Abstract
Background & Aims:
Incidence rates for hepatocellular carcinoma (HCC) increased rapidly in the United States (US) since the 1990s, but have plateaued or started to decrease in other industrialized countries. It unclear if and when a similar trend will be observed in the US. We examined trends in HCC incidence rates in the US by age, sex, and race/ethnicity of patients.
Methods:
We calculated age-adjusted HCC incidence rates using data from the Surveillance, Epidemiology, and End Results program of cancer registries from 1992 through 2015. We estimated incidence rates by 10-year age group and used joinpoint regression to quantify the magnitude and direction of trends, overall and by sex and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and Asian/Pacific Islander).
Results:
HCC incidence increased by 4.8% per year from 1992 through 2010 (from 4.1 per 100,000 to 9.4 per 100,000) but then started to plateau (annual percent change, −0.7; 95% CI, −2.0 to 0.7). Incidence rates steadily increased among persons 60 years or older in all racial/ethnic groups except Asian/Pacific Islanders 70–79 years old. In contrast, incidence rates decreased in younger and middle-aged adults, in men and women of all races/ethnicities, beginning in the mid-2000s. Rates decreased by 6.2% per year in persons 40–49 years old and by 10.3% per year in persons 50–59 years old. Annual decreases in incidence were larger among middle-aged blacks (17.2% decrease per year since 2012) compared to adults of the same age inother racial/ethnic groups.
Conclusions:
In an analysis of data from the Surveillance, Epidemiology, and End Results program of cancer registries from 1992 through 2015, we found the incidence of HCC to be decreasing among younger and middle-aged adults in the US, regardless of sex or race or ethnicity. It is unclear whether current decreases in incidence will reduce the burden of HCC in the future.
Keywords: SEER, liver cancer, prevalence, epidemiology
Introduction
Liver cancer is a leading cause of cancer-related death worldwide.1 Hepatocellular carcinoma (HCC), the most common form of primary liver cancer, occurs most often in the background of cirrhosis;2 risk factors include hepatitis B (HBV) and hepatitis C (HCV) virus infections, alcohol use, and nonalcoholic fatty liver disease (NAFLD). HCC incidence rates have increased dramatically in the U.S. over the past three decades,3, 4 but recent data suggest rates may have plateaued or begun to decline in some European and Asian countries.5
Decreasing rates in Asian countries may be due, in part, to reduced aflatoxin exposure, improvements in HBV vaccination programs, and treatment for both HBV and HCV infections.6 Although HBV is the leading cause of HCC globally, HCV is the most common risk factor for HCC in the U.S. and accounts for the largest increases in incidence since the 1990s.6,7 Efforts to increase HCV screening uptake among the high-risk baby boomer cohort, combined with the use of direct-acting antivirals, has led to a greater number of patients achieving sustained viral response, which has been shown to considerably reduce HCC risk.8 However, despite declines in HCV infection, HCC rates are projected to continue to rise in the U.S.,9 given the aging population, increasing alcohol consumption,10 and growing prevalence of obesity and fatty liver disease among younger adults.11 Trends in HCC risk factors may also differ by race/ethnicity, and minority populations tend to have higher prevalence of obesity and metabolic syndrome compared to whites.12, 13 Therefore, the cumulative effect of changes in prevalence of HCC risk factors may impact projected incidence rates quite differently in population subgroups and over time.
Although prior studies have described trends in HCC incidence rates, to our knowledge, few have examined age-related differences by race/ethnicity and sex, particularly among younger and middle-aged adults (age < 60 years). In this study, we estimated HCC incidence rates during 1992-2015 using population-based data from the Surveillance, Epidemiology and End Results (SEER) program.
Methods
We derived HCC incidence using data from the National Cancer Institute’s SEER program of cancer registries from 1992 to 2015. SEER routinely collects information on patient demographics and tumor characteristics for all cancers diagnosed in defined geographic regions. SEER 13 registries include Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, rural Georgia, and Alaska Native, representing approximately 14% of the U.S. population. HCC was defined anatomically as located in the liver (International Classification of Disease for Oncology, Third Edition [ICD-O-3] topography code C22) and histologically as hepatocellular carcinoma (ICD-O-3 morphology codes 8170-8175). We estimated age-adjusted (to the 2000 U.S. standard population) and age-specific (10-year age groups) incidence using SEER*Stat (version 8.3.5) as incidence rates per 100,000 persons. Corresponding 95% confidence intervals were calculated as modified gamma intervals.14
We used Joinpoint Regression Program (version 4.6.0) to quantify the magnitude and direction of incidence trends, overall and by 10-year age group, allowing a maximum of four joinpoints. The joinpoint model uses permutation analysis to fit a series of joined straight lines on a logarithmic scale to observed rates.15 The slope of the line segment between joinpoints is equivalent to the annual percent change (APC). Two-sided P values of less than 0.05 were considered to indicate statistical significance, whereby the APC is significantly different from zero.
