Abstract
Cadherins constitute a family of transmembrane proteins that mediate calcium-dependent cell-cell adhesion. The extracellular domain of cadherins consists of extracellular cadherin (EC) domains, separated by calcium binding sites. The EC interacts with other cadherin molecules in cis and in trans to mechanically hold apposing cell surfaces together. CDH2 encodes N-cadherin, whose essential roles in neural development include neuronal migration and axon pathfinding. However, CDH2 has not yet been linked to a Mendelian neurodevelopmental disorder. Here, we report de novo heterozygous pathogenic variants (seven missense, two frameshift) in CDH2 in nine individuals with a syndromic neurodevelopmental disorder characterized by global developmental delay and/or intellectual disability, variable axon pathfinding defects (corpus callosum agenesis or hypoplasia, mirror movements, Duane anomaly), and ocular, cardiac, and genital anomalies. All seven missense variants (c.1057G>A [p.Asp353Asn]; c.1789G>A [p.Asp597Asn]; c.1789G>T [p.Asp597Tyr]; c.1802A>C [p.Asn601Thr]; c.1839C>G [p.Cys613Trp]; c.1880A>G [p.Asp627Gly]; c.2027A>G [p.Tyr676Cys]) result in substitution of highly conserved residues, and six of seven cluster within EC domains 4 and 5. Four of the substitutions affect the calcium-binding site in the EC4-EC5 interdomain. We show that cells expressing these variants in the EC4-EC5 domains have a defect in cell-cell adhesion; this defect includes impaired binding in trans with N-cadherin-WT expressed on apposing cells. The two frameshift variants (c.2563_2564delCT [p.Leu855Valfs∗4]; c.2564_2567dupTGTT [p.Leu856Phefs∗5]) are predicted to lead to a truncated cytoplasmic domain. Our study demonstrates that de novo heterozygous variants in CDH2 impair the adhesive activity of N-cadherin, resulting in a multisystemic developmental disorder, that could be named ACOG syndrome (agenesis of corpus callosum, axon pathfinding, cardiac, ocular, and genital defects).
Keywords: CDH2, N-cadherin, cell-cell adhesion, intellectual disability, cardiac defects, eye defects, genital defects, corpus callosum, ACOG
Main Text
Cell-cell adhesion is a dynamic and tightly regulated process involved in key biological processes, from tissue morphogenesis during early stages of development to the maintenance of adult tissue integrity.1, 2 The cadherin superfamily is one of the major families of cell-adhesion molecules and mediates cell adhesion in a calcium (Ca2+)-binding-dependent manner. Cadherins are classified into classical cadherins (type I and II), desmosomal cadherins, protocadherins (clustered and non-clustered), and atypical members, including FATs, flamingos or celsrs, and calsyntenins.3 They are typically characterized by one to 34 extracellular cadherin (EC) domain repeats, which are responsible for the adhesive properties of the molecules.4 Classical cadherins are composed of an adhesive extracellular domain, a transmembrane region, and a cytoplasmic tail.4 The extracellular domain consists of five EC repeats (EC1–5) that mediate cell adhesion through the formation of a lattice both in cis (between molecules of the same cell) and in trans (between molecules of apposed cells) interactions to form adherens junctions.4, 5 The intracellular domain binds to p120 and β-catenin which, in turn, connects via alpha-catenin to the actin cytoskeleton, enabling multiple signaling cascades that control cell differentiation, proliferation, migration, and apoptosis.6 Consistent with these pleiotropic functions, abnormalities in critical domains of cadherins have been associated with a number of human diseases,7 such as cancer8 and neurodevelopmental disorders, including intellectual disability (ID),9, 10, 11 autism spectrum disorder (ASD),12 attention deficit hyperactivity disorder (ADHD),13 epilepsy,14 and psychiatric disorders.15 Some of these have been recognized as Mendelian disorders, linked to pathogenic variants in the following cadherins: CDH1 (blepharocheilodontic syndrome [MIM: 119580], gastric cancer [MIM: 137215], breast cancer [MIM: 114480]), CDH3 (ectodermal dysplasia, ectrodactyly, and macular dystrophy [MIM: 225280], congenital hypotrichosis with juvenile macular dystrophy [MIM: 601553]), CDH11 (Elsahy-Waters syndrome [MIM 211380]), PCDH12 (microcephaly, seizure, spasticity and brain calcifications [MIM: 251280]), PCDH19 (early infantile epileptic encephalopathy [MIM: 300088]) and CDH23 (autosomal recessive deafness [MIM: 601386], Usher syndrome type 1D/F [MIM: 601067], pituitary adenoma [MIM: 617540]). CDH2 encodes cadherin-2, a type I (classical) cadherin, also known as N-cadherin because of its high level of expression in neural tissue, where it was first identified.16 N-cadherin has an important role in the early steps of neural development, including in the proliferation and differentiation of neural progenitor cells,17 the formation of the neural tube,18 synaptogenesis,19, 20 neuronal migration, and axon elongation.21, 22 In recent years, rare single-nucleotide polymorphisms (SNPs) in CDH2 have been suggested to confer susceptibility to psychiatric disorders.23, 24, 25 N-cadherin is also widely expressed in many other tissues outside the nervous system; such tissues include the heart,26 where it plays a crucial role in mechanical coupling and chemical communication between cardiomyocytes.27 Recently, two rare heterozygous CDH2 variants encoding ectodomain residues have been linked to arrhythmogenic cardiomyopathy.28, 29 Furthermore, several somatic CDH2 variants are reported in COSMIC, a database of somatic mutations found in human cancer.30
Despite its significant biological role in neurodevelopment, CDH2 has not yet been directly associated with a Mendelian neurodevelopmental disorder. In this study, we report on nine subjects harboring de novo heterozygous CDH2 variants, causing a syndromic neurodevelopmental disorder whose main clinical features are global developmental delay (GDD) and/or ID; corpus callosum agenesis or hypoplasia; craniofacial dysmorphisms; and ocular, cardiac, and genital anomalies.
In a Montreal Children’s Hospital research program dedicated to investigating individuals affected by brain malformations, whole-exome sequencing (WES) was performed in a child with agenesis of the corpus callosum (ACC); interhemispheric cyst; mild ID and ASD; Duane anomaly (i.e., characteristic abnormal eye movements due to aberrant innervation); craniofacial dysmorphic features; cryptorchidism; and mild tricuspid regurgitation (Figures 1I and 1J and subject 2 in Tab1e 1 and Table S1). The child was previously reported in a case series of individuals with ACC and interhemispheric cysts.31 This study was approved by the Montreal Children’s Hospital ethics committee, and informed consent was obtained from parents.
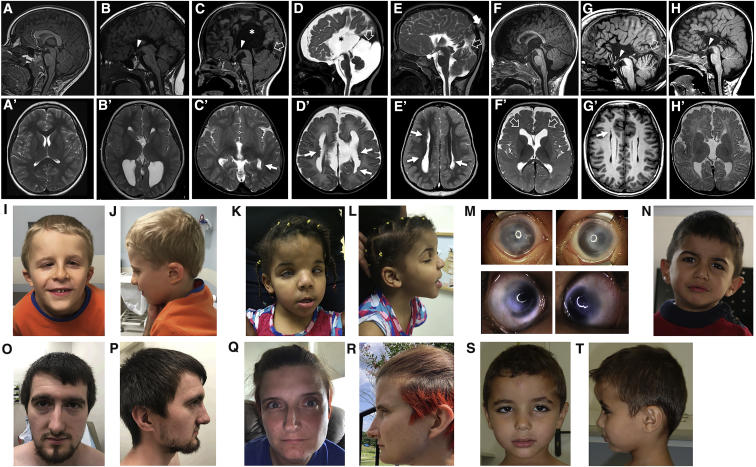
Figure 1.
Neuroradiologic and Facial Features of Individuals with De Novo CDH2 Variants
Brain MRI, sagittal T1-weighted (A) and axial T2-weighted (A′) images of a normal subject. Brain MRIs from subject 1 at 7 years (B and B′), subject 2 at 2 years (C and C′), subject 3 at 13 days (D and D′), subject 4 at 4 years (E and E′), subject 5 at 6 months (F and F′), subject 8 at 26 years (G and G′), and subject 9 at 5 months (H and H′) show complete agenesis (B, C, D, E, G, and H) or mild hypoplasia (F) of the corpus callosum; there is an interhemispheric cyst communicating with the III ventricle in two subjects (asterisks in [B] and [D]). In addition, there is hypo-dysplasia of the tentorium in four cases (C, D, E, and G, empty arrows) associated with an atretic parietal cephalocele in one subject (E, arrow). Note the hypothalamic adhesion in subjects 1, 2, 4, 8 and 9 (B, C, E, G, and H, arrowheads), as well as the megacisterna magna in subjects 3 and 8 (D and G). Axial T2 images reveal multiple nodular periventricular heterotopias in four subjects (C′, D′, E′, and G′, arrows), and mild frontal ventriculomegaly in one subject (F′, empty arrows). Photographs of subjects 2 (I and J) and 4 (K and L) demonstrate prominent forehead and frontal bossing, downslanting palpebral fissures, a thin upper lip, and low-set and thick helices. Subject 4 (M) also has Peters anomaly with clouding of the cornea, as well as hypertelorism and epicanthal folds. Subjects 5 (N) and 9 (S and T) have thick earlobes but other major dysmorphisms. Subjects 7 (O and P) and 8 (Q and R) show common craniofacial features, including deep-set eyes, a thin upper lip, a pointed chin, and slightly low-set and posteriorly rotated ears with attached earlobes.
Table 1.
