;16(3):663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zenebe Y, Adem Y, Mekonnen D, Derbie A, Bereded F, Bantie M, et al. Profile of tuberculosis and its response to anti-TB drugs among tuberculosis patients treated under the TB control programme at Felege-Hiwot Referral Hospital, Ethiopia. BMC Public Health 2016;16(688). 016–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Worku S, Derbie A, Mekonnen D, Biadglegne F. Treatment outcomes of tuberculosis patients under directly observed treatment short-course at Debre Tabor General Hospital, northwest Ethiopia: nine-years retrospective study. Infectious diseases of poverty 2018;7(1):018–0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ypma RJ, Altes HK, van Soolingen D, Wallinga J, van Ballegooijen WM. A sign of superspreading in tuberculosis: highly skewed distribution of genotypic cluster sizes. Epidemiology 2013:395–400. [DOI] [PubMed] [Google Scholar]
- [9].Girum T, Muktar E, Lentiro K, Wondiye H, Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Tropical diseases, travel medicine and vaccines 2018;4(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Eshetie S, Alebel A, Wagnew F, Geremew D, Fasil A, Sack U. Current treatment of multidrug resistant tuberculosis in Ethiopia: an aggregated and individual patients’ data analysis for outcome and effectiveness of the current regimens. BMC Infect Dis 2018;18(1):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jha P, Jain A, Arora K, Bose M. Genetic association study suggests a role for SP110 variants in lymph node tuberculosis but not pulmonary tuberculosis in north Indians. Hum Immunol 2011;72(7):576–80. [DOI] [PubMed] [Google Scholar]
- [12].Fox GJ, Sy DN, Nhung NV, Yu B, Ellis MK, Van Hung N, et al. Polymorphisms of SP110 are associated with both pulmonary and extra-pulmonary tuberculosis among the Vietnamese. PLoS One 2014;9(7):e99496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liang L, Zhao Y-l, Yue J, Liu J-f, Han M, Wang H, et al. Association of SP110 gene polymorphisms with susceptibility to tuberculosis in a Chinese population. Infect Genet Evol 2011;11(5):934–9. [DOI] [PubMed] [Google Scholar]
- [14].Li X, Yang Y, Zhou F, Zhang Y, Lu H, Jin Q, et al. SLC11A1 (NRAMP1) polymorphisms and tuberculosis susceptibility: updated systematic review and metaanalysis. PLoS One 2011;6(1):e15831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bellamy R, Ruwende C, Corrah T, Mcadam KP, Whittle HC, Hill AV. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N Engl J Med 1998;338(10):640–4. [DOI] [PubMed] [Google Scholar]
- [16].Motsinger-Reif AA, Antas PR, Oki NO, Levy S, Holland SM, Sterling TR. Polymorphisms in IL-1ß, vitamin D receptor Fok 1, and Toll-like receptor 2 are associated with extrapulmonary tuberculosis. BMC Med Genet 2010;11(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cailhol J, Decludt B, Che D. Sociodemographic factors that contribute to the development of extrapulmonary tuberculosis were identified. J Clin Epidemiol 2005;58(10):1066–71. [DOI] [PubMed] [Google Scholar]
- [18].Click ES, Moonan PK, Winston CA, Cowan LS, Oeltmann JE. Relationship between Mycobacterium tuberculosis phylogenetic lineage and clinical site of tuberculosis. Clin Infect Dis 2012;54(2):211–9. [DOI] [PubMed] [Google Scholar]
- [19].Garda de Viedma D, Lorenzo G, Cardona P-J, Alonso Rodriguez N, Gordillo S, Ruiz Serrano MJ, et al. Association between the infectivity of Mycobacterium tuberculosis strains and their efficiency for extrarespiratory infection. J Infect Dis 2005;192(12):2059–65. [DOI] [PubMed] [Google Scholar]