To account for differences by race/ethnicity and sex, we also conducted joinpoint analyses (by 10-year age group) in four racial/ethnic groups and for men and women. Racial/ethnic groups included: non-Hispanic white (white), non-Hispanic black (black), Hispanic, and Asian/ Pacific Islander. Hispanic ethnicity is based on the NAACCR Hispanic/Latino Identification Algorithm (version 2.2.1), which uses Spanish/Hispanic origin, last name, maiden name, birthplace, and race to indirectly and directly assign ethnicity.16, 17 The NAACR Asian Pacific Islander Identification Algorithm (version 1.2.1)18 uses a similar combination of variables to classify cases as Asian/Pacific Islander.
Results
Overall incidence trends
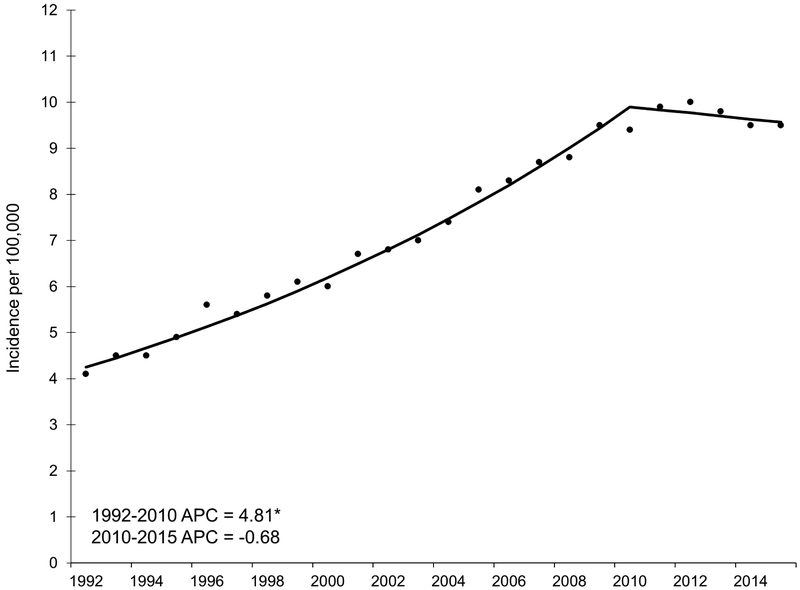
There were 51,188 incident HCC cases diagnosed among adults (ages 20-85+ years) during the study period. The overall incidence rate was 7.7 per 100,000, increasing from 4.1 per 100,000 in 1992 to 9.5 per 100,000 in 2015 (incidence rate ratio [IRR] 2.32, 95% CI 2.16, 2.50).
Across all age groups, incidence increased by 4.8% per year from 1992 to 2010 (Figure 1). Rates plateaued starting in 2010; although not statistically significant, the APC (−0.7, 95% CI −2.0, 0.7) suggested rates slightly decreased from 2010 to 2015.
Figure 1.
Annual percent change (APC) in age-adjusted incidence rates of hepatocellular carcinoma, SEER 13, 1992 – 2015.
NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test.