Clinical and Neuroradiological Features of Individuals with De Novo CDH2 Pathogenic Variants
| Subjects | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject 6 | Subject 7 | Subject 8 | Subject 9 |
|---|---|---|---|---|---|---|---|---|---|
| CDH2 variant (GenBank: NM_001792.5) | c.1057G>A p.Asp353Asn |
c.1789G>A p.Asp597Asn |
c.1789G>T p.Asp597Tyr |
c.1802A>C p.Asn601Thr |
c.1839C>G p.Cys613Trp |
c.1880A>G p.Asp627Gly |
c.2027A>G p.Tyr676Cys |
c.2563_2564delCT p.Leu855Valfs∗4 |
c.2564_2567dup p.Leu856Phefs∗5 |
| Inheritance | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo |
| Gender | female | male | female | female | male | male | male | female | male |
| Current age, years (deceased) | 13 | 8 | (2 months) | 5 | 3.5 | 11 | 23 | 37 | 12 |
| Neurodevelopment | Gross and fine motor delay | GDD | − | GDD | GDD | GDD | GDD | GDD | fine motor and language delay |
| Intellectual disability | borderline or low average IQ | mild ID | − | moderate ID | moderate ID | no | no | low average IQ | mild ID |
| Neuropsychiatric issues | + | + | − | + | + | + | + | + | − |
| Epilepsy | − | − | − | + | + | − | − | − | − |
| Head circumference | +3.3 SD | +0.9 SD | −0.5 SD | +2.6 SD | −0.5 SD | +2.4 SD | NA | +3.3 SD | −1.7 SD |
| Axial hypotonia | − | + | − | − | + | + | − | − | − |
| Appendicular hypertonia | − | + | − | − | + | + | − | − | − |
| Hyposmia | + | − | − | − | − | − | + | + | − |
| Sensorineural hearing loss | − | + | − | − | − | − | − | − | + |
| Roving eye movements | − | − | + | − | + | − | − | − | − |
| Dysmorphisms | − | + | + | + | minor | + | + | + | minor |
| ACC | + | + | + | + | mild CC hypoplasia | + | − | + | + |
| Hypothalamic adhesion | + | + | − | + | − | − | − | + | + |
| Interhemispheric cyst | − | + | + | − | − | − | − | − | − |
| PNH | − | + | + | + | − | − | − | + | − |
| Tentorium hypo−dysplasia | − | + | + | + | − | − | − | + | − |
| Cardiovascular abnormalities | + AV canal defect |
+ mild tricuspidal regurgitation |
+ AV canal defect, mild hypoplastic aortic arch |
− | + dextrocardia, right pulmonary artery hypoplasia, atrial flutter |
− | + small pericardial effusion |
+ aortic coarctation |
+ aortic coarctation |
| Eye abnormalities | + strabismus, bilateral cataracts |
+ Duane anomaly, right ptosis, bilateral upgaze limitation |
− | + Peters anomaly, right esotropia |
− | + Peters anomaly, strabismus, nystagmus |
+ myopia |
+ strabismus, mild hyperopia |
+ astigmatism, hyperopia |
| Urogenital malformations | − | micropenis | − | − | − | right cryptorchidism | bilateral cryptorchidism | − | bilateral cryptorchidism |
| Other | salt hypogenusia, C5-C6 partial fusion, imperforate anus. de novo missense in DNM1 (p.Leu134Met) |
− | − | aplasia cutis congenita at vertex, shoulder Sprengel type deformity | pulmonary sequestration | − | mirror movements, umbilical and inguinal hernias, bilateral absence of shoulder muscles, hip dysplasia, scoliosis, hyperlordosis, joint hypermobility, pes planus | scapular winging, right shoulder Sprengel type deformity, mild scoliosis, noise sensitivity |
Abbreviations are as follows: ADHD, attention deficit hyperactivity disorder; AV, atrioventricular; GDD, global developmental delay; ID, intellectual disability; CC, corpus callosum; ACC, agenesis of corpus callosum; PNH, periventricular nodular heterotopia; NA, not available; SD, standard deviation.
Genomic DNA extracted from blood samples of the affected child and his parents was captured with the Agilent SureSelectXT CRE kit and sequenced on the Illumina HiSeq platform at the McGill University and Genome Québec Innovation Center. Sequence processing and alignment to GRCh37, variant annotation, filtering, and prioritization were performed via an in-house implemented pipeline that includes publicly available tools and was done according to the GATK’s best practices. We performed segregation and filtering analyses by using an in-house script to retain non-synonymous exonic and splicing variants with a minor-allele frequency (MAF) ≤ 0.001 in the gnomAD database. For compound heterozygous variants, we considered variants with a MAF ≤ 0.005. The list of all rare variants is available in Table S2.
This stepwise filtering failed to identify pathogenic variants in any OMIM genes, despite the fact that we considered all possible patterns of inheritance. On the basis of our suspicion of an axon pathfinding defect in our subject presenting with ACC and Duane anomaly, we next prioritized variants in genes known to have important functions in neuronal development. Accordingly, we retained a de novo heterozygous CDH2 variant (GenBank: NM_001792.5: c.1789G>A [p.Asp597Asn]) that was absent in gnomAD. Sanger sequencing confirmed that it was heterozygous in the proband and absent in his parents. This missense variant results in the substitution of a phylogenetically conserved amino acid (Figure 2B) and is predicted to result in a deleterious effect according to in silico tools (Table S3). Furthermore, the CDH2 gnomAD Z score predicts intolerance for missense variations (2.09) (positive Z scores indicate increased constraint and therefore intolerance to variation), in line with a residual variation intolerance score (RVIS) (%ExAC v2 RVIS: −1.0396 [12.3558%]) (a negative score means that less common functional variations are observed in this gene than would be expected, suggesting that it is intolerant to variation and is among the 12.3558% most intolerant of human genes), and CDH2 is predicted to be potentially associated with dominant conditions according to a linear discriminant analysis (LDA) score of 3.5 (> 0.8 corresponds to a “very likely dominant” class) by the DOMINO algorithm.32
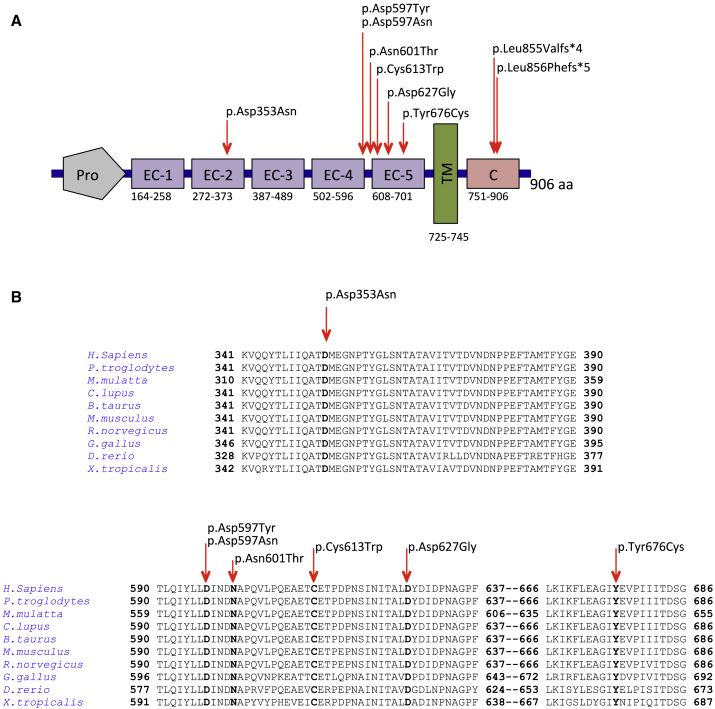
Figure 2.
Localization and Conservation of De Novo Variants in CDH2
(A) A schematic depiction of CDH2 shows the cadherin-Pro region (Pro), five extracellular cadherin domain repeats (EC-1 to EC-5), the transmembrane region (TM), and the cytoplasmic tail (C). Arrowheads above the protein show the positions of the variants.
(B) The HomoloGene-generated amino acid alignment of human CDH2 and its predicted orthologs shows the conservation of the affected residues.
We next queried CDH2 in GeneMatcher, a freely accessible website that enables the identification of individuals with variants in candidate genes,33 and ascertained eight additional cases, all of whom had an overlapping phenotype (Table 1, Table S1, and Supplemental Note). All CDH2 variants were found by clinical (subjects 3, 4, 5) or research (subjects 1, 6, 7, 8, 9) exome sequencing according to standard protocols, after written informed consent was obtained from probands and/or families. In all subjects, WES data analysis excluded the presence of other functionally relevant variants compatible with known Mendelian disorders, except that subject 4 carried a maternally inherited pathogenic GALT variant (GenBank: NM_000155.3, c.1802A>C [p.Ser135Leu]), responsible for the autosomal recessive Galactosemia (MIM: 230400) and that subject 1 harbored a de novo DNM1 variant (GenBank: NM_004408, c.400C>A [p.Leu134Met]), associated with an autosomal dominant epileptic encephalopathy (MIM: 616346). Although the DNM1 variant is classified as pathogenic according to the ACMG guidelines, it was felt not to explain subject 1’s phenotype because the major DNM1-encephalopathy-associated features, such as ID, epilepsy, and microcephaly,34 were absent (Table 1 and Table S1). All subjects harbored de novo CDH2 variants, which included six missense (c.1057G>A [p.Asp353Asn]; c.1789G>T [p.Asp597Tyr]; c.1802A>C [p.Asn601Thr]; c.1839C>G [p.Cys613Trp]; c.1880A>G [p.Asp627Gly]; and c.2027A>G, [p.Tyr676Cys]) and two distal frameshift (c.2563_2564delCT [p.Leu855Valfs∗4] and c.2564_2567dupTGTT [p.Leu856Phefs∗5]) variants, all of which are absent in gnomAD and are predicted to have a deleterious effect by several bioinformatics tools (Table S3).
CDH2 consists of 906 amino acids and is structurally divided into a cadherin pro-region; five EC repeats of about 110 residues, each of which fold into seven anti-parallel β strands arranged into two β sandwich folds; a transmembrane region; and a cytoplasmic tail. Three Ca2+ ions bind to the highly conserved linker domains between each EC domain, stabilizing the whole structure and ensuring its proper conformation. This facilitates trans interactions with other cadherin molecules from apposing membranes.35, 36 N-cadherin binding capacity is highly sensitive to extracellular Ca2+ concentration changes, as revealed by the partial loss of homophilic interaction upon rapid removal of extracellular Ca2+.37
Remarkably, six of the seven missense variants we identified (c.1789G>A [p.Asp597Asn], c.1789G>T [p.Asp597Tyr], c.1802A>C [p.Asn601Thr], c.1839C>G [p.Cys613Trp], c.1880A>G [p.Asp627Gly], and c.2027A>G [p.Tyr676Cys]) affect residues of the EC4-EC5 linker region and EC5, which are highly conserved among vertebrate orthologs (Figures 2A and 2B). Three variants result in amino acid substitutions that are in the calcium-binding site between EC4 and EC5, whereas the other three are in EC5 (Figure 2A). These amino acid changes lie in protein regions that are intolerant to missense variations according to the tolerance landscape of CDH2 variants, as depicted by MetaDome38 (Figure S1). To assess the clustering of these variants, we calculated their geometric mean distance.39 Compared to the gnomAD-reported mutational density of variants along the transcript, the observed variants in the EC4-EC5 calcium-binding site and EC5 are significantly clustered (p = 1.37 × 10−4), suggesting that there is a common effect on N-cadherin activity and that this effect is critically related to a region spanning about 80 amino acids.