- [20].Brites D, Gagneux S. Co-evolution of Mycobacterium tuberculosis and Homo sapiens. Immunol Rev 2015;264(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Singh P, Cole ST. Mycobacterium leprae: genes, pseudogenes and genetic diversity. Future Microbiol 2011;6(1):57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zingue D, Bouam A, Tian RB, Drancourt M. Buruli ulcer, a prototype for ecosystem-related infection, caused by Mycobacterium ulcerans. Clin Microbiol Rev 2018;31(1). e00045–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Porvaznik I, Solovic I, Mokry J. Non-tuberculous mycobacteria: classification, diagnostics, and therapy. Respiratory Treatment and Prevention 2016:19–25. Springer. [DOI] [PubMed] [Google Scholar]
- [24].Primm TP, Lucero CA, Falkinham JO. Health impacts of environmental mycobacteria. Clin Microbiol Rev 2004;17(1):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Riojas MA, McGough KJ, Rider-Riojas CJ, Rastogi N, Hazbôn MH. Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis. Int J Syst Evol Microbiol 2017;68(1):324–32. [DOI] [PubMed] [Google Scholar]
- [26].Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, et al. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol 2006;6(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gagneux S, Small PM. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis 2007;7(5):328–37. [DOI] [PubMed] [Google Scholar]
- [28].Coll FM, Guerra-Assuncao AJ, Glynn RJ, Perdigao J, Viveiros M, Portugal I, Pain A, Martin N, Clark GT. A robust SNP barcode for typingMycobacterium tuberculosis complex strains. Nat Commun 2014;5(4812):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Comas I, Hailu E, Kiros T, Bekele S, Mekonnen W, Gumi B, et al. Population genomics of Mycobacterium tuberculosis in Ethiopia contradicts the virgin soil hypothesis for human tuberculosis in Sub-Saharan Africa. Curr Biol 2015;25(24):3260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McDougall I, Brown FH, Fleagle JG. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature 2005;433(7027):733. [DOI] [PubMed] [Google Scholar]
- [31].Gagneux S. Ecology and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol 2018;16(4):202. [DOI] [PubMed] [Google Scholar]
- [32].Pasipanodya JG, Moonan PK, Vecino E, Miller TL, Fernandez M, Slocum P, et al. Allopatric tuberculosis host-pathogen relationships are associated with greater pulmonary impairment. Infect Genet Evol 2013;16:433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fenner L, Egger M, Bodmer T, Furrer H, Ballif M, Battegay M, et al. HIV infection disrupts the sympatric host-pathogen relationship in human tuberculosis. PLoS Genet 2013;9(3):e1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cole S, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nat Genet 1998;393(6685):537. [DOI] [PubMed] [Google Scholar]
- [35].Coscolla M, Gagneux S, editors. Consequences of genomic diversity in Mycobacterium tuberculosis. Semin Immunol. Elsevier; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jagielski T, Van Ingen J, Rastogi N, Dziadek J, Mazur PK, JJBri Bielecki. Current methods in the molecular typing of Mycobacterium tuberculosis and other mycobacteria. 2014. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Driscoll JR. Spoligotyping for molecular epidemiology of the Mycobacterium tuberculosis complex. Molecular Epidemiology of Microorganisms. Springer; 2009. p. 117–28. [DOI] [PubMed] [Google Scholar]
- [38].Galagan JE. Genomic insights into tuberculosis. Nat Rev Genet 2014;15(5):307. [DOI] [PubMed] [Google Scholar]
- [39].Stucki D, Brites D, Jeljeli L, Coscolla M, Liu Q, Trauner A, et al. Mycobacterium tuberculosis lineage 4 comprises globally distributed and geographically restricted sublineages. Science 2016;48(12):1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gagneux S. Strain variation in the Mycobacterium tuberculosis complex- its role in biology, epidemiology and control. Switzerland: Springer International Publishing; 2017. p. 319. [Google Scholar]