Trends by age
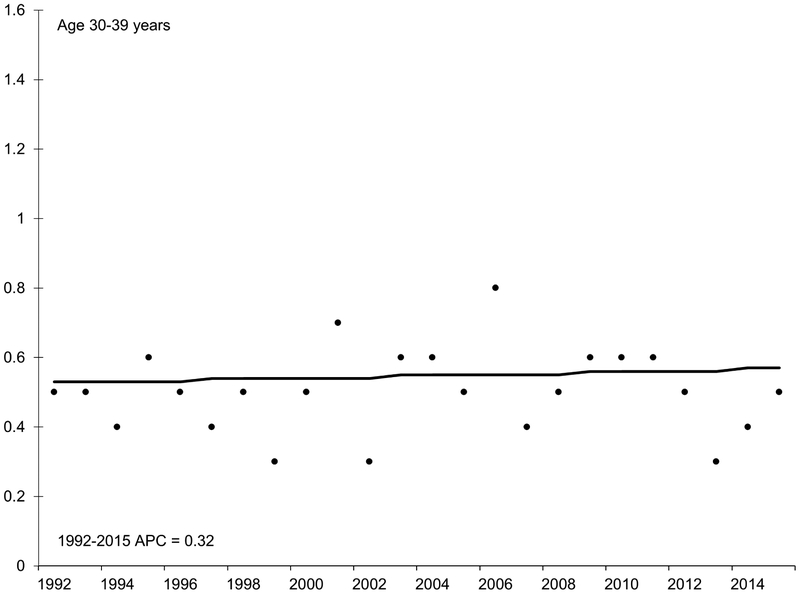
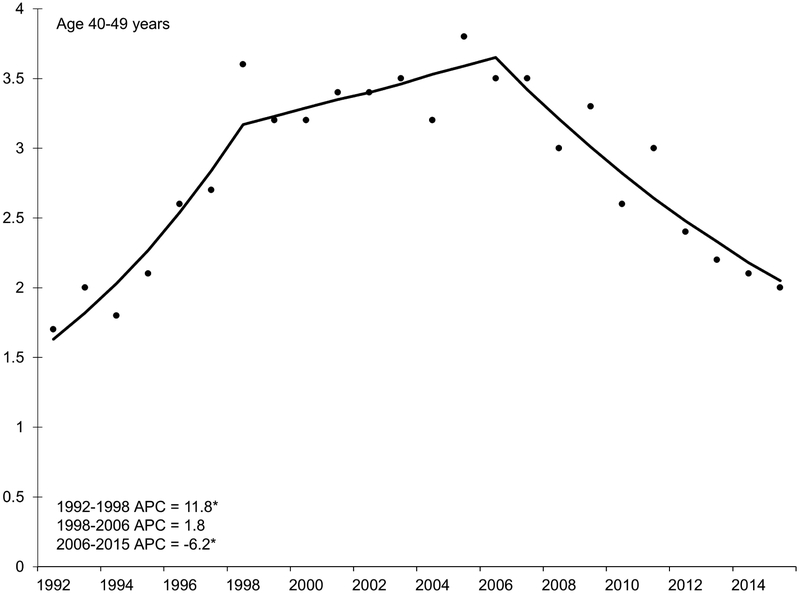
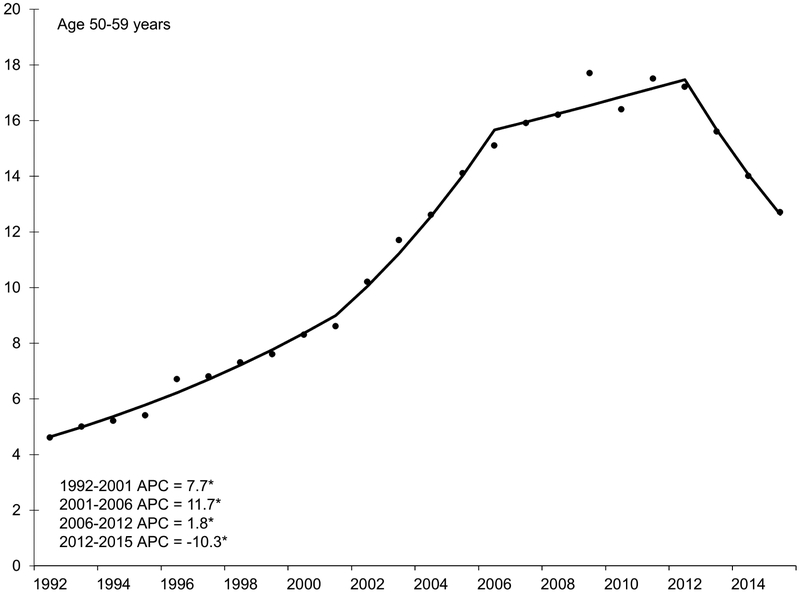
With the exception of the youngest age group (20-29 years), HCC incidence rates increased during the 1990s and early 2000s (Figure 2). Incidence began to decline in middle-aged adults in the mid-2000s, decreasing by 6.2% per year in adults age 40-49 years and by 10.3% per year in adults age 50-59 years. Specifically, rates decreased from 3.5 per 100,000 in 2006 to 2.0 per 100,000 in 2015 among the 40-49 year age group (IRR 0.57, 95% CI 0.45, 0.72). The 50-59 year age group experienced a similar decrease during this time period but of larger magnitude (from 15.1 to 12.7 per 100,000; IRR 0.84, 95% CI 0.76, 0.93).