The c.1057G>A (p.Asp353Asn) missense variant affects an EC2 residue that is highly conserved among vertebrate orthologs (Figure 2B). The proximal region of EC2 along with EC1 has been largely studied in the homophilic interaction of opposing cadherins5, 40 and together they constitute the “minimal essential unit” for CDH2-mediated cell adhesion.41 The two frameshift variants (c.2563_2564delCT [p.Leu855Valfs∗4] and c.2564_2567dupTGTT [p.Leu856Phefs∗5]) are predicted to result in a truncated protein with a shortened cytoplasmic tail. The variants encode mRNAs that are not predicted to undergo mRNA decay according to the 50-nucleotide rule.42 The cytoplasmic tail of classical cadherins, including N-cadherin, is well known to stabilize cadherins on the cell surface through its link to the actin cytoskeleton; it thereby ensures proper cell adhesions and clustering.43, 44, 45
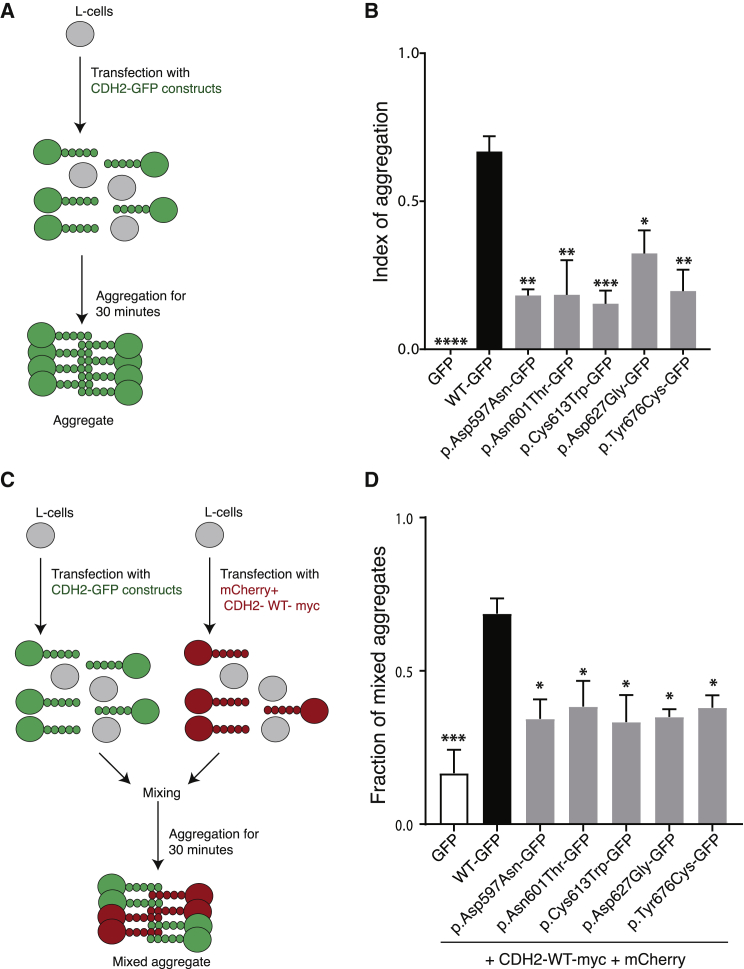
To test whether the variants lying in the EC4-EC5 linker region and EC5 could affect N-cadherin-mediated cell adhesion, we generated an N-cadherin-GFP fusion protein with the GFP tag at the C-terminal of the protein; this tag does not alter the cis and trans binding capability of the protein.36, 41 We then used the N-cadherin-GFP construct to generate five of the six point mutations identified in the subjects by PCR mutagenesis. p.Asp597Tyr was not generated because the same amino acid is substituted in (p.Asp597Asn). To investigate whether the N-cadherin variants affect cell adhesion, we performed an aggregation assay (Figure 3A). We transfected L cells with one of the following constructs: N-cadherin-WT-GFP or the variants N-cadherin-Asp597Asn-GFP, N-cadherin-Asn601Thr-GFP, N-cadherin-Cys613Trp-GFP, N-cadherin-Asp627Gly-GFP, or N-cadherin-Tyr676Cys-GFP. Similar protein amounts of N-cadherin-WT-GFP or the variants were observed by immunoblot analysis (Figure S2A), as well as similar cell-surface protein amounts, as assessed by immunofluorescence under non-permeabilizing conditions (Figure S2B). 48 h after transfection, we assessed the cell-aggregation capability in the presence of Ca2+ (Figure S3A). Expression of the N-cadherin-WT-GFP promoted the formation of aggregates in relation to the GFP negative control. However, cells expressing the N-cadherin variants displayed reduced aggregation capabilities in comparison to N-cadherin-WT-GFP. Cells expressing N-cadherin variants formed smaller aggregates than those expressing N-cadherin-WT-GFP (Figure S3A), resulting in a significantly lower index of aggregation than that for cells expressing N-cadherin-WT-GFP (p value ≤ 0.05) (Figure 3B). These results indicate that the N-cadherin variants have defective self-interaction.
Figure 3.
N-cadherin Variants Have Impaired Cell-Cell Adhesion and Reduced trans Adhesion to N-cadherin-WT
(A) Schematic representation of the cell-aggregation assay. L cells were transfected with empty GFP, N-cadherin-WT, and N-cadherin variants. Aggregation was then performed for 30 min in the presence of calcium.
(B) Bar graph of the mean ± SE of the index of aggregation for N-cadherin-WT (black) and N-cadherin variants (gray) (n = 5 experiments). Cell aggregation was evaluated after 30 min of aggregation (T30) by the index (N0-N30)/N0, where N0 is number of GFP-positive cells or aggregates at T0, and N30 is the number of GFP-positive cells or aggregates at T30. The N-cadherin-GFP variants have a lower index of aggregation than N-cadherin-WT-GFP cells do. One-way ANOVA post-tests were performed for each variant versus N-cadherin-WT (∗∗∗∗p ≤ 0.0001; ∗∗∗p ≤ 0.001; ∗∗p ≤ 0.01; ∗p ≤ 0.05).
(C) Schematic representation of the mixed-cell aggregation assay evaluating trans interaction with N-cadherin-WT. L cells were transfected with empty GFP, N-cadherin-WT, and N-cadherin variants or with N-cadherin-WT-Myc+mCherry. Cells were then mixed in a 1:1 ratio, and aggregation was performed for 30 min in the presence of calcium.
(D) Bar graph of the mean ± SE of the fraction of mixed aggregates for N-cadherin-WT (black) and N-cadherin (gray) variants (n = 3 experiments). The fraction of mixed aggregates was evaluated after 30 min of aggregation (T30) by the following index: number of mixed aggregates/number of total aggregates. Cells expressing N-cadherin WT-myc+mCherry form mixed aggregates with cells expressing N-cadherin-WT-GFP, but they form fewer mixed aggregates with cells expressing the N-cadherin variants. One-way ANOVA post-test were performed for each variant versus N-cadherin-WT (∗∗∗p ≤ 0.001; ∗p ≤ 0.05).
Next, to determine whether the variants had impaired binding in trans with N-cadherin-WT-GFP, we performed a mixed aggregation assay. We transfected L cells with N-cadherin-WT-Myc+mCherry or with N-cadherin-WT-GFP, N-cadherin-Asp597Asn-GFP, N-cadherin-Asn601Thr-GFP, N-cadherin-Cys613Trp-GFP, N-cadherin-Asp627Gly-GFP, or N-cadherin-Tyr676Cys-GFP variants. 48 h post-transfection, cells expressing N-cadherin-WT-Myc+mCherry were mixed in a 1:1 ratio with the cells expressing N-cadherin-WT-GFP or N-cadherin-variants and cell aggregation capability was assessed (Figure 3C). N-cadherin-WT-Myc+mCherry formed mixed aggregates with N-cadherin-WT-GFP. In contrast, when we mixed N-cadherin-WT-Myc+mCherry cells with cells expressing N-cadherin-variants, fewer mixed aggregates were formed (Figure S3B). As expected, N-cadherin-WT-Myc+mCherry cells still formed clusters of self-aggregates. Analysis of the fraction of mixed aggregates showed that all the CDH2 substitutions had significantly reduced adhesion in trans with the N-cadherin-WT-Myc+mCherry, with respect to the N-cadherin-WT-GFP (p value ≤ 0.05) (Figure 3D). Taken together, these findings indicate that the CDH2 variants impair the cell-adhesion function of N-cadherin by affecting both self-binding and trans-binding with N-cadherin-WT.
Our results indicate that the EC4-EC5 region of N-cadherin is important for cell adhesion. Multiple studies have identified the distal EC1 domain as being critical for trans interactions between cadherins on opposing membranes and for cell-cell adhesion.46, 47 In contrast, little is known about the functional role of the proximal EC domains, EC4, and EC5 in the cis and trans cadherin interaction.
To visualize the identified amino acid substitutions and predict their effect on 3D protein structure, we generated a CDH2 model by using SWISS-MODEL and displayed the amino acids by using Chimera UCSF (Figures S4 and S5). Three amino acid substitutions in the calcium binding site (p.Asp597Asn, p.Asp597Tyr, and p.Asn601Thr) and one in EC5 (p.Asp627Gly) were predicted to modify the calcium-binding pocket compared to the that in the wild-type residues and thereby to potentially alter the ectodomain stability mediated by the calcium ions (Figures S4E, S4I, S4M, and S4N). The substitutions of the polar and charged Asp597 by either the uncharged Asn or the aromatic Tyr were predicted to abolish the H bond that the wild-type Asp597 forms with Asp629 and Asn633 and to interfere with Arg561 (Figures S4M and S4N). The substitution of the Asn601 by the Thr in the loop between EC4 and EC5 (Figures S4H and S4I) is also predicted to alter the Ca2+-binding pocket. Similar findings are observed when Asp627 is substituted by the small glycine (Figures S4D and S4E). Our results implicating changes in Ca2+ binding are consistent with E-cadherin studies showing that disruption of the calcium binding site between EC4 and EC5 is sufficient to disrupt the trans association between E-cadherin of apposing cells.36
Cys613 and Tyr676 are in EC5, and the substitution of the small Cys with the aromatic Trp at position 613 is predicted to interfere with Cys701 and disrupt the H bond formed by the wild-type Cys613 and Cys701 (Figures S4F and S4G). The substitution of Tyr676 with Cys is predicted to disrupt the H bond between the wild-type Tyr676 and Glu672 (Figures S4B and S4C). Altogether, these changes might result in altered protein conformation and explain the defective adhesion observed in L cells expressing N-cadherin mutants.
Although Asp353 lies 20 amino acids from the EC2–3 interdomain, the predicted folding of CDH2 in 3D modeling (Figure S5) shows that it is proximal to the calcium-binding site of the EC1-EC2 interdomain. The substitution of the negatively charged Asp353 with the neutral Asn is predicted to abolish the H bond between the wild-type Asp and Asn261 without altering the calcium binding pocket.