- [41].Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. In Pract 2010;42(6):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Coscolla M, Copin R, Sutherland J, Gehre F, De Jong B, Owolabi O, et al. M. tuberculosis T cell epitope analysis reveals paucity of antigenic variation and identifies rare variable TB antigens. Cell Host Microbe 2015;18(5):538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Meehan CJ, Moris P, Kohl TA, Pecerska J, Akter S, Merker M, et al. The relationship between transmission time and clustering methods in. Mycobacterium tuberculosis epidemiology 2018;37:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Blower SM, Mclean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, Moss AR. The intrinsic transmission dynamics of tuberculosis epidemics. 1995;1:815 8. [DOI] [PubMed] [Google Scholar]
- [45].Bennett DE, Onorato IM, Ellis BA, Crawford JT, Schable B, Byers R, et al. DNA fingerprinting of Mycobacterium tuberculosis isolates from epidemiologically linked case pairs. Emerg Infect Dis 2002;8(11):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jerene D, Melese M, Kassie Y, Alem G, Daba SH, Hiruye N, et al. The yield of a tuberculosis household contact investigation in two regions of Ethiopia. Int J Tuberc Lung Dis 2015;19(8):898–903. [DOI] [PubMed] [Google Scholar]
- [47].Gashu Z, Jerene D, Ensermu M, Habte D, Melese M, Hiruy N, et al. The yield of community-based “retrospective” tuberculosis contact investigation in a high burden setting in Ethiopia. PLoS One 2016;11(8):e0160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hiruy N, Melese M, Habte D, Jerene D, Gashu Z, Alem G, et al. Comparison of the yield of tuberculosis among contacts of multidrug-resistant and drug-sensitive tuberculosis patients in Ethiopia using GeneXpert as a primary diagnostic test. Int J Infect Dis 2018;71:4–8. [DOI] [PubMed] [Google Scholar]
- [49].Borrell S, Espanol M, Orcau A, Tudô G, March F, Cayla JA, et al. Factors associated with differences between conventional contact tracing and molecular epidemiology in study of tuberculosis transmission and analysis in the city of Barcelona, Spain. J Clin Microbiol 2009;47(1):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mathema B, Andrews JR, Cohen T, Borgdorff MW, Behr M, Glynn JR, et al. Drivers of tuberculosis transmission. J Infect Dis 2017;216(suppl_6):S644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tulu B, Ameni G. Spoligotyping based genetic diversity of Mycobacterium tuberculosis in Ethiopia: a systematic review. BMC Infect Dis 2018;18(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gallo V, Egger M, McCormack V, Farmer PB, Ioannidis JP, Kirsch-Volders M, et al. STrengthening the reporting of OBservational studies in epidemiology-molecular epidemiology (STROBE-ME): an extension of the STROBE statement. Mutagenesis 2011;27(1):17–29. [DOI] [PubMed] [Google Scholar]
- [53].[Internet] Critical Appraisal Tools. Checklist for analytical cross sectional studies. University of Adelaide; Joanna Briggs Institute; 2018. [cited cited 2018 15 October 2018]. Available from:. http://joannabriggs.org/research/critical-appraisal-tools.html. [Google Scholar]
- [54].Hamblion EL, Le Menach A, Anderson LF, Lalor MK, Brown T, Abubakar I, et al. Recent TB transmission, clustering and predictors of large clusters in London, 2010–2012: results from first 3 years of universal MIRU-VNTR strain typing. Thorax 2016;71(8):749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Borgdorff MW, Nagelkerke N, van Soolingen D, de Haas PE, Veen J, van Embden JD. Analysis of tuberculosis transmission between nationalities in The Netherlands in the period 1993–1995 using DNA fingerprinting. Am J Epidemiol 1998;147(2):187–95. [DOI] [PubMed] [Google Scholar]
- [56].Tanaka MMFA. Methods of quantifying and visualising outbreaks of tuberculosis using genotypic information. Infect Genet Evol 2005;5:35–43. [DOI] [PubMed] [Google Scholar]