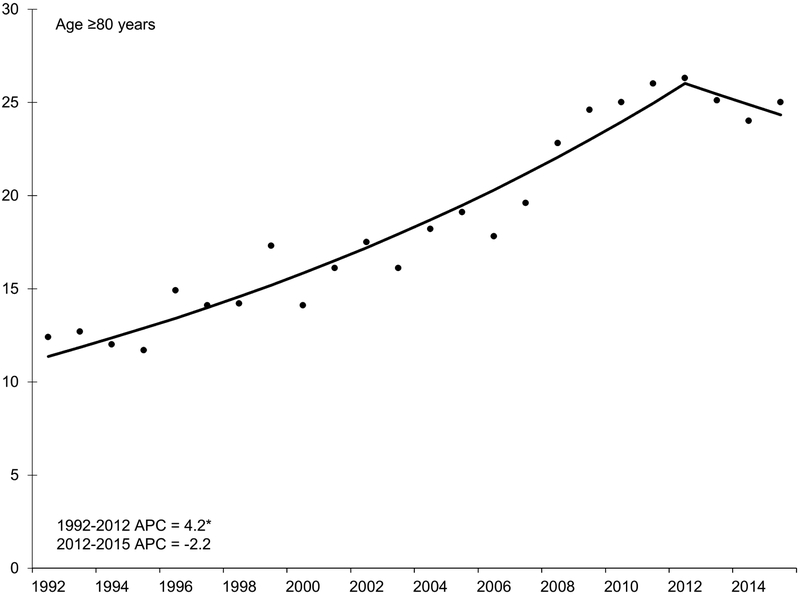
Figure 2.
Annual percent change (APC) in age-specific incidence rates of hepatocellular carcinoma, SEER 13, 1992 – 2015.
NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test. APC in 20-29 year age group could not be estimated because the standard error was available and/or number of cases too small. Y-axis scale varies across figures to demonstrate trend.
There were continued increases in incidence among adults older than age 60 years. From the early 1990s through about 2010, incidence rates increased by 3.8% and 4.2% per year in adults age 70-79 and 80-89 years, respectively. Starting in 2006, rates increased rapidly in 60-69 year olds by 6.6% per year. Increasing rates appeared to slow after 2010, particularly for adults age ≥70 years; however, this trend was not statistically significant.
We observed a shift in the distribution of stage at diagnosis during the study period, with an increasing proportion of patients detected at a localized stage across all age groups (Supplementary Table 1). For example, in the 40-49 year age group, the proportion of cases diagnosed as local stage increased from 30.7% in 1992-98 to 49.4% in 2006-15.
Trends by race/ethnicity
We observed similar incidence trends across race/ethnicity (Table 1, Supplemental Figures 1-4). Rates consistently decreased in adults ages 40-49 and 50-59 years starting in the mid-2000s. These annual decreases in incidence were larger in middle-aged blacks compared to adults of the same age inother racial/ethnic groups. For example, after 2012, rates decreased among 50-59 year olds by 12.2% per year in non-Hispanic whites, 17.2% per year in non-Hispanic blacks, 12.4% per year in Hispanics, and 8.3% per year in Asian/Pacific Islanders. There were similar decreases in 40-49 year olds, but these declines generally started earlier, around 2006. With the exception of Asian/Pacific Islanders age 70-79 years, incidence rates steadily increased among older (age ≥60 years) adults across all racial/ethnic groups.
Table 1.