A summary of the clinical features of affected individuals is presented in Table 1, Table S1, and the Supplemental Note. All subjects exhibited developmental delay, and half (4/8) had mild to moderate ID. Two individuals met diagnostic criteria for ASD, one displayed self-injurious behaviors, and another had several neuropsychiatric issues, including auditory hallucinations. Seven of nine subjects had ACC, which was prenatally diagnosed in all cases (Figure 1). One individual had corpus callosum hypoplasia. Additionally, periventricular nodular heterotopias were detected in four subjects. Hypothalamic adhesion was identified in five subjects, and two individuals had an intherhemispheric cyst communicating with the III ventricle. Four subjects had hypo-dysplastic tentorium, two subjects had megacisterna magna, and another showed an atretic parietal cephalocele. Only one subject had a normal brain MRI. A wide range of congenital cardiac defects were found in the majority of individuals (6/9); such defects include a defect of the atrioventricular canal (n = 2), aortic coarctation (n = 2), dextrocardia with right pulmonary artery hypoplasia (n = 1), and tricuspid regurgitation (n = 1). Five of seven of these congenital cardiac defects were diagnosed prenatally. In addition, one subject had pericardial effusion. Two individuals developed seizures. Half of the cohort exhibited congenital eye defects, including Peters anomaly (n = 2), unilateral ptosis with Duane anomaly (n = 1), congenital cataracts (n = 1), and strabismus (n = 3), all requiring surgical intervention. Hyposmia was reported in three subjects, one of which also had hypogeusia. Interestingly, subject 7 had congenital mirror movements (MMs), which are involuntary movements that occur on one side of the body but that mirror voluntary movements made on the opposite side.48 MMs reflect aberrant neuronal wiring and axon guidance.49, 50 Three individuals showed shoulder deformities (bilateral absence of shoulder muscles [n = 1] and Sprengel type deformity [n = 2]). All but one male had genital anomalies, including cryptorchidism (n = 3) and micropenis (n = 1). Four subjects had macrocephaly, and one had relative macrocephaly at last evaluation. Consent for published images (Figure 1) was obtained from all parents and legal guardians. In those for whom photos were available, common craniofacial dysmorphisms in two subjects (2, 4) included a broad and prominent forehead, downslanting palpebral fissures, thin upper lips, and low-set ears with thick helices and earlobes, whereas subjects 7 and 8 displayed deep-set eyes, slightly low-set and posteriorly rotated ears with attached earlobes, thin upper lips, and a pointed chin. Two subjects (5 and 9) did not show major craniofacial dysmorphisms.
Of note, subject 1, who was found to also harbor a de novo variant in DNM1, displayed an overlapping phenotype with our cohort. It remains to be determined whether the imperforate anus and vertebral anomalies observed in this individual are features of the CDH2 spectrum; they are not usually observed in the DNM1 epileptic encephalopathy.
We have identified de novo pathogenic variants in CDH2 in nine individuals with a strikingly similar clinical and radiologic phenotype characterized by developmental delay/ID (8/8), callosal malformations (8/9), cardiac (7/9) and ocular (7/9) abnormalities, and characteristic facial dysmorphisms. Six of the seven missense variants, of which two affect the same amino acid, cluster in the same protein region, namely EC4-EC5. Our in vitro assays demonstrated that the EC4-EC5 variants result in defective CDH2 function, supporting a possible dominant-negative effect (DNE). Our cohort also includes one individual with a missense variant in EC2 and two individuals with distal and neighboring frameshift variants leading to a premature stop of translation of the cytoplasmic tail and predicted to result in a truncated CDH2 protein. On the basis of previous evidence supporting the DNE of truncating variants affecting the cytoplasmic domain of other cadherins51 or other proteins,52 we speculate a similar pathomechanism for our variants, though a loss of function mechanism is also possible. Given that the EC2 domain is critical for N-cadherin-mediated adhesion,41 the EC2 missense variant is likely to also act through a DNE.
The neurodevelopmental features of the affected individuals, including ACC, periventricular nodular heterotopias, hyposmia, MMs, and Duane anomaly, support previous evidence regarding the critical role of CDH2 in neuronal migration and axon pathfinding. Indeed, N-cadherin is essential for establishing dynamic adhesions between migrating neurons and radial glial cells during glia-dependent migration.53, 54 Knockdown studies in vivo either by RNAi53 or by in utero electroporation55 have shown that depletion of N-cadherin impairs the neuronal attachment of migrating neurons along the radial glial fibers in the mouse developing cerebral cortex. N-cadherin overexpression also perturbs neuronal migration.55 N-cadherin knock-out mice die during embryonic stages;56 however, mice with a conditional inactivation of N-cadherin in the cerebral cortex display cortical disorganization and lack a corpus callosum,57 paralleling the ACC and periventricular nodular heterotopias observed in our subjects. In addition, N-cadherin plays a critical role for the proper direction and collective migration of facial branchiomotor neurons from the developing hindbrain.58 Lastly, knockdown of N-cadherin in the developing chicken optic tectum affects axonal length, formation of multipolar neurons, and neuronal migration.22 Taken together, the impaired cell-cell adhesion that we observed in vitro most likely underlies a neuronal migration or axon pathfinding defect in vivo during early development and results in ACC, hyposmia, Duane anomaly, abnormal shoulder muscle innervation, nodular heterotopia, and MMs in our subjects. MMs have been linked to genes (DCC,59 NTN1,60 DNAL4,61 RAD5162) encoding axon-guidance receptors and ligands that tightly regulate the spatiotemporal development of corticospinal tract and corpus callosum. Monoallelic DCC variants can cause congenital MMs (MIM: 157600) in association with abnormal midline crossing of the corticospinal tract, isolated ACC (MIM: 217990), or both.59, 63 These features partly overlap the neuroradiological features and movement disorders of our subjects. Although CDH2 and MM genes have been linked so far to distinct pathways and gene regulatory networks (according to KEGG, geneMANIA, STRING, and TRUUST databases),64, 65, 66, 67 a previous study revealed that overexpression of truncated DCC constructs in neuroblastoma cells diminished N-cadherin protein amounts (together with alpha- and beta-catenin) and impaired calcium-dependent cell adhesion, pointing to a possible functional link between DCC and the activity of N-cadherin and catenin.68 Further studies will shed light on the regulatory framework and the possible interaction between CDH2 and MM genes.
The presence of mild to moderate ID and/or ASD in three subjects is consistent with the pivotal role of the cadherin superfamily in synapse structure, stability, and plasticity.69, 70, 71 Dendrite and synapse morphogenesis are in fact key determinants of neuronal connectivity, whose impairment underlies the pathogenesis of many neurodevelopmental disorders, such as ID, ASD, and epilepsy.72, 73 N-cadherin is known to regulate presynaptic function at glutamatergic synapses74 and control presynaptic-vesicle clustering through a trans-synaptic mechanism, promoting postsynaptic accumulation of synaptic organizing molecules, such as neuroligin-1.75 Although some knockdown studies of N-cadherin19, 74, 75, 76 showed defects in synapse structure, others revealed an imbalance between excitatory and inhibitory synaptic markers,57, 77 suggesting a critical role for N-cadherin in maintaining synaptic homeostasis and plasticity.77 Furthermore, it has recently been revealed that CDH2 cooperates with other synaptic organizers to stimulate both presynaptic and postsynaptic differentiation.20 After the association of canine compulsive disorder with its orthologous CDH2,23 a few CDH2 SNPs were identified in humans with obsessive compulsive disorder and Tourette disorder23, 24, 25 and were shown to reduce CDH2 protein amounts in HEK293 cells.23 Among the CDH2 variants related to neuropsychiatric disorders, only c.2118C>A (p.Asn706Lys; rs775358499) is rare (MAF < 0.001, allele count in gnomAD is 1) and lies between EC5 and the transmembrane domain. Altogether, the neurodevelopmental features of our subjects are consistent with the important role of CDH2 in synaptogenesis, expanding the list of cadherin superfamily genes associated with neurodevelopmental disorders.15
Interestingly, seven of nine individuals showed eye anomalies. Although ptosis and Duane anomaly could be explained by an axon-guidance defect,78 Peters anomaly (found in two out of six individuals) is consistent with N-cadherin’s pivotal role in the development of the anterior segment of the eye.79, 80
Moreover, the muscle anomalies observed in three subjects most likely reflect an axon-pathfinding defect that results in a failure of proper innervation.81 Furthermore, the finding of hyposmia in three subjects might point to an axon-pathfinding role of CDH2 in the olfactory system, as previously suggested82 and is consistent with the fact that other adhesion molecules have key roles in the guidance of olfactory neurons.83 In addition, CDH2 is required for the proper axon-axon interactions between atonal and amos olfactory receptor neurons (ORNs) in Drosophila,84 suggesting a role for CDH2 in glomeruli homeostasis.
Heterozygous variants affecting ectodomains of CDH2 have been recently associated with arrhythmogenic right ventricular cardiomyopathy (ARVC), an inherited cardiomyopathy that is characterized by fibrofatty replacement of the right ventricular myocardium and that predisposes individuals to ventricular arrhythmia and sudden death.85, 86 ARVC is primarily caused by pathogenic variants in genes encoding proteins of the desmosomes,87 a type of intercellular junction that, along with adherens and gap junctions, forms intercalated discs within cardiomyocytes. A missense CDH2 variant (c.686A>C [p.Gln229Pro]) was first identified by WES in multiple individuals with ARVC in a three-generation family.29 Subsequently, another missense variant, c.1219G>A (p.Asp407Asn), was found in several ARVC-affected subjects from two unrelated families,28, 29 further supporting the candidate role of CDH2 in ARVC pathogenesis. These findings are in line with the critical role of CDH2 in cardiac development and function, as shown by N-cadherin knockout mouse models that display dissolution of the adherens junctions in the intercalated discs and develop dilated cardiomyopathy and ventricular arrhythmia.88 Accordingly, it is not surprising that seven of nine subjects in our cohort display a wide range of cardiac abnormalities, including complete atrioventricular canal defect (subjects 1 and 3), aortic coarctation (subjects 8 and 9), dextrocardia and right pulmonary artery hypoplasia (subject 5), pericardium effusion (subject 7), and mild tricuspid regurgitation (subject 2). Of note, the finding of dextrocardia in subject 5 is consistent with the pivotal role of CDH2 in establishing the left-to-right axis during gastrulation of chicken embryos.89 Subject 3 died after pulseless electrical activity at eight weeks of life, and subject 5 experienced atrial flutter requiring extracorporeal circulation at birth. Although the other two individuals had no cardiac abnormalities, periodic cardiac reassessment, including electrocardiogram and standard echocardiogram, becomes strongly warranted in all subjects with pathogenic CDH2 variants, according to the recent updated guidelines for ARVC.90
Interestingly, three of five males showed cryptorchidism, and one showed micropenis. Of note, CDH2 is highly expressed in the testis,91 where it is known to mediate Sertoli cell-germ cell adhesion.92 Remarkably, mice with a conditional knockout of CDH2 in Sertoli cells showed compromised blood-testis barrier function and spermatogenesis failure, suggesting a crucial role of CDH2 in the function of Sertoli cells.93
The finding of hearing loss in two subjects (2 and 9) further supports previous evidence regarding a CDH2 role in the morphogenesis of the otic vesicle in zebrafish.94
Furthermore, the well-known association of cadherins with cell migration and invasion in many types of cancers unavoidably raises the question whether germline CDH2 variants could confer susceptibility to malignancy. To date, 499 somatic CDH2 variants have been reported in COSMIC. Among these, two affect the same residues of subject 1 and 6 but result in different amino acid changes (c.1057G>T [p.Asp353Tyr] [COSM: 1236400] and c.1879G>A [p.Asp627Asn] [COSM: 3524707], respectively). Several studies, mainly focused on transcriptional or post-transcriptional regulation, showed that CDH2 upregulation leads to transepithelial spreading of melanoma,95 hematological malignancies,96 and high histopathological grade of glioma.97 High CDH2 protein amounts are also a poor prognostic factor in pancreatic and gallbladder cancer.98, 99 Further studies are needed to shed light on the possible oncologic risk in patients harboring germline CDH2 variants.
