Renal cell carcinoma (RCC) has long been termed a radioresistant histology owing to older data demonstrating a lack of benefit with conventional fractionated radiation.1, 2 More modern series have demonstrated that high-dose regimens delivered using stereotactic body radiation therapy (SBRT) offer impressive tumor control.3, 4 Preservation of normal kidney function is a priority when using radiation therapy (RT). Investigators have previously noted decreased renal function after SBRT, especially in patients with pre-existing renal disease.5, 6 The effectiveness of SBRT correlates with accurate tumor delineation and avoidance of high-dose radiation to normal tissue. Furthermore, treatment planning must compensate for respiratory motion of renal tumors.
We report a case illustrating a patient with metastatic RCC who responded on systemic therapy with nivolumab and underwent SBRT to the primary renal mass for a cytoreductive effect. This SBRT regimen was delivered using a magnetic resonance (MR) imaging (MRI)-guided RT system that allows for MR-based simulation imaging, real-time tumor tracking, and respiratory gating. We further highlight the implications of this novel system in treatment planning, treatment delivery, and potential outcomes.
Case Report
An 83-year-old man presented to the emergency department with acute shortness of breath and history of progressive dyspnea over the prior 2 months. A computed tomography (CT) scan of the chest revealed a large right-sided pleural effusion, multiple bilateral pulmonary nodules, and a superior left kidney lesion. The patient received a thoracentesis with removal of 1.5 L of pleural fluid and a biopsy of a right-sided pleural based nodule via CT guidance. Pathology confirmed a diagnosis of clear cell RCC, and pazopanib was initiated. After 2 months of therapy, the patient developed elevated transaminases indicative of pazopanib-induced hepatotoxicity. His systemic therapy was subsequently switched to nivolumab.
Approximately 4 months after diagnosis, the patient developed 4 brain metastases, which were treated with stereotactic radiosurgery. After 1 year of nivolumab, the patient was reimaged and found to have resolution of intracranial disease, stable pulmonary nodules, response of the primary left kidney mass, and no radiographic evidence of new sites of disease. Given that the largest burden of disease was in the left kidney, cytoreductive nephrectomy was discussed, but the patient declined surgery. After 29 cycles of nivolumab, his disease remained stable (Fig. 1A and 1B), and he consented to local treatment of the left renal primary using SBRT.
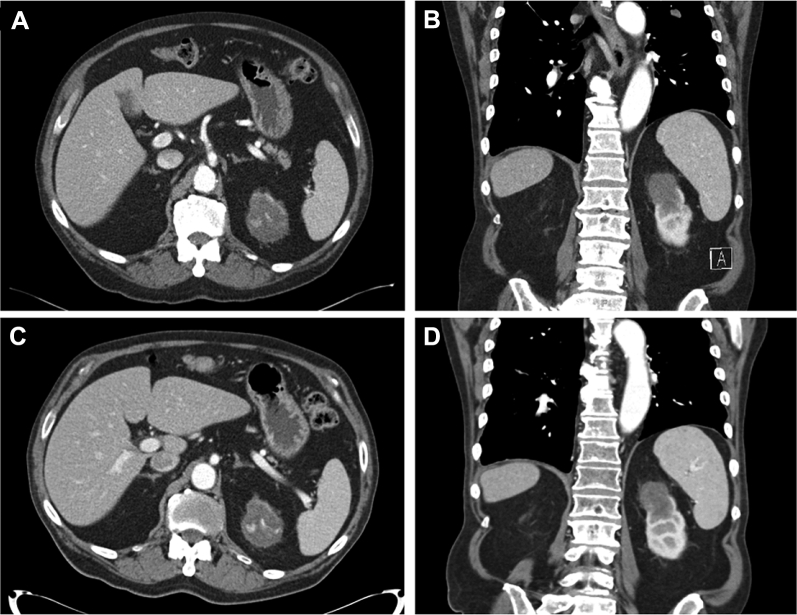
Figure 1.
