Abstract
Purpose
The aim of this study was to report the treatment planning feasibility of dose escalation to suspicious lymph nodes (LNs) for a series of men who underwent pretreatment [18F]fluciclovine positron emission tomography (PET)/magnetic resonance imaging (MRI).
Methods and Materials
Cases of men with prostate cancer who enrolled in a clinical trial of pretreatment [18F]fluciclovine PET who had suspicious LNs were selected. Pelvic LNs <1 cm were defined as positive based on [18F]fluciclovine-PET if their maximum standardized uptake value (SUVmax) was ≥1.3-fold greater than the reference blood pool SUVmean, and LNs ≥1 cm were defined as positive if the SUV was greater than the reference SUV bone marrow reference. For each case, a radiation treatment plan was generated to deliver 70 Gy to the prostate and proximal seminal vesicles, 60.2 Gy to the PET-positive LNs, and 50.4 Gy to the elective nodal regions, simultaneously in 28 fractions of 2.5 Gy, 2.15 Gy, and 1.8 Gy, respectively. Treatment planning goals were defined a priori. The resulting target volume and organ-at-risk dosimetry were compared with the original treatment plan.
Results
Four cases were identified, with between 1 and 5 [18F]fluciclovine PET–positive LNs each. Goals for the prostate and elective nodal target volumes were successfully met in all cases. The goal of covering more than 90% of the positive LN planning target volume by the prescription dose of 60.2 Gy was met in 3 of the 4 cases. This goal was not met in 1 case, but 100% of clinical target volume was covered by 60.2 Gy. The primary organ-at-risk tradeoff was that a small volume (0.5-8.2 cm3) of small bowel would receive ≥54 Gy in each case.
Conclusions
These preliminary results suggest that [18F]fluciclovine PET/MRI directed dose escalation of suspicious pelvic LNs is likely feasible in the setting of definitive radiation therapy. The potential clinical benefit of dose escalating [18F]fluciclovine PET–positive LNs should be investigated in a prospective clinical trial.
Introduction
Disease control outcomes for men with high-risk prostate cancer remain suboptimal, with 10-year biochemical failure rates of 25% to 40% in modern trials of dose escalated radiation therapy and androgen suppression.1, 2 Because the risk of pelvic lymph node (LN) involvement is recognized for men with high-risk prostate cancer, one strategy to improve outcomes has been the use of elective pelvic irradiation, but this approach is controversial because randomized trials have failed to confirm a benefit.3, 4 Historical patterns of failures studies have not identified pelvic LNs as a typical site of failure after radiation therapy5, 6; however, this has recently been challenged. A major limitation of prior studies is that imaging has largely consisted of computed tomography (CT) and magnetic resonance imaging (MRI), which are able to localize the site of recurrence in less than half of men who have a biochemical failure.7 Recent studies incorporating molecular imaging, including [11C]acetate, [18F]fluciclovine, [11C]choline, and 68Ga- and 18F-labeled prostate specific membrane antigen (PSMA) positron emission tomography (PET), have significantly higher detection rates compared