Overall, the association of callosal anomalies, congenital heart defects, and ocular and urogenital anomalies with variants in CDH2 suggests that pathogenic variants in CDH2 are causative of a recognizable syndrome, for which we suggest the name ACOG syndrome (agenesis of corpus callosum, axon pathfinding, cardiac, ocular, and genital defects). Moreover, the prenatal diagnosis of ACC in association with congenital heart defects should motivate clinicians to consider CDH2 in the differential diagnosis of ACC spectrum,100 and this should orient them toward providing prompt prenatal genetic counselling.
In summary, our study demonstrates that de novo heterozygous variants in CDH2 result in a multisystemic developmental disorder, primarily involving the nervous, cardiac, ophthalmologic, and genital systems. Many of these pathogenic variants cluster between the EC4 and EC5 domains and result in defective CDH2-mediated cell-cell adhesion, suggesting a previously unrecognized critical role of this region in CDH2 function. Future clinical, genetic, and functional studies are warranted to delineate the phenotypic spectrum and provide further insights into the pathogenic mechanisms related to CDH2 variants.
Consortia
Collaborators of The Undiagnosed Diseases Network (UDN) include Maria T. Acosta, David R. Adams, Pankaj Agrawal, Mercedes E. Alejandro, Patrick Allard, Justin Alvey, Ashley Andrews, Euan A. Ashley, Mahshid S. Azamian, Carlos A. Bacino, Guney Bademci, Eva Baker, Ashok Balasubramanyam, Dustin Baldridge, Jim Bale, Deborah Barbouth, Gabriel F. Batzli, Pinar Bayrak-Toydemir, Alan H. Beggs, Gill Bejerano, Hugo J. Bellen, Jonathan A. Bernstein, Gerard T. Berry, Anna Bican, David P. Bick, Camille L. Birch, Stephanie Bivona, John Bohnsack, Carsten Bonnenmann, Devon Bonner, Braden E. Boone, Bret L. Bostwick, Lorenzo Botto, Lauren C. Briere, Elly Brokamp, Donna M. Brown, Matthew Brush, Elizabeth A. Burke, Lindsay C. Burrage, Manish J. Butte, John Carey, Olveen Carrasquillo, Ta Chen Peter Chang, Hsiao-Tuan Chao, Gary D. Clark, Terra R. Coakley, Laurel A. Cobban, Joy D. Cogan, F. Sessions Cole, Heather A. Colley, Cynthia M. Cooper, Heidi Cope, William J. Craigen, Precilla D’Souza, Surendra Dasari, Mariska Davids, Jyoti G. Dayal, Esteban C. Dell’Angelica, Shweta U. Dhar, Naghmeh Dorrani, Daniel C. Dorset, Emilie D. Douine, David D. Draper, Laura Duncan, David J. Eckstein, Lisa T. Emrick, Christine M. Eng, Cecilia Esteves, Tyra Estwick, Liliana Fernandez, Carlos Ferreira, Elizabeth L. Fieg, Paul G. Fisher, Brent L. Fogel, Irman Forghani, Laure Fresard, William A. Gahl, Rena A. Godfrey, Alica M. Goldman, David B. Goldstein, Jean-Philippe F. Gourdine, Alana Grajewski, Catherine A. Groden, Andrea L. Gropman, Melissa Haendel, Rizwan Hamid, Neil A. Hanchard, Nichole Hayes, Frances High, Ingrid A. Holm, Jason Hom, Alden Huang, Yong Huang, Rosario Isasi, Fariha Jamal, Yong-hui Jiang, Jean M. Johnston, Angela L. Jones, Lefkothea Karaviti, Emily G. Kelley, Dana Kiley, David M. Koeller, Isaac S. Kohane, Jennefer N. Kohler, Deborah Krakow, Donna M. Krasnewich, Susan Korrick, Mary Koziura, Joel B. Krier, Jennifer E. Kyle, Seema R. Lalani, Byron Lam, Brendan C. Lanpher, Ian R. Lanza, C. Christopher Lau, Jozef Lazar, Kimberly LeBlanc, Brendan H. Lee, Hane Lee, Roy Levitt, Shawn E. Levy, Richard A. Lewis, Sharyn A. Lincoln, Pengfei Liu, Xue Zhong Liu, Nicola Longo, Sandra K. Loo, Joseph Loscalzo, Richard L. Maas, Ellen F. Macnamara, Calum A. MacRae, Valerie V. Maduro, Marta M. Majcherska, May Christine V. Malicdan, Laura A. Mamounas, Teri A. Manolio, Rong Mao, Thomas C. Markello, Ronit Marom, Gabor Marth, Beth A. Martin, Martin G. Martin, Julian A. Martínez-Agosto, Shruti Marwaha, Thomas May, Jacob McCauley, Allyn McConkie-Rosell, Colleen E. McCormack, Alexa T. McCray, Thomas O. Metz, Matthew Might, Eva Morava-Kozicz, Paolo M. Moretti, Marie Morimoto, John J. Mulvihill, David R. Murdock, Avi Nath, Stan F. Nelson, J. Scott Newberry, John H. Newman, Sarah K. Nicholas, Donna Novacic, Devin Oglesbee, James P. Orengo, Laura Pace, Stephen Pak, J. Carl Pallais, Christina GS. Palmer, Jeanette C. Papp, Neil H. Parker, John A. Phillips III, Jennifer E. Posey, John H. Postlethwait, Lorraine Potocki, Barbara N. Pusey, Aaron Quinlan, Archana N. Raja, Genecee Renteria, Chloe M. Reuter, Lynette Rives, Amy K. Robertson, Lance H. Rodan, Jill A. Rosenfeld, Robb K. Rowley, Maura Ruzhnikov, Ralph Sacco, Jacinda B. Sampson, Susan L. Samson, Mario Saporta, Judy Schaechter, Timothy Schedl, Kelly Schoch, Daryl A. Scott, Lisa Shakachite, Prashant Sharma, Vandana Shashi, Kathleen Shields, Jimann Shin, Rebecca Signer, Catherine H. Sillari, Edwin K. Silverman, Janet S. Sinsheimer, Kathy Sisco, Kevin S. Smith, Lilianna Solnica-Krezel, Rebecca C. Spillmann, Joan M. Stoler, Nicholas Stong, Jennifer A. Sullivan, Shirley Sutton, David A. Sweetser, Holly K. Tabor, Cecelia P. Tamburro, Queenie K.-G. Tan, Mustafa Tekin, Fred Telischi, Willa Thorson, Cynthia J. Tifft, Camilo Toro, Alyssa A. Tran, Tiina K. Urv, Matt Velinder, Dave Viskochil, Tiphanie P. Vogel, Colleen E. Wahl, Nicole M. Walley, Chris A. Walsh, Melissa Walker, Jennifer Wambach, Jijun Wan, Lee-kai Wang, Michael F. Wangler, Patricia A. Ward, Katrina M. Waters, Bobbie-Jo M. Webb-Robertson, Daniel Wegner, Monte Westerfield, Matthew T. Wheeler, Anastasia L. Wise, Lynne A. Wolfe, Jeremy D. Woods, Elizabeth A. Worthey, Shinya Yamamoto, John Yang, Amanda J. Yoon, Guoyun Yu, Diane B. Zastrow, Chunli Zhao, and Stephan Zuchner.
Declaration of Interests
K.McW. and A.B. are employees of GeneDx, Inc.
Acknowledgments
We thank Laurens van de Wiel from the Radboud Institute for Molecular Life Sciences (Nijmegen, the Netherlands) for his assistance in calculating significance of spatial clustering of de novo variants. M.S. holds a clinician-scientist award from Fonds de Recherche du Québec Santé (FRQS). A.A.’s training fellowship was supported by the Mel Hoppenheim Fund in Pediatric Neurology (Montreal Children’s Foundation) and the Rotary Foundation (global grant GC1641420 scholarship sponsored by the Rotary district 2032, Genoa, Italy). Work performed in the M.S. laboratory was supported by funding from the Canadian Institutes of Health Research (CIHR) and Sick Kids Foundation (NI16-028). Work performed in the F.C. laboratory was supported by funding from the CIHR (FDN334023), the FRQS, and the Canada Foundation for Innovation (CFI 33768). F.C. holds the Canada Research Chair in Developmental Neurobiology. Research reported in this manuscript was in part supported by the National Institutes of Health Common Fund, through the Office of Strategic Coordination and Office of the NIH Director under award number 2U01HG007690. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Sequencing centers for subject 1 and subject 8 were the University of Washington Center for Mendelian Genomics and the Broad Institute of MIT and Harvard.
Published: October 3, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.09.005.
Contributor Information
Frédéric Charron, Email: frederic.charron@ircm.qc.ca.
Myriam Srour, Email: myriam.srour@mcgill.ca.