Trends in hepatocellular carcinoma incidence rates by race/ethnicity and 10-year age group, SEER 13, 1992-2015
| Race/ethnicity | Age (y) | N | Trend 1 | Trend 2 | Trend 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | APC | 95% CI | Years | APC | 95% CI | Years | APC | 95% CI | |||
| Non-Hispanic white | 20-29 | 96 | -- | -- | -- | ||||||
| 30-39 | 179 | -- | -- | -- | |||||||
| 40-49 | 1,354 | 1992-98 | 19.0* | 8.3, 30.8 | 1998-09 | −0.2 | −2.4, 2.1 | 2009-15 | −12.2* | −18.9, −5.1 | |
| 50-59 | 5,897 | 1992-08 | 11.7* | 10.6, 12.8 | 2008-12 | 0.4 | −6.6, 7.9 | 2012-15 | −9.0* | −15.8, −1.6 | |
| 60-69 | 6,607 | 1992-04 | 2.5* | 1.3, 3.8 | 2004-15 | 8.7* | 7.7, 9.7 | ||||
| 70-79 | 5,434 | 1992-15 | 2.6* | 2.2, 3.0 | |||||||
| ≥80 | 2,994 | 1992-15 | 3.2* | 2.6, 3.8 | |||||||
| Non-Hispanic black | 20-29 | 34 | -- | -- | -- | ||||||
| 30-39 | 111 | -- | -- | -- | |||||||
| 40-49 | 544 | 1992-05 | 2.2 | −1.3, 5.8 | 2005-15 | −10.4* | −15.4, −5.1 | ||||
| 50-59 | 2,206 | 1992-06 | 11.2* | 9.2, 13.3 | 2006-12 | −1.2 | −5.9, 3.7 | 2012-15 | −17.2* | −26.7, −6.4 | |
| 60-69 | 2,246 | 1992-03 | 3.8* | 0.1, 7.6 | 2003-15 | 9.8* | 7.9, 11.7 | ||||
| 70-79 | 957 | 1992-15 | 2.5* | 1.6, 3.4 | |||||||
| ≥80 | 361 | 1992-15 | 1.1 | −0.7, 2.9 | |||||||
| Hispanic | 20-29 | 41 | -- | -- | -- | ||||||
| 30-39 | 102 | 1992-15 | 0.5 | −2.9, 4.0 | |||||||
| 40-49 | 904 | 1992-06 | 7.1* | 4.3, 10.0 | 2006-15 | −8.2* | −11.6, −4.6 | ||||
| 50-59 | 2,783 | 1992-05 | 8.0* | 6.3, 9.8 | 2005-12 | 3.3* | 0.4, 6.3 | 2012-15 | −12.4* | −19.4, −4.8 | |
| 60-69 | 2,742 | 1992-15 | 4.4* | 3.8, 5.1 | |||||||
| 70-79 | 1,956 | 1992-15 | 2.8* | 1.9, 3.6 | |||||||
| ≥80 | 815 | 1992-15 | 2.8* | 1.3, 4.2 | |||||||
| Asian/Pacific | 20-29 | 59 | -- | -- | -- | ||||||
| Islander | 30-39 | 322 | 1992-15 | −2.3* | −4.0, −0.6 | ||||||
| 40-49 | 1,183 | 1992-05 | 0.8 | −1.6, 3.2 | 2005-15 | −6.5* | −9.5, −3.4 | ||||
| 50-59 | 2,630 | 1992-11 | 1.4* | 0.4, 2.4 | 2011-15 | −8.3* | −15.4, −0.6 | ||||
| 60-69 | 3,384 | 1992-15 | −0.3 | −1.0, 0.5 | |||||||
| 70-79 | 3,048 | 1992-07 | 2.6* | 1.4, 3.8 | 2007-15 | −3.6* | −5.8, −1.4 | ||||
| ≥80 | 1,503 | 1992-94 | −20.2 | −57.2, 48.7 | 1994-15 | 2.2* | 1.1, 3.4 | ||||
APC, annual percent change; y, years; CI, confidence interval
-- indicates the APC could not be estimated because standard error not available and/or number of cases too small
NOTE: Each trend corresponds to the slope of the line segment between joinpoints. Asterisk denotes the APC is statistically significantly different from zero (P <0.05).
Trends by sex
We noted a similar pattern by sex (Table 2), whereby incidence rates decreased among men and women in the 40-49 year age group starting in the mid-2000s. The rate of decline appeared similar in the two groups (men: APC −6.7, 95% CI −8.7, −4.6; women: APC −8.3, 95% CI −14.8, −1.3). Rates decreased by 10.7% per year among 50-59-year-old men starting in 2012. There was not a statistically significant decline in women of this age group, however the APC (−6.3, 95% −17.6, 6.5) estimate is consistent with declining rates from 2011 to 2015.
Table 2.