- [57].Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat 1950:607–11. [Google Scholar]
- [58].DerSimonian R, Laird N. Meta-analysis in clinical trials. Contr Clin Trials 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- [59].Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in metaanalyses. BMJ Br Med J (Clin Res Ed) 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Agonafir M, Lemma E, Wolde-Meskel D, Goshu S, Santhanam A, Girmachew F, et al. Phenotypic and genotypic analysis of multidrug-resistant tuberculosis in Ethiopia. Int J Tuberc Lung Dis 2010;14(10):1259–65. [PubMed] [Google Scholar]
- [61].Ali S, Beckert P, Haileamlak A, Wieser A, Pritsch M, Heinrich N, et al. Drug resistance and population structure of M. tuberculosis isolates from prisons and communities in Ethiopia. 2016;16(1):687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ameni G, Tadesse K, Hailu E, Deresse Y, Medhin G, Aseffa A, et al. Transmission of Mycobacterium tuberculosis between farmers and cattle in central Ethiopia. PLoS One 2013;8(10):e76891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bedewi Z, Worku A, Mekonnen Y, Yimer G, Medhin G, Mamo G, et al. Molecular typing of Mycobacterium tuberculosis complex isolated from pulmonary tuberculosis patients in central Ethiopia. BMC Infect Dis 2017;17(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Belay M, Ameni G, Bjune G, Couvin D, Rastogi N, Abebe F. Strain diversity of Mycobacterium tuberculosis isolates from pulmonary tuberculosis patients in Afar pastoral region of Ethiopia. BioMed Res Int 2014;2014:238532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Biadglegne F, Merker M, Sack U, Rodloff AC, Niemann S. Tuberculous lymphadenitis in Ethiopia predominantly caused by strains belonging to the Delhi/CAS lineage and newly identified Ethiopian clades of the Mycobacterium tuberculosis complex. PLoS One 2015;10(9):e0137865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chemeda A, Mihret A, Abebe T, Worku A, Ameni G. Genotyping of mycobacterium tuberculosis isolated from pulmonary tuberculosis patients among people living with HIV in Addis Ababa: cross-sectional study. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 2018;12:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Debebe T, Admassu A, Mamo G, Ameni G. Molecular characterization of Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients in Felege Hiwot Referral Hospital, northwest Ethiopia. J Microbiol Immunol Infect 2014;47(4):333–8. [DOI] [PubMed] [Google Scholar]
- [68].Deribew A, Abebe G, Apers L, Abdissa A, Deribe F, Woldemichael K, et al. Prevalence of pulmonary TB and spoligotype pattern of Mycobacterium tuberculosis among TB suspects in a rural community in Southwest Ethiopia. BMC Infect Dis 2012;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Diriba B, Berkessa T, Mamo G, Tedla Y, Ameni G. Spoligotyping of multidrug-resistant Mycobacterium tuberculosis isolates in Ethiopia. Int J Tuberc Lung Dis 2013;17(2):246–50. [DOI] [PubMed] [Google Scholar]
- [70].Disassa H, Tafess K, Worku A, Ameni G. A preliminary study on molecular characterization ofMycobacterium tuberculosis in Benishangul Gumuz region, Western Ethiopia. British Microbiol Res J 2015;10(6):1–10. 10.9734/BMRJ/2015/20032. [DOI] [Google Scholar]
- [71].Firdessa R, Berg S, Hailu E, Schelling E, Gumi B, Erenso G, et al. Mycobacterial lineages causing pulmonary and extrapulmonary tuberculosis, Ethiopia. Emerg Infect Dis 2013;19(3):460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Garedew L, Mihret A, Ameni G. Molecular typing of mycobacteria isolated from extrapulmonary tuberculosis patients at Debre Birhan Referral Hospital, central Ethiopia. Scand J Infect Dis 2013;45(7):512–8. [DOI] [PubMed] [Google Scholar]