(A) Axial computed tomography (CT) scan of an abdomen demonstrating a 4.3 × 5.6 cm exophytic mass in the upper pole of the left kidney with internal calcifications before radiation. (B) Coronal view of the CT scan of the abdomen before radiation. (C) Axial CT scan of the abdomen after radiation demonstrating stable size of primary renal mass. (D) Coronal view of CT scan of the abdomen after radiation.
The patient's baseline renal function was assessed using a renal scintigraphy before starting SBRT. The left kidney contributed 34% of total renal function. In addition, baseline creatinine was 1.20 mg/dL, and estimated glomerular filtration rate was 55 mL/min/1.73 m2.
Stereotactic body radiation therapy
The patient received a 4-dimensional CT simulation lying supine in a body immobilization device with the left arm up. MRI coils were placed under the immobilization device, and the patient received MR simulation with the MRI-guided RT system (ViewRay MRIdian System, Oakwood Village, OH). CT and MR images were transferred to the RT planning system, where images were registered and contouring was performed. The primary contouring dataset was the MRI data obtained using a fast gradient echo sequence (TrueFISP) as set by the manufacturer of the MRI-guided RT system. The treating radiation oncologist contoured the gross tumor volume (GTV), defined as the left kidney mass visible on the MRI set. Planning tumor volume (PTV) was created using a margin of 5 mm (Fig. 2A and 2B). Organs at risk (OARs) were also delineated and included stomach, small bowel, large bowel, kidneys, liver, and spinal cord.
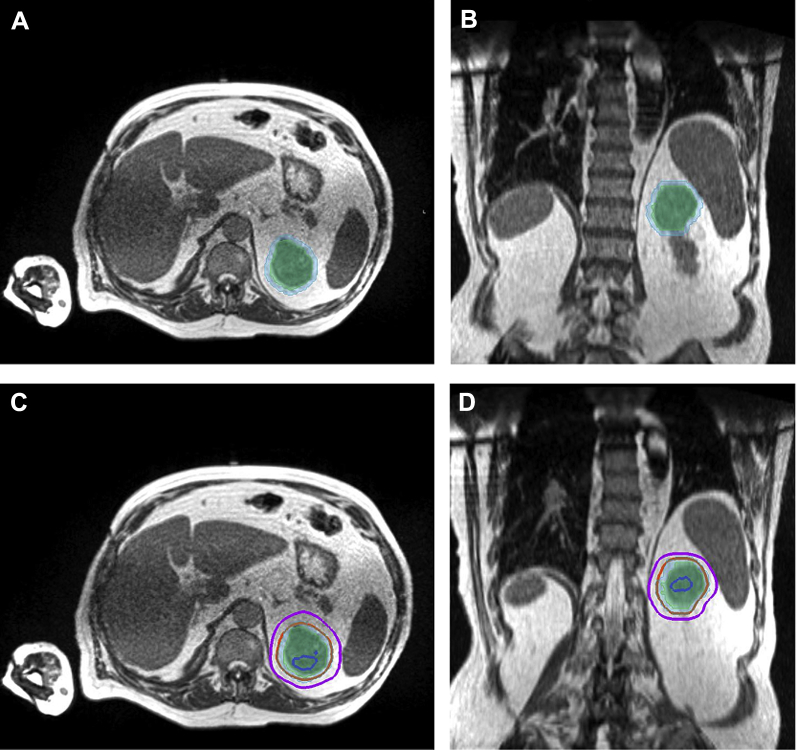
Figure 2.
(A) Axial simulation magnetic resonance imaging (MRI) scan of the abdomen demonstrating gross tumor volume (GTV) shaded in light green and planning target volume (PTV) in light blue. (B) Coronal view of MRI scan of the abdomen with treatment volumes. (C) Axial MRI scan of the abdomen with treatment volumes and isodose lines: blue, 120%; orange, 100%; magenta, 80%. (D) Coronal view of MRI scan of the abdomen with treatment volumes and isodose lines.