Trends in hepatocellular carcinoma incidence rates by sex and 10-year age group, SEER 13, 1992-2015
| Sex | Age (y) | N | Trend 1 | Trend 2 | Trend 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | APC | 95% CI | Years | APC | 95% CI | Years | APC | 95% CI | |||
| Male | 20-29 | 152 | 1992-15 | 2.0 | −0.7, 4.8 | ||||||
| 30-39 | 551 | 1992-11 | 1.4 | −0.6, 3.5 | 2011-15 | −19.3 | −34.9, 0.1 | ||||
| 40-49 | 3,361 | 1992-98 | 12.0* | 7.6, 16.6 | 1998-06 | 1.3 | −1.8, 4.6 | 2006-15 | −6.7* | −8.7, −4.6 | |
| 50-59 | 11,448 | 1992-07 | 9.7* | 8.9, 10.4 | 2007-12 | 1.2 | −4.0, 6.5 | 2012-15 | −10.7* | −17.7, −3.1 | |
| 60-69 | 11,776 | 1992-04 | 3.1* | 2.0, 4.2 | 2004-15 | 6.9* | 5.7, 8.2 | ||||
| 70-79 | 7,754 | 1992-08 | 3.3* | 2.8, 3.7 | 2008-15 | 1.0 | −0.6, 2.6 | ||||
| ≥80 | 3,405 | 1992-15 | 3.1* | 2.4, 3.8 | |||||||
| Female | 20-29 | 82 | -- | -- | -- | ||||||
| 30-39 | 173 | 1992-15 | 0.7 | −2.3, 3.9 | |||||||
| 40-49 | 681 | 1992-07 | 6.1* | 3.1, 9.1 | 2007-15 | −8.3* | −14.8, −1.3 | ||||
| 50-59 | 2,309 | 1992-11 | 5.9* | 4.7, 7.2 | 2011-15 | −6.3 | −17.6, 6.5 | ||||
| 60-69 | 3,411 | 1992-02 | 6.6* | 4.6, 8.6 | 2002-05 | −7.8 | −27.5, 17.3 | 2005-15 | 6.0* | 4.0, 8.0 | |
| 70-79 | 3,767 | 1992-15 | 3.4* | 2.7, 4.0 | |||||||
| ≥80 | 2,318 | 1992-15 | 4.0* | 3.3, 4.7 | |||||||
APC, annual percent change; y, years; CI, confidence interval
-- indicates the APC could not be estimated because standard error not available and/or number of cases too small
NOTE: Each trend corresponds to the slope of the line segment between joinpoints. Asterisk denotes the APC is statistically significantly different from zero (P <0.05).
Discussion
Contrary to the well-known increases in HCC incidence over the past three decades, we observed a clear pattern of decreasing incidence rates among younger and middle-aged adults (age 40-49 and 50-59 years) in both men and women, and all racial/ethnic groups, starting in the mid-2000s. Interestingly, decreasing rates were most prominent among non-Hispanic blacks in this age group, a contrast with marked racial disparities in older ages, among whom incidence rates have increased. Temporal trends in incidence likely reflect the varying influence of risk factors involved in HCC pathogenesis, and possible differences in mechanisms by age or race/ethnicity, raising questions about future disease burden. Combined with changing demographics in the U.S., it is unclear whether the declining rates observed among younger and middle-aged adults will persist or rates will increase in the future as previously projected9.
Although many have reported increases in HCC incidence,9, 19, 20 less attention has been paid to decreasing rates after 2006, particularly among 40- and 50-year olds. We observed decreases in this age group of about 10% per year, with even larger annual declines among non-Hispanic blacks. This is in contrast to recent and alarming reports of rising incidence rates of other gastrointestinal cancers (e.g., colorectal, gallbladder, and pancreatic) among younger adults.21 The declines we observed in this age group occurred while incidence rates have continued to increase in older adults and likely reflect population changes in chronic HCV infection. Incidence rates have already begun to decline in other industrialized countries where a large proportion of HCC diagnoses are related to HCV, including high-income Asian-Pacific countries and Western Europe.6, 22 In the U.S., the aging of the baby boomer cohort has resulted in increases in HCC incidence among older adults. Conversely, the declines in HCC incidence among younger adults may be related to reductions in the risk of transfusion-transmitted HCV infection23 and increased awareness of high risk behaviors (e.g., needle sharing).24 In the future, efforts to improve HCV screening uptake and the advent of new, highly effective HCV treatments may play a role in continued declines in HCC incidence.