Undiagnosed Diseases Network:
Maria T. Acosta, David R. Adams, Pankaj Agrawal, Mercedes E. Alejandro, Patrick Allard, Justin Alvey, Ashley Andrews, Euan A. Ashley, Mahshid S. Azamian, Carlos A. Bacino, Guney Bademci, Eva Baker, Ashok Balasubramanyam, Dustin Baldridge, Jim Bale, Deborah Barbouth, Gabriel F. Batzli, Pinar Bayrak-Toydemir, Alan H. Beggs, Gill Bejerano, Hugo J. Bellen, Jonathan A. Bernstein, Gerard T. Berry, Anna Bican, David P. Bick, Camille L. Birch, Stephanie Bivona, John Bohnsack, Carsten Bonnenmann, Devon Bonner, Braden E. Boone, Bret L. Bostwick, Lorenzo Botto, Lauren C. Briere, Elly Brokamp, Donna M. Brown, Matthew Brush, Elizabeth A. Burke, Lindsay C. Burrage, Manish J. Butte, John Carey, Olveen Carrasquillo, Ta Chen Peter Chang, Hsiao-Tuan Chao, Gary D. Clark, Terra R. Coakley, Laurel A. Cobban, Joy D. Cogan, F. Sessions Cole, Heather A. Colley, Cynthia M. Cooper, Heidi Cope, William J. Craigen, Precilla D’Souza, Surendra Dasari, Mariska Davids, Jyoti G. Dayal, Esteban C. Dell’Angelica, Shweta U. Dhar, Naghmeh Dorrani, Daniel C. Dorset, Emilie D. Douine, David D. Draper, Laura Duncan, David J. Eckstein, Lisa T. Emrick, Christine M. Eng, Cecilia Esteves, Tyra Estwick, Liliana Fernandez, Carlos Ferreira, Elizabeth L. Fieg, Paul G. Fisher, Brent L. Fogel, Irman Forghani, Laure Fresard, William A. Gahl, Rena A. Godfrey, Alica M. Goldman, David B. Goldstein, Jean-Philippe F. Gourdine, Alana Grajewski, Catherine A. Groden, Andrea L. Gropman, Melissa Haendel, Rizwan Hamid, Neil A. Hanchard, Nichole Hayes, Frances High, Ingrid A. Holm, Jason Hom, Alden Huang, Yong Huang, Rosario Isasi, Fariha Jamal, Yong-hui Jiang, Jean M. Johnston, Angela L. Jones, Lefkothea Karaviti, Emily G. Kelley, Dana Kiley, David M. Koeller, Isaac S. Kohane, Jennefer N. Kohler, Deborah Krakow, Donna M. Krasnewich, Susan Korrick, Mary Koziura, Joel B. Krier, Jennifer E. Kyle, Seema R. Lalani, Byron Lam, Brendan C. Lanpher, Ian R. Lanza, C. Christopher Lau, Jozef Lazar, Kimberly LeBlanc, Brendan H. Lee, Hane Lee, Roy Levitt, Shawn E. Levy, Richard A. Lewis, Sharyn A. Lincoln, Pengfei Liu, Xue Zhong Liu, Nicola Longo, Sandra K. Loo, Joseph Loscalzo, Richard L. Maas, Ellen F. Macnamara, Calum A. MacRae, Valerie V. Maduro, Marta M. Majcherska, May Christine V. Malicdan, Laura A. Mamounas, Teri A. Manolio, Rong Mao, Thomas C. Markello, Ronit Marom, Gabor Marth, Beth A. Martin, Martin G. Martin, Julian A. Martínez-Agosto, Shruti Marwaha, Thomas May, Jacob McCauley, Allyn McConkie-Rosell, Colleen E. McCormack, Alexa T. McCray, Thomas O. Metz, Matthew Might, Eva Morava-Kozicz, Paolo M. Moretti, Marie Morimoto, John J. Mulvihill, David R. Murdock, Avi Nath, Stan F. Nelson, J. Scott Newberry, John H. Newman, Sarah K. Nicholas, Donna Novacic, Devin Oglesbee, James P. Orengo, Laura Pace, Stephen Pak, J. Carl Pallais, Christina G.S. Palmer, Jeanette C. Papp, Neil H. Parker, John A. Phillips, III, Jennifer E. Posey, John H. Postlethwait, Lorraine Potocki, Barbara N. Pusey, Aaron Quinlan, Archana N. Raja, Genecee Renteria, Chloe M. Reuter, Lynette Rives, Amy K. Robertson, Lance H. Rodan, Jill A. Rosenfeld, Robb K. Rowley, Maura Ruzhnikov, Ralph Sacco, Jacinda B. Sampson, Susan L. Samson, Mario Saporta, Judy Schaechter, Timothy Schedl, Kelly Schoch, Daryl A. Scott, Lisa Shakachite, Prashant Sharma, Vandana Shashi, Kathleen Shields, Jimann Shin, Rebecca Signer, Catherine H. Sillari, Edwin K. Silverman, Janet S. Sinsheimer, Kathy Sisco, Kevin S. Smith, Lilianna Solnica-Krezel, Rebecca C. Spillmann, Joan M. Stoler, Nicholas Stong, Jennifer A. Sullivan, Shirley Sutton, David A. Sweetser, Holly K. Tabor, Cecelia P. Tamburro, Queenie K.-G. Tan, Mustafa Tekin, Fred Telischi, Willa Thorson, Cynthia J. Tifft, Camilo Toro, Alyssa A. Tran, Tiina K. Urv, Matt Velinder, Dave Viskochil, Tiphanie P. Vogel, Colleen E. Wahl, Nicole M. Walley, Chris A. Walsh, Melissa Walker, Jennifer Wambach, Jijun Wan, Lee-kai Wang, Michael F. Wangler, Patricia A. Ward, Katrina M. Waters, Bobbie-Jo M. Webb-Robertson, Daniel Wegner, Monte Westerfield, Matthew T. Wheeler, Anastasia L. Wise, Lynne A. Wolfe, Jeremy D. Woods, Elizabeth A. Worthey, Shinya Yamamoto, John Yang, Amanda J. Yoon, Guoyun Yu, Diane B. Zastrow, Chunli Zhao, and Stephan Zuchner
Web Resources
GATK, https://software.broadinstitute.org/gatk/best-practices/
Kyoto Encyclopedia of Genes and Genomes, https://www.genome.jp/kegg/
OMIM, https://www.omim.org/
PhosphoSitePlus, https://www.phosphosite.org/proteinAction.action?id=4611&showAllSites=true
STRING, https://string-db.org
SWISS-MODEL, https://swissmodel.expasy.org/
TRRUST, https://www.grnpedia.org/trrust/key_regulator/1f988df38f.php
UCSF Chimera, https://www.cgl.ucsf.edu/chimera/
Supplemental Data
References
- 1.Goodwin K., Nelson C.M. Generating tissue topology through remodeling of cell-cell adhesions. Exp. Cell Res. 2017;358:45–51. doi: 10.1016/j.yexcr.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wickström S.A., Niessen C.M. Cell adhesion and mechanics as drivers of tissue organization and differentiation: local cues for large scale organization. Curr. Opin. Cell Biol. 2018;54:89–97. doi: 10.1016/j.ceb.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Priest A.V., Shafraz O., Sivasankar S. Biophysical basis of cadherin mediated cell-cell adhesion. Exp. Cell Res. 2017;358:10–13. doi: 10.1016/j.yexcr.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Tiwari P., Mrigwani A., Kaur H., Kaila P., Kumar R., Guptasarma P. Structural-mechanical and biochemical functions of classical cadherins at cellular junctions: A review and some hypotheses. Adv. Exp. Med. Biol. 2018;1112:107–138. doi: 10.1007/978-981-13-3065-0_9. [DOI] [PubMed] [Google Scholar]
- 5.Brasch J., Harrison O.J., Honig B., Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 2012;22:299–310. doi: 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yulis M., Kusters D.H.M., Nusrat A. Cadherins: cellular adhesive molecules serving as signalling mediators. J. Physiol. 2018;596:3883–3898. doi: 10.1113/JP275328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Amraoui A., Petit C. Cadherin defects in inherited human diseases. Prog. Mol. Biol. Transl. Sci. 2013;116:361–384. doi: 10.1016/B978-0-12-394311-8.00016-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q., Peng C., Song J., Zhang Y., Chen J., Song Z., Shou X., Ma Z., Peng H., Jian X. Germline mutations in CDH23, encoding cadherin-related 23, are associated with both familial and sporadic pituitary adenomas. Am. J. Hum. Genet. 2017;100:817–823. doi: 10.1016/j.ajhg.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhalla K., Luo Y., Buchan T., Beachem M.A., Guzauskas G.F., Ladd S., Bratcher S.J., Schroer R.J., Balsamo J., DuPont B.R. Alterations in CDH15 and KIRREL3 in patients with mild to severe intellectual disability. Am. J. Hum. Genet. 2008;83:703–713. doi: 10.1016/j.ajhg.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoumid J., Stichelbout M., Jourdain A.S., Frenois F., Lejeune-Dumoulin S., Alex-Cordier M.P., Lebrun M., Guerreschi P., Duquennoy-Martinot V., Vinchon M. Blepharocheilodontic syndrome is a CDH1 pathway-related disorder due to mutations in CDH1 and CTNND1. Genet. Med. 2017;19:1013–1021. doi: 10.1038/gim.2017.11. [DOI] [PubMed] [Google Scholar]
- 11.Taskiran E.Z., Karaosmanoglu B., Koşukcu C., Doğan O.A., Taylan-Şekeroğlu H., Şimşek-Kiper P.O., Utine E.G., Boduroğlu K., Alikaşifoğlu M. Homozygous indel mutation in CDH11 as the probable cause of Elsahy-Waters syndrome. Am. J. Med. Genet. A. 2017;173:3143–3152. doi: 10.1002/ajmg.a.38495. [DOI] [PubMed] [Google Scholar]
- 12.Crepel A., De Wolf V., Brison N., Ceulemans B., Walleghem D., Peuteman G., Lambrechts D., Steyaert J., Noens I., Devriendt K. Association of CDH11 with non-syndromic ASD. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014;165B:391–398. doi: 10.1002/ajmg.b.32243. [DOI] [PubMed] [Google Scholar]
- 13.Rivero O., Sich S., Popp S., Schmitt A., Franke B., Lesch K.P. Impact of the ADHD-susceptibility gene CDH13 on development and function of brain networks. Eur. Neuropsychopharmacol. 2013;23:492–507. doi: 10.1016/j.euroneuro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Smith L., Singhal N., El Achkar C.M., Truglio G., Rosen Sheidley B., Sullivan J., Poduri A. PCDH19-related epilepsy is associated with a broad neurodevelopmental spectrum. Epilepsia. 2018;59:679–689. doi: 10.1111/epi.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawi Z., Tong J., Dark C., Yates H., Johnson B., Bellgrove M.A. The role of cadherin genes in five major psychiatric disorders: A literature update. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2018;177:168–180. doi: 10.1002/ajmg.b.32592. [DOI] [PubMed] [Google Scholar]
- 16.Hatta K., Takagi S., Fujisawa H., Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev. Biol. 1987;120:215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto Y., Sakane F., Hashimoto K. N-cadherin-based adherens junction regulates the maintenance, proliferation, and differentiation of neural progenitor cells during development. Cell Adhes. Migr. 2015;9:183–192. doi: 10.1080/19336918.2015.1005466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalasani K., Brewster R.M. N-cadherin-mediated cell adhesion restricts cell proliferation in the dorsal neural tube. Mol. Biol. Cell. 2011;22:1505–1515. doi: 10.1091/mbc.E10-08-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aiga M., Levinson J.N., Bamji S.X. N-cadherin and neuroligins cooperate to regulate synapse formation in hippocampal cultures. J. Biol. Chem. 2011;286:851–858. doi: 10.1074/jbc.M110.176305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagata M., Duan X., Sanes J.R. Cadherins interact with synaptic organizers to promote synaptic differentiation. Front. Mol. Neurosci. 2018;11:142. doi: 10.3389/fnmol.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu C., Funahashi Y., Watanabe T., Takano T., Nakamuta S., Namba T., Kaibuchi K. Radial glial cell-neuron interaction directs axon formation at the opposite side of the neuron from the contact site. J. Neurosci. 2015;35:14517–14532. doi: 10.1523/JNEUROSCI.1266-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C., Li X., Guan L., Li S., Qiao L., Lin J. Effects of N-cadherin on neuronal migration during chicken optic tectum development. Histochem. Cell Biol. 2019;151:239–248. doi: 10.1007/s00418-018-1733-2. [DOI] [PubMed] [Google Scholar]