An SBRT treatment plan was developed to deliver 4000 cGy in 5 daily fractions (Fig. 2C and 2D). Dose constraints were used during planning for the following OARs: stomach (V36 Gy [OAR volume receiving 36 Gy] <0.5 mL, V18 Gy < 10 mL), small bowel (V20 Gy < 5 mL, Dmax [maximum dose to volume] <35 Gy), large bowel (V36 Gy < 0.5 mL, V25 Gy < 20 mL), kidneys (V16 Gy < 60%), liver (mean dose <18 Gy, V21 Gy < 700 mL), and spinal cord (V25 Gy < 0.5 mL). The treatment was prescribed with purposeful hotspots within the GTV. PTV coverage was not compromised due to OARs. The treatment plan delivered 95% prescription dose to 99% of the PTV. OAR doses were as follows: stomach (V36 Gy = 0 mL, V18 Gy = 6 mL), small bowel (V20 Gy = 0 mL), large bowel (V36 Gy = 0 mL, V25 Gy = 0 mL), kidneys (V16 Gy = 10%), liver (mean dose = 2 Gy, V21 Gy = 0 cmL), and spinal cord (V25 Gy = 0 mL).
All treatments were performed on the MRI-guided RT machine using a 0.35T magnet, Co-60 delivery, and real-time MRI acquisition. During daily set-up for treatment, a volumetric MRI was obtained to visualize the left kidney and OARs. RT was delivered using the end-expiration gating technique. The treatment target was the GTV with a 5-mm expansion acting as the gating boundary (same as PTV). Typical gating margins are 3 to 5 mm. The treating physician selected a larger gating margin so that beam-off time could be minimized. This was an important consideration for an elderly patient who had difficulty maintaining arm-up position for substantial treatment durations. Figure 3A demonstrates the GTV within the gating boundary resulting in radiation delivery, and Figure 3B demonstrates the GTV moving outside the boundary resulting in the beams turning off and temporarily stopping delivery. The average delivery time for each fraction was 14.8 minutes, and the average on-table time for the patient was 33 minutes. The average duty cycle was 75%.
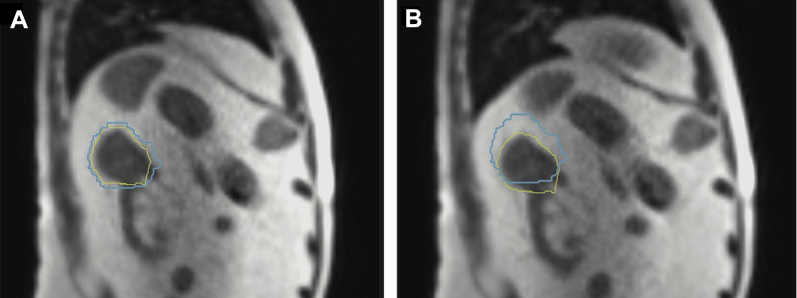
Figure 3.
Sagittal magnetic resonance image view of respiratory-gated radiation delivery with gross tumor volume (yellow) within 3-mm gating boundary (light blue) (A) and outside gating boundary (B).
During treatment, the patient experienced some mild fatigue but did not report any nausea, vomiting, diarrhea, or skin irritation. He presented for follow-up at 5 weeks after completion of therapy and continued to report fatigue without any new symptoms. CT of the chest, abdomen, and pelvis was performed at 6 weeks, demonstrating stable size of the primary renal mass and stable metastatic disease. At 8 weeks after RT, the patient decided to stop nivolumab because of continued fatigue on therapy and proceeded with close observation. Imaging was repeated at 6 months after RT and demonstrated stable disease at the primary (Fig. 1C and 1D) and metastatic sites. In addition, the patient received a repeat evaluation of his kidney function after SBRT. At 6 months after treatment, the patient's kidney function remained stable with creatinine of 1.20 mg/dL and estimated glomerular filtration rate of 54 mL/min/1.73 m2.