- [73].Garedew L, Mihret A, Mamo G, Abebe T, Firdessa R, Bekele Y, et al. Strain diversity of mycobacteria isolated from pulmonary tuberculosis patients at Debre Birhan Hospital, Ethiopia. Int J Tuberc Lung Dis 2013;17(8):1076–81. [DOI] [PubMed] [Google Scholar]
- [74].Gebrezgabiher G, Romha G, Ameni G. Spoligotyping of mycobacterium tuberculosis isolates from tuberculosis diagnosed patients at dilla university referral hospital and other private clinics, Southern Ethiopia. Asian Pacific Journal of Tropical Disease 2015;5(4):329–33. [Google Scholar]
- [75].Getahun M, Ameni G, Kebede A, Yaregal Z, Hailu E, Medihn G, et al. Molecular typing and drug sensitivity testing of Mycobacterium tuberculosis isolated by a community-based survey in Ethiopia. BMC Public Health 2015;15:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gumi B, Schelling E, Berg S, Firdessa R, Erenso G, Mekonnen W, et al. Zoonotic transmission of tuberculosis between pastoralists and their livestock in South-East Ethiopia. EcoHealth 2012;9(2):139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Korma W, Mihret A, Hussien J, Anthony R, Lakew M, Aseffa A. Clinical, molecular and drug sensitivity pattern of mycobacterial isolates from extra-pulmonary tuberculosis cases in Addis Ababa, Ethiopia. BMC Infect Dis 2015;15:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Maru M, Mariam SH, Airgecho T, Gadissa E, AJTr Aseffa, treatment. Prevalence of tuberculosis, drug susceptibility testing, and genotyping of mycobacterial isolates from pulmonary tuberculosis patients in Dessie, Ethiopia. 2015. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mekonnen A, Merker M, Collins JM, Addise D, Aseffa A, Petros B, et al. Molecular epidemiology and drug resistance patterns of mycobacterium tuberculosis complex isolates from university students and the local community in Eastern Ethiopia. PLoS One 2018;13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mihret A, Bekele Y, Aytenew M, Assefa Y, Abebe M, Wassie L, et al. Modern lineages of Mycobacterium tuberculosis in Addis Ababa, Ethiopia: implications for the tuberculosis control programe. Afr Health Sci 2012;12(3):339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Molina-Moya B, Agonafir M, Blanco S, Dacombe R, Gomgnimbou MK, Spinasse L, et al. Microbead-based spoligotyping of Mycobacterium tuberculosis from Ziehl-Neelsen-stained microscopy preparations in Ethiopia. Sci Rep 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nuru A, Mamo G, Worku A, Admasu A, Medhin G, Pieper R, et al. Genetic diversity of Mycobacterium tuberculosis complex isolated from tuberculosis patients in bahir dar city and its surroundings, northwest Ethiopia. BioMed Res Int 2015;2015:174732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Nuru A, Mamo G, Zewude A, Mulat Y, Yitayew G, Admasu A, et al. Preliminary investigation of the transmission of tuberculosis between farmers and their cattle in smallholder farms in northwestern Ethiopia: a cross-sectional study. BMC Res Notes 2017;10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Tadesse M, Abebe G, Bekele A, Bezabih M, de Rijk P, Meehan CJ, et al. The predominance of Ethiopian specific Mycobacterium tuberculosis families and minimal contribution of Mycobacterium bovis in tuberculous lymphadenitis patients in Southwest Ethiopia. Infect Genet Evol 2017;55:251–9. [DOI] [PubMed] [Google Scholar]