Consistent with others,4, 20, 25 we observed increases in HCC incidence among older adults, and incidence rates in this population were generally higher among racial/ethnic minorities compared to non-Hispanic whites. Specifically, non-Hispanic blacks, Hispanics, and Asian/Pacific Islanders had higher HCC incidence rates, perhaps because the prevalence of HCC risk factors differs across these population subgroups. In our prior work, we have shown a much higher proportion of blacks are diagnosed with HCV-related HCC, while a higher proportion of whites and Hispanics have alcohol- or NASH-related HCC.26 Diagnostic factors may also account for some of the observed increases in HCC incidence over time by increasing case ascertainment, including increased HCC screening utilization over time27 and improvements in imaging technology.28
Our findings point to a number of possible scenarios that may occur in the future. Small changes in the relative presence or absence of risk factors, in the setting of changing demographics in U. S., may substantially alter the burden of HCC. Continued efforts to screen and treat viral hepatitis may result in further declines in incidence among younger adults. Alternatively, although rates have decreased consistently in this population since the mid-2000s, these declines may be thwarted by increases in metabolic syndrome (e.g., obesity, diabetes) and non-alcoholic fatty liver disease,11 particularly among Hispanics and the youngest age group (age <40 years). Prolonged periods of exposure to obesity and insulin resistance, which now begin as early as childhood, may contribute to higher HCC incidence rates in the future. Recent studies have similarly described increasing alcohol consumption among younger adults, which may also contribute to increases in liver-related morbidity and mortality.10, 29 Further, the opioid crisis has led to dramatic increases in the incidence of acute HCV infection related to injection drug use (increases of 133% since 2004),30 and the largest increases have occurred among young whites (age 18-39 years). Although current HCC incidence rates in the youngest age groups remain low, increasing prevalence of HCV infection may increase risk of HCC later in life. The complexity and multifactorial nature of HCC pathogenesis, associated risk factors, and co-factors that accelerate progression, underscore the difficulties in precisely predicting future HCC incidence trends.
We acknowledge limitations of cancer registry data. Although our findings raise the possibility that age-related differences in HCC incidence rates may be due to the underlying etiology of liver disease, cancer registries do not systematically collect this information. Additionally, we could not observe HCC incidence rates after direct-acting antivirals for HCV became widely available in 2014 because of the time lag between diagnosis and registry reporting. While we obtained detailed data stratified by race/ethnicity, the number of American Indian/Alaskan Natives (AI/AN) with HCC was very small and thus not included in our analyses. Lastly, the data reflect HCC incidence rates in adults age ≥20 years, and incidence rates are low in the youngest age group, limiting our ability to draw definitive conclusions in this subgroup.
In summary, we observed declining HCC incidence rates among younger and middle-aged adults across all racial/ethnic groups, a contrast to continued increases observed among adults over the age of 60 years. Differential incidence rates by age may be related to differences in risk factors and underlying disease etiology, specifically HCV, which may substantially differ by time period and race/ethnicity.
Supplementary Material
Supplementary Figure 1. Annual percent change (APC) in age-specific incidence rates of hepatocellular carcinoma among non-Hispanic whites, SEER 13, 1992 – 2015.
NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test. APC in 20-29 and 30-39 year age groups could not be estimated because the standard error not available and/or number of cases too small. Y-axis scale varies across figures to demonstrate trend.
Supplementary Figure 2. Annual percent change (APC) in age-specific incidence rates of hepatocellular carcinoma among non-Hispanic blacks, SEER 13, 1992 – 2015.
NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test. APC in 20-29 and 30-39 year age groups could not be estimated because the standard error not available and/or number of cases too small. Y-axis scale varies across figures to demonstrate trend.
Supplementary Figure 3. Annual percent change (APC) in age-specific incidence rates of hepatocellular carcinoma among Hispanics, SEER 13, 1992 – 2015.
NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test. APC in 20-29 year age group could not be estimated because the standard error not available and/or number of cases too small. Y-axis scale varies across figures to demonstrate trend.
Supplementary Figure 4. Annual percent change (APC) in age-specific incidence rates of hepatocellular carcinoma among Asian/Pacific Islanders, SEER 13, 1992 – 2015. NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test. APC in 20-29 year age group could not be estimated because the standard error not available and/or number of cases too small. Y-axis scale varies across figures to demonstrate trend.
Background
Changes in the prevalence of risk factors for hepatocellular carcinoma (HCC) may impact incidence rates differently in population subgroups and over time.
Findings
HCC incidence rates were previously increasing in all populations but started to plateau in 2010. Although incidence rates continue to increase among older adults (age ≥60), declines were observed among younger adults, in both men and women and all races/ethnicities.