Discussion
Cytoreductive nephrectomy in certain subgroups of patients with metastatic RCC may improve overall survival. Flanigan et al performed a randomized trial demonstrating that surgery followed by interferon therapy improved survival over interferon therapy alone.7 Mickisch et al demonstrated favorable time to progression and survival outcomes in the combined surgery and interferon arm of their study as well.8 However, both studies were performed before the advent of modern targeted systemic therapy options. Although there is no published randomized evidence to support cytoreductive surgery in patients receiving targeted therapies, large modern retrospective studies have demonstrated survival outcomes favoring surgery in this setting.9, 10 However, patients with RCC with brain metastases generally have a guarded prognosis, and invasive surgery may not be a viable option for this subset of patients. Alternatives to surgery include cryoablation and radiofrequency ablation, but these are limited to smaller tumor volumes and are still invasive procedures.11 Thus, in patients who are medically inoperable or decline invasive procedures, SBRT may be an option to deliver noninvasive therapy to the primary renal tumor.
Currently, SBRT is a feasible treatment modality for nonmetastatic RCC, as described by the International Radiosurgery Oncology Consortium for Kidney consensus statement, and provides excellent long-term local control.12, 13 This technique allows for high doses of radiation to be delivered to the tumor while attempting to spare surrounding normal tissue. Most RT clinics use fluoroscopic imaging and cone beam CT to help align patients for SBRT treatments. Unfortunately, the ability of both prior imaging modalities to discern tumor from normal kidney and surrounding OARs can be poor in the abdomen.14 MRI-guided RT provides excellent soft-tissue contrast, which reduces this uncertainty. In addition to improving accuracy of patient set-up, MRI can be continuously acquired during treatment, without additional ionizing radiation, to confirm tumor location within the gating boundary when the beam is on. This eliminates the need for fiducial marker placement in a classically highly vascular tumor. Treatment with this novel system may improve the therapeutic ratio by improving tumor coverage while reducing unnecessary dose to OARs. This is of particular importance with SBRT treatments where high doses of radiation are delivered with sharp dose fall off outside the target volumes.
This case demonstrates that MRI-guided SBRT is a reasonable approach to locally ablate the primary renal tumor in the setting of stable metastatic RCC with concurrent nivolumab. Kerkhof et al initially reported the technical feasibility of MRI-guided radiation for renal tumor ablation by evaluating renal movement during free-breathing and breath-hold imaging.15 Correa et al reported a prospective phase 1 dose-escalation study for conventional SBRT to the primary renal mass in metastatic patients with RCC; they were able to escalate to a regimen of 35 Gy delivered in 5 fractions with acceptable toxicity outcomes.16 Our patient was treated to 40 Gy in 5 fractions with no reported toxicity at 6-month follow-up, stable renal function, and no evidence of progression.
Of note, the benefits of SBRT may go beyond local control. Radiation delivered in large fraction sizes is known to boost the antitumor immune response in preclinical studies.17, 18 Singh et al showed that SBRT to the primary renal mass in patients with metastatic RCC can alter immunomodulatory markers in the primary tumor.19 The combination of high dose per fraction and immunotherapy in animal models has demonstrated responses in both irradiated and nonirradiated sites of disease, suggesting a role for SBRT in improving local and distant control.20 As a result, prospective clinical evaluation of the combination of SBRT with immunotherapy in patients with RCC is underway (NCT02855203).
To our knowledge, this is the first report of MRI-guided SBRT for the primary mass in a patient with metastatic RCC. A prior case report demonstrated use of the MRI-guided system on a spleen tumor with a more conventionally fractionated radiation regimen.21 In the present report, we detail the simulation and treatment planning parameters used for MRI-guided SBRT to the kidney at our institution. Additional prospective studies are needed to identify subgroups of patients with RCC who may benefit from SBRT to not only metastatic disease, but also the primary site.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Fischer-Valuck reports grants and personal fees from ViewRay, Inc, outside the submitted work. Dr Green reports grants and personal fees from ViewRay, Inc, outside the submitted work.