- 23.Moya P.R., Dodman N.H., Timpano K.R., Rubenstein L.M., Rana Z., Fried R.L., Reichardt L.F., Heiman G.A., Tischfield J.A., King R.A. Rare missense neuronal cadherin gene (CDH2) variants in specific obsessive-compulsive disorder and Tourette disorder phenotypes. Eur. J. Hum. Genet. 2013;21:850–854. doi: 10.1038/ejhg.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazaryan L., Bertelsen B., Padmanabhuni S.S., Debes N.M., LuCamp, Have C.T., Tümer Z. Association study between CDH2 and Gilles de la Tourette syndrome in a Danish cohort. Psychiatry Res. 2015;228:974–975. doi: 10.1016/j.psychres.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 25.McGregor N.W., Lochner C., Stein D.J., Hemmings S.M. Polymorphisms within the neuronal cadherin (CDH2) gene are associated with obsessive-compulsive disorder (OCD) in a South African cohort. Metab. Brain Dis. 2016;31:191–196. doi: 10.1007/s11011-015-9693-x. [DOI] [PubMed] [Google Scholar]
- 26.Mu L.M., Wang W.F., Zheng H., Guo Z.K., Zhang G.M. Expression of N-cadherin in myocardial tissues during the development of a rat heart. Genet. Mol. Res. 2015;14:9882–9889. doi: 10.4238/2015.August.19.22. [DOI] [PubMed] [Google Scholar]
- 27.Zuppinger C., Eppenberger-Eberhardt M., Eppenberger H.M. N-Cadherin: structure, function and importance in the formation of new intercalated disc-like cell contacts in cardiomyocytes. Heart Fail. Rev. 2000;5:251–257. doi: 10.1023/A:1009809520194. [DOI] [PubMed] [Google Scholar]
- 28.Turkowski K.L., Tester D.J., Bos J.M., Haugaa K.H., Ackerman M.J. Whole exome sequencing with genomic triangulation implicates CDH2-encoded N-cadherin as a novel pathogenic substrate for arrhythmogenic cardiomyopathy. Congenit. Heart Dis. 2017;12:226–235. doi: 10.1111/chd.12462. [DOI] [PubMed] [Google Scholar]
- 29.Mayosi B.M., Fish M., Shaboodien G., Mastantuono E., Kraus S., Wieland T., Kotta M.C., Chin A., Laing N., Ntusi N.B. Identification of Cadherin 2 (CDH2) Mutations in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ Cardiovasc Genet. 2017;10:10. doi: 10.1161/CIRCGENETICS.116.001605. [DOI] [PubMed] [Google Scholar]
- 30.Forbes S.A., Beare D., Bindal N., Bamford S., Ward S., Cole C.G., Jia M., Kok C., Boutselakis H., De T. COSMIC: High-resolution cancer genetics using the catalogue of somatic mutations in cancer. Curr. Protoc. Hum. Genet. 2016;91:10.11.1–10.11.37. doi: 10.1002/cphg.21. [DOI] [PubMed] [Google Scholar]
- 31.Uccella S., Accogli A., Tortora D., Mancardi M.M., Nobili L., Berloco B., Morana G., Striano P., Capra V., Srour M. Dissecting the neurological phenotype in children with callosal agenesis, interhemispheric cysts and malformations of cortical development. J. Neurol. 2019;266:1167–1181. doi: 10.1007/s00415-019-09247-7. [DOI] [PubMed] [Google Scholar]
- 32.Quinodoz M., Royer-Bertrand B., Cisarova K., Di Gioia S.A., Superti-Furga A., Rivolta C. DOMINO: Using machine learning to predict genes associated with dominant disorders. Am. J. Hum. Genet. 2017;101:623–629. doi: 10.1016/j.ajhg.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: A matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Spiczak S., Helbig K.L., Shinde D.N., Huether R., Pendziwiat M., Lourenço C., Nunes M.E., Sarco D.P., Kaplan R.A., Dlugos D.J., Epi4K Consortium. EuroEPINOMICS-RES NLES Working Group DNM1 encephalopathy: A new disease of vesicle fission. Neurology. 2017;89:385–394. doi: 10.1212/WNL.0000000000004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prasad A., Pedigo S. Calcium-dependent stability studies of domains 1 and 2 of epithelial cadherin. Biochemistry. 2005;44:13692–13701. doi: 10.1021/bi0510274. [DOI] [PubMed] [Google Scholar]
- 36.Klingelhöfer J., Laur O.Y., Troyanovsky R.B., Troyanovsky S.M. Dynamic interplay between adhesive and lateral E-cadherin dimers. Mol. Cell. Biol. 2002;22:7449–7458. doi: 10.1128/MCB.22.21.7449-7458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S.A., Tai C.Y., Mok L.P., Mosser E.A., Schuman E.M. Calcium-dependent dynamics of cadherin interactions at cell-cell junctions. Proc. Natl. Acad. Sci. USA. 2011;108:9857–9862. doi: 10.1073/pnas.1019003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiel L., Baakman C., Gilissen D., Veltman J.A., Vriend G., Gilissen C. MetaDome: Pathogenicity analysis of genetic variants through aggregation of homologous human protein domains. Hum. Mutat. 2019;40:1030–1038. doi: 10.1002/humu.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lelieveld S.H., Wiel L., Venselaar H., Pfundt R., Vriend G., Veltman J.A., Brunner H.G., Vissers L.E.L.M., Gilissen C. Spatial clustering of de novo missense mutations identifies candidate neurodevelopmental disorder-associated genes. Am. J. Hum. Genet. 2017;101:478–484. doi: 10.1016/j.ajhg.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison O.J., Bahna F., Katsamba P.S., Jin X., Brasch J., Vendome J., Ahlsen G., Carroll K.J., Price S.R., Honig B., Shapiro L. Two-step adhesive binding by classical cadherins. Nat. Struct. Mol. Biol. 2010;17:348–357. doi: 10.1038/nsmb.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shan W., Yagita Y., Wang Z., Koch A., Fex Svenningsen A., Gruzglin E., Pedraza L., Colman D.R. The minimal essential unit for cadherin-mediated intercellular adhesion comprises extracellular domains 1 and 2. J. Biol. Chem. 2004;279:55914–55923. doi: 10.1074/jbc.M407827200. [DOI] [PubMed] [Google Scholar]
- 42.Lykke-Andersen S., Jensen T.H. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nat. Rev. Mol. Cell Biol. 2015;16:665–677. doi: 10.1038/nrm4063. [DOI] [PubMed] [Google Scholar]
- 43.Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- 44.Yap A.S., Niessen C.M., Gumbiner B.M. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J. Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratheesh A., Yap A.S. A bigger picture: classical cadherins and the dynamic actin cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012;13:673–679. doi: 10.1038/nrm3431. [DOI] [PubMed] [Google Scholar]
- 46.Harrison O.J., Corps E.M., Berge T., Kilshaw P.J. The mechanism of cell adhesion by classical cadherins: the role of domain 1. J. Cell Sci. 2005;118:711–721. doi: 10.1242/jcs.01665. [DOI] [PubMed] [Google Scholar]
- 47.Vunnam N., Pedigo S. Sequential binding of calcium leads to dimerization in neural cadherin. Biochemistry. 2011;50:2973–2982. doi: 10.1021/bi101872b. [DOI] [PubMed] [Google Scholar]
- 48.Cincotta M., Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clin. Neurophysiol. 2008;119:744–762. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 49.Peng J., Charron F. Lateralization of motor control in the human nervous system: genetics of mirror movements. Curr. Opin. Neurobiol. 2013;23:109–118. doi: 10.1016/j.conb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Nugent A.A., Kolpak A.L., Engle E.C. Human disorders of axon guidance. Curr. Opin. Neurobiol. 2012;22:837–843. doi: 10.1016/j.conb.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee C.H., Gumbiner B.M. Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Dev. Biol. 1995;171:363–373. doi: 10.1006/dbio.1995.1288. [DOI] [PubMed] [Google Scholar]
- 52.Veitia R.A., Caburet S., Birchler J.A. Mechanisms of Mendelian dominance. Clin. Genet. 2018;93:419–428. doi: 10.1111/cge.13107. [DOI] [PubMed] [Google Scholar]
- 53.Kawauchi T., Sekine K., Shikanai M., Chihama K., Tomita K., Kubo K., Nakajima K., Nabeshima Y., Hoshino M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Sakane F., Miyamoto Y. N-cadherin regulates the proliferation and differentiation of ventral midbrain dopaminergic progenitors. Dev. Neurobiol. 2013;73:518–529. doi: 10.1002/dneu.22077. [DOI] [PubMed] [Google Scholar]
- 55.Shikanai M., Nakajima K., Kawauchi T. N-cadherin regulates radial glial fiber-dependent migration of cortical locomoting neurons. Commun. Integr. Biol. 2011;4:326–330. doi: 10.4161/cib.4.3.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radice G.L., Rayburn H., Matsunami H., Knudsen K.A., Takeichi M., Hynes R.O. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 57.Kadowaki M., Nakamura S., Machon O., Krauss S., Radice G.L., Takeichi M. N-cadherin mediates cortical organization in the mouse brain. Dev. Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 58.Rebman J.K., Kirchoff K.E., Walsh G.S. Cadherin-2 is required cell autonomously for collective migration of facial branchiomotor neurons. PLoS ONE. 2016;11:e0164433. doi: 10.1371/journal.pone.0164433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srour M., Rivière J.B., Pham J.M., Dubé M.P., Girard S., Morin S., Dion P.A., Asselin G., Rochefort D., Hince P. Mutations in DCC cause congenital mirror movements. Science. 2010;328:592. doi: 10.1126/science.1186463. [DOI] [PubMed] [Google Scholar]