Implications for patient care
Changes in HCC incidence rates are related to the varying prevalence of risk factors, which may differ by age, race/ethnicity, sex, and time period. Understanding trends in HCC incidence can inform future resource needs and help target efforts for primary prevention and early detection.
Abbreviations
- APC
annual percent change
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IRR
incidence rate ratio
- NAFLD
nonalcoholic fatty liver disease
- SEER
Surveillance, Epidemiology and End Result
Footnotes
Disclosures: The authors declare no conflicts of interest or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Kim WR, Coelho R, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol 2011;9:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124:2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 2015;19:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Burden of Disease Liver Cancer C. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncology 2017;3:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology (Baltimore, Md.) 2014;60:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanwal F, Kramer J, Asch SM, et al. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017;153:996–1005.e1. [DOI] [PubMed] [Google Scholar]
- 9.Petrick JL, Kelly SP, Altekruse SF, et al. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016;34:1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ 2018;362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the united states, 2011-2012. JAMA 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hales CM CM, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth, 2015-2016. NCHS Data Brief 2017;288. [PubMed] [Google Scholar]
- 14.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006;15:547–69. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 16.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among US Hispanic/Latino populations. Cancer 2006;107:1711–1742. [DOI] [PubMed] [Google Scholar]
- 17.Howe HL. NAACCR Guideline for Enhancing Hispanic-Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2]. [Google Scholar]
- 18.Boscoe FP. nAaCCR Asian/Pacific Islander Identification Algorithm [NAPIIA v1. 2.1]: Enhancing the Specificity of Identification. 2009. [Google Scholar]
- 19.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–50. [DOI] [PubMed] [Google Scholar]
- 20.Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sung H, Siegel RL, Rosenberg PS, et al. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 2019. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Imai Y, Hiramatsu N, et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med 2008;148:820–6. [DOI] [PubMed] [Google Scholar]
- 23.Donahue JG, Munoz A, Ness PM, et al. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med 1992;327:369–73. [DOI] [PubMed] [Google Scholar]
- 24.Kwiatkowski CF, Fortuin Corsi K, Booth RE. The association between knowledge of hepatitis C virus status and risk behaviors in injection drug users. Addiction 2002;97:1289–94. [DOI] [PubMed] [Google Scholar]
- 25.White DL, Thrift AP, Kanwal F, et al. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology 2017;152:812–820.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rich NE, Hester C, Odewole M, et al. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2019; 17:551–559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi DT, Kum HC, Park S, et al. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2019;17:976–987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leoni S, Piscaglia F, Golfieri R, et al. The impact of vascular and nonvascular findings on the noninvasive diagnosis of small hepatocellular carcinoma based on the EASL and AASLD criteria. Am J Gastroenterol 2010;105:599–609. [DOI] [PubMed] [Google Scholar]
- 29.Patrick ME, Terry-McElrath YM, Miech RA, et al. Age-Specific Prevalence of Binge and High-Intensity Drinking Among U.S. Young Adults: Changes from 2005 to 2015. Alcoholism: Clinical and Experimental Research 2017;41:1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zibbell JE, Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. American Journal of Public Health 2018;108:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Annual percent change (APC) in age-specific incidence rates of hepatocellular carcinoma among non-Hispanic whites, SEER 13, 1992 – 2015.
NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test. APC in 20-29 and 30-39 year age groups could not be estimated because the standard error not available and/or number of cases too small. Y-axis scale varies across figures to demonstrate trend.
Supplementary Figure 2. Annual percent change (APC) in age-specific incidence rates of hepatocellular carcinoma among non-Hispanic blacks, SEER 13, 1992 – 2015.
NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test. APC in 20-29 and 30-39 year age groups could not be estimated because the standard error not available and/or number of cases too small. Y-axis scale varies across figures to demonstrate trend.
Supplementary Figure 3. Annual percent change (APC) in age-specific incidence rates of hepatocellular carcinoma among Hispanics, SEER 13, 1992 – 2015.
NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test. APC in 20-29 year age group could not be estimated because the standard error not available and/or number of cases too small. Y-axis scale varies across figures to demonstrate trend.
Supplementary Figure 4. Annual percent change (APC) in age-specific incidence rates of hepatocellular carcinoma among Asian/Pacific Islanders, SEER 13, 1992 – 2015. NOTE: An asterisk denotes the APC is statistically significantly different from zero (P <0.05) using a two-sided test. APC in 20-29 year age group could not be estimated because the standard error not available and/or number of cases too small. Y-axis scale varies across figures to demonstrate trend.