References
- 1.Juusela H., Malmio K., Alfthan O. Preoperative irradiation in the treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1977;11:277–281. doi: 10.3109/00365597709179965. [DOI] [PubMed] [Google Scholar]
- 2.van der Werf-Messing B.H. Carcinoma of the kidney. Cancer. 1973;32:1056–1061. doi: 10.1002/1097-0142(197311)32:5<1056::aid-cncr2820320505>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Svedman C., Sandström P., Pisa P. A prospective phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45:870–875. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 4.Siva S., Pham D., Gill S. A systematic review of stereotactic radiotherapy ablation for primary renal cell carcinoma. BJU Int. 2012;110:E737–E743. doi: 10.1111/j.1464-410X.2012.11550.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang J.H., Cheung P., Erler D. Stereotactic ablative body radiotherapy for primary renal cell carcinoma in non-surgical candidates: Initial clinical experience. Clin Oncol. 2016;28:e109–e114. doi: 10.1016/j.clon.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Siva S., Jackson P., Kron T. Impact of stereotactic radiotherapy on kidney function in primary renal cell carcinoma: Establishing a dose-response relationship. Radiother Oncol. 2016;118:540–546. doi: 10.1016/j.radonc.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Flanigan R.C., Salmon S.E., Blumenstein B.A. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 8.Mickisch G.H., Garin A., van Poppel H. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 9.Heng D.Y., Wells J.C., Rini B.I. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2014;66:704–710. doi: 10.1016/j.eururo.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Hanna N., Sun M., Meyer C.P. Survival analyses of patients with metastatic renal cancer treated with targeted therapy with or without cytoreductive nephrectomy: A national cancer data base study. J Clin Oncol. 2016;34:3267–3275. doi: 10.1200/JCO.2016.66.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network Kidney Cancer (version 2.2019) https://www.nccn.org/professionals/physician_gls/pdf/kidney_blocks.pdf Available at: Accessed February 9, 2019.
- 12.Siva S., Ellis R.J., Ponsky L. Consensus statement from the International Radiosurgery Oncology Consortium for Kidney for primary renal cell carcinoma. Future Oncol. 2016;12:637–645. doi: 10.2217/fon.16.2. [DOI] [PubMed] [Google Scholar]
- 13.Siva S., Louie A.V., Warner A. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK) Cancer. 2018;124:934–942. doi: 10.1002/cncr.31156. [DOI] [PubMed] [Google Scholar]
- 14.Noel C.E., Parikh P.J., Spencer C.R. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol. 2015;54:1474–1482. doi: 10.3109/0284186X.2015.1062541. [DOI] [PubMed] [Google Scholar]
- 15.Kerkhof E.M., Raaymakers B.W., van Vulpen M. A new concept for non-invasive renal tumour ablation using real-time MRI-guided radiation therapy. BJU Int. 2011;107:63–68. doi: 10.1111/j.1464-410X.2010.09458.x. [DOI] [PubMed] [Google Scholar]
- 16.Correa R.J.M., Ahmad B., Warner A. A prospective phase I dose-escalation trial of stereotactic ablative radiotherapy (SABR) as an alternative to cytoreductive nephrectomy for inoperable patients with metastatic renal cell carcinoma. Radiat Oncol. 2018;13:47. doi: 10.1186/s13014-018-0992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finkelstein S.E., Timmerman R., McBride W.H. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol. 2011;2011:439752. doi: 10.1155/2011/439752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siva S., Kothari G., Muacevic A. Radiotherapy for renal cell carcinoma: Renaissance of an overlooked approach. Nat Rev Urol. 2017;14:549–563. doi: 10.1038/nrurol.2017.87. [DOI] [PubMed] [Google Scholar]
- 19.Singh A.K., Winslow T.B., Kermany M.H. A pilot study of stereotactic body radiation therapy combined with cytoreductive nephrectomy for metastatic renal cell carcinoma. Clin Cancer Res. 2017;23:5055–5065. doi: 10.1158/1078-0432.CCR-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S.S., Dong H., Liu X. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015;3:610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer-Valuck B.W., Green O., Mazur T. Magnetic resonance image guided radiation therapy for primary splenic diffuse large B-cell lymphoma. A teaching case. 2017;7:e23–e26. doi: 10.1016/j.prro.2016.06.004. [DOI] [PubMed] [Google Scholar]