- 60.Méneret A., Franz E.A., Trouillard O., Oliver T.C., Zagar Y., Robertson S.P., Welniarz Q., Gardner R.J.M., Gallea C., Srour M. Mutations in the netrin-1 gene cause congenital mirror movements. J. Clin. Invest. 2017;127:3923–3936. doi: 10.1172/JCI95442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed I., Mittal K., Sheikh T.I., Vasli N., Rafiq M.A., Mikhailov A., Ohadi M., Mahmood H., Rouleau G.A., Bhatti A. Identification of a homozygous splice site mutation in the dynein axonemal light chain 4 gene on 22q13.1 in a large consanguineous family from Pakistan with congenital mirror movement disorder. Hum. Genet. 2014;133:1419–1429. doi: 10.1007/s00439-014-1475-8. [DOI] [PubMed] [Google Scholar]
- 62.Depienne C., Bouteiller D., Méneret A., Billot S., Groppa S., Klebe S., Charbonnier-Beaupel F., Corvol J.C., Saraiva J.P., Brueggemann N. RAD51 haploinsufficiency causes congenital mirror movements in humans. Am. J. Hum. Genet. 2012;90:301–307. doi: 10.1016/j.ajhg.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marsh A.P., Heron D., Edwards T.J., Quartier A., Galea C., Nava C., Rastetter A., Moutard M.L., Anderson V., Bitoun P. Mutations in DCC cause isolated agenesis of the corpus callosum with incomplete penetrance. Nat. Genet. 2017;49:511–514. doi: 10.1038/ng.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franz M., Rodriguez H., Lopes C., Zuberi K., Montojo J., Bader G.D., Morris Q. GeneMANIA update 2018. Nucleic Acids Res. 2018;46(W1):W60–W64. doi: 10.1093/nar/gky311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Mering C., Huynen M., Jaeggi D., Schmidt S., Bork P., Snel B. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 2003;31:258–261. doi: 10.1093/nar/gkg034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han H., Shim H., Shin D., Shim J.E., Ko Y., Shin J., Kim H., Cho A., Kim E., Lee T. TRRUST: a reference database of human transcriptional regulatory interactions. Sci. Rep. 2015;5:11432. doi: 10.1038/srep11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reyes-Múgica M., Meyerhardt J.A., Rzasa J., Rimm D.L., Johnson K.R., Wheelock M.J., Reale M.A. Truncated DCC reduces N-cadherin/catenin expression and calcium-dependent cell adhesion in neuroblastoma cells. Lab. Invest. 2001;81:201–210. doi: 10.1038/labinvest.3780228. [DOI] [PubMed] [Google Scholar]
- 69.Seong E., Yuan L., Arikkath J. Cadherins and catenins in dendrite and synapse morphogenesis. Cell Adhes. Migr. 2015;9:202–213. doi: 10.4161/19336918.2014.994919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mendez P., De Roo M., Poglia L., Klauser P., Muller D. N-cadherin mediates plasticity-induced long-term spine stabilization. J. Cell Biol. 2010;189:589–600. doi: 10.1083/jcb.201003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bozdagi O., Wang X.B., Nikitczuk J.S., Anderson T.R., Bloss E.B., Radice G.L., Zhou Q., Benson D.L., Huntley G.W. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J. Neurosci. 2010;30:9984–9989. doi: 10.1523/JNEUROSCI.1223-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keller R., Basta R., Salerno L., Elia M. Autism, epilepsy, and synaptopathies: a not rare association. Neurol. Sci. 2017;38:1353–1361. doi: 10.1007/s10072-017-2974-x. [DOI] [PubMed] [Google Scholar]
- 73.Guang S., Pang N., Deng X., Yang L., He F., Wu L., Chen C., Yin F., Peng J. Synaptopathology Involved in Autism Spectrum Disorder. Front. Cell. Neurosci. 2018;12:470. doi: 10.3389/fncel.2018.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jüngling K., Eulenburg V., Moore R., Kemler R., Lessmann V., Gottmann K. N-cadherin transsynaptically regulates short-term plasticity at glutamatergic synapses in embryonic stem cell-derived neurons. J. Neurosci. 2006;26:6968–6978. doi: 10.1523/JNEUROSCI.1013-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stan A., Pielarski K.N., Brigadski T., Wittenmayer N., Fedorchenko O., Gohla A., Lessmann V., Dresbach T., Gottmann K. Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc. Natl. Acad. Sci. USA. 2010;107:11116–11121. doi: 10.1073/pnas.0914233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bozdagi O., Shan W., Tanaka H., Benson D.L., Huntley G.W. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 77.Nikitczuk J.S., Patil S.B., Matikainen-Ankney B.A., Scarpa J., Shapiro M.L., Benson D.L., Huntley G.W. N-cadherin regulates molecular organization of excitatory and inhibitory synaptic circuits in adult hippocampus in vivo. Hippocampus. 2014;24:943–962. doi: 10.1002/hipo.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitman M.C., Engle E.C. Ocular congenital cranial dysinnervation disorders (CCDDs): insights into axon growth and guidance. Hum. Mol. Genet. 2017;26(R1):R37–R44. doi: 10.1093/hmg/ddx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leonard M., Zhang L., Zhai N., Cader A., Chan Y., Nowak R.B., Fowler V.M., Menko A.S. Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens. Dev. Biol. 2011;349:363–377. doi: 10.1016/j.ydbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Logan C.M., Rajakaruna S., Bowen C., Radice G.L., Robinson M.L., Menko A.S. N-cadherin regulates signaling mechanisms required for lens fiber cell elongation and lens morphogenesis. Dev. Biol. 2017;428:118–134. doi: 10.1016/j.ydbio.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johansen J., Halpern M.E., Keshishian H. Axonal guidance and the development of muscle fiber-specific innervation in Drosophila embryos. J. Neurosci. 1989;9:4318–4332. doi: 10.1523/JNEUROSCI.09-12-04318.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akins M.R., Greer C.A. Axon behavior in the olfactory nerve reflects the involvement of catenin-cadherin mediated adhesion. J. Comp. Neurol. 2006;499:979–989. doi: 10.1002/cne.21147. [DOI] [PubMed] [Google Scholar]
- 83.Schwarting G.A., Henion T.R. Regulation and function of axon guidance and adhesion molecules during olfactory map formation. J. Cell. Biochem. 2011;112:2663–2671. doi: 10.1002/jcb.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okumura M., Kato T., Miura M., Chihara T. Hierarchical axon targeting of Drosophila olfactory receptor neurons specified by the proneural transcription factors Atonal and Amos. Genes Cells. 2016;21:53–64. doi: 10.1111/gtc.12321. [DOI] [PubMed] [Google Scholar]
- 85.Watkins H., Ashrafian H., Redwood C. Inherited cardiomyopathies. N. Engl. J. Med. 2011;364:1643–1656. doi: 10.1056/NEJMra0902923. [DOI] [PubMed] [Google Scholar]
- 86.Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A., Calkins H., Corrado D., Cox M.G., Daubert J.P. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur. Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delmar M., McKenna W.J. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ. Res. 2010;107:700–714. doi: 10.1161/CIRCRESAHA.110.223412. [DOI] [PubMed] [Google Scholar]
- 88.Kostetskii I., Li J., Xiong Y., Zhou R., Ferrari V.A., Patel V.V., Molkentin J.D., Radice G.L. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ. Res. 2005;96:346–354. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- 89.García-Castro M.I., Vielmetter E., Bronner-Fraser M. N-Cadherin, a cell adhesion molecule involved in establishment of embryonic left-right asymmetry. Science. 2000;288:1047–1051. doi: 10.1126/science.288.5468.1047. [DOI] [PubMed] [Google Scholar]
- 90.Protonotarios A., Elliott P.M. Arrhythmogenic cardiomyopathies (ACs): diagnosis, risk stratification and management. Heart. 2019;105:1117–1128. doi: 10.1136/heartjnl-2017-311160. [DOI] [PubMed] [Google Scholar]
- 91.Andersson A.M., Edvardsen K., Skakkebaek N.E. Expression and localization of N- and E-cadherin in the human testis and epididymis. Int. J. Androl. 1994;17:174–180. doi: 10.1111/j.1365-2605.1994.tb01239.x. [DOI] [PubMed] [Google Scholar]
- 92.Newton S.C., Blaschuk O.W., Millette C.F. N-cadherin mediates Sertoli cell-spermatogenic cell adhesion. Dev. Dyn. 1993;197:1–13. doi: 10.1002/aja.1001970102. [DOI] [PubMed] [Google Scholar]
- 93.Jiang X., Ma T., Zhang Y., Zhang H., Yin S., Zheng W., Wang L., Wang Z., Khan M., Sheikh S.W. Specific deletion of Cdh2 in Sertoli cells leads to altered meiotic progression and subfertility of mice. Biol. Reprod. 2015;92:79. doi: 10.1095/biolreprod.114.126334. [DOI] [PubMed] [Google Scholar]
- 94.Babb-Clendenon S., Shen Y.C., Liu Q., Turner K.E., Mills M.S., Cook G.W., Miller C.A., Gattone V.H., 2nd, Barald K.F., Marrs J.A. Cadherin-2 participates in the morphogenesis of the zebrafish inner ear. J. Cell Sci. 2006;119:5169–5177. doi: 10.1242/jcs.03299. [DOI] [PubMed] [Google Scholar]
- 95.Ciołczyk-Wierzbicka D., Laidler P. The inhibition of invasion of human melanoma cells through N-cadherin knock-down. Med. Oncol. 2018;35:42. doi: 10.1007/s12032-018-1104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mrozik K.M., Blaschuk O.W., Cheong C.M., Zannettino A.C.W., Vandyke K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer. 2018;18:939. doi: 10.1186/s12885-018-4845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asano K., Duntsch C.D., Zhou Q., Weimar J.D., Bordelon D., Robertson J.H., Pourmotabbed T. Correlation of N-cadherin expression in high grade gliomas with tissue invasion. J. Neurooncol. 2004;70:3–15. doi: 10.1023/b:neon.0000040811.14908.f2. [DOI] [PubMed] [Google Scholar]
- 98.Yi S., Yang Z.L., Miao X., Zou Q., Li J., Liang L., Zeng G., Chen S. N-cadherin and P-cadherin are biomarkers for invasion, metastasis, and poor prognosis of gallbladder carcinomas. Pathol. Res. Pract. 2014;210:363–368. doi: 10.1016/j.prp.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 99.Su Y., Li J., Witkiewicz A.K., Brennan D., Neill T., Talarico J., Radice G.L. N-cadherin haploinsufficiency increases survival in a mouse model of pancreatic cancer. Oncogene. 2012;31:4484–4489. doi: 10.1038/onc.2011.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leombroni M., Khalil A., Liberati M., D’Antonio F. Fetal midline anomalies: Diagnosis and counselling Part 1: Corpus callosum anomalies. Eur. J. Paediatr. Neurol. 2018;22:951–962. doi: 10.1016/j.ejpn.2018.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.