- [85].Tessema B, Beer J, Merker M, Emmrich F, Sack U, Rodloff AC, et al. Molecular epidemiology and transmission dynamics of Mycobacterium tuberculosis in Northwest Ethiopia: new phylogenetic lineages found in Northwest Ethiopia. BMC Infect Dis 2013;13:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tilahun M, Ameni G, Desta K, Zewude A, Yamuah L, Abebe M, et al. Molecular epidemiology and drug sensitivity pattern of Mycobacterium tuberculosis strains isolated from pulmonary tuberculosis patients in and around Ambo Town, Central Ethiopia. PLoS One 2018;13(2):e0193083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Workalemahu B, Berg S, Tsegaye W, Abdissa A, Girma T, Abebe M, et al. Genotype diversity of Mycobacterium isolates from children in Jimma, Ethiopia. BMC Res Notes 2013;6:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yimer SA, Hailu E, Derese Y, Bjune GA, Holm-Hansen C. Spoligotyping of Mycobacterium tuberculosis isolates among pulmonary tuberculosis patients in Amhara Region, Ethiopia. APMIS 2013;121(9):878–85. [DOI] [PubMed] [Google Scholar]
- [89].Yimer SA, Norheim G, Namouchi A, Zegeye ED, Kinander W, Tonjum T, et al. Mycobacterium tuberculosis lineage 7 strains are associated with prolonged patient delay in seeking treatment for pulmonary tuberculosis in Amhara Region, Ethiopia. J Clin Microbiol 2015;53(4):1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zewdie O, Mihret A, Ameni G, Worku A, Gemechu T, Abebe T. Molecular typing of mycobacteria isolated from tuberculous lymphadenitis cases in Addis Ababa, Ethiopia. Int J Tuberc Lung Dis 2016;20(11):1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Beiranvand R, Karimi A, Delpisheh A, Sayehmiri K, Soleimani S, Ghalavandi S. Correlation assessment of climate and geographic distribution of tuberculosis using geographical information system (GIS). Iran J Public Health 2016;45(1):86. [PMC free article] [PubMed] [Google Scholar]
- [92].Hershberg R, Lipatov M, Small PM, Sheffer H, Niemann S, Homolka S, et al. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol 2008;6(12):e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Brites D, Gagneux S. Co-evolution of M ycobacterium tuberculosis and H omo sapiens. Immunol Rev 2015;264(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet 2013;45(10):1176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Pagani L, Kivisild T, Tarekegn A, Ekong R, Plaster C, Romero IG, et al. Ethiopian genetic diversity reveals linguistic stratification and complex influences on the Ethiopian gene pool. Am J Hum Genet 2012;91(1):83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Theus S, Cave M, Eisenach K. Intracellular Macrophage Growth Rates and Cytokine Profiles of Mycobacterium tuberculosis Strains with Different Transmission Dynamics. J Infect Dis 2005;191:453–60. [DOI] [PubMed] [Google Scholar]
- [97].Stein RA. Super-spreaders in infectious diseases. Int J Infect Dis 2011;15(8):e510–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gopinath SLJS, Bouley DM, Elias JE, Monack DM, Yohannes AG. Role of disease-associated tolerance in infectious superspreaders. Proc Natl Acad Sci 2014;111(41):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Chase-Topping MGD, Low C, Matthews L, Woolhouse M. Super-shedding and the link between human infection and livestock carriage of Escherichia coliO157. Nat Rev Microbiol 2008;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].VanderWaal KL, Ezenwa VO. Heterogeneity in pathogen transmission: mechanisms and methodology. Funct Ecol 2016;30(10):1606–22. [Google Scholar]
- [101].Borgdorff MW, Nagelkerke NJ, De Haas PE, van Soolingen D. Transmission of Mycobacterium tuberculosis depending on the age and sex of source cases. Am J Epidemiol 2001;154(10):934–43. [DOI] [PubMed] [Google Scholar]
- [102].Murray M. Determinants of cluster distribution in the molecular epidemiology of tuberculosis. Proc Natl Acad Sci 2002;99(3):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Stucki D, Ballif M, Egger M, Furrer H, Altpeter E, Battegay M, et al. Standard genotyping overestimates transmission of Mycobacterium tuberculosis among immigrants in a low-incidence country. J Clin Microbiol 2016;54(7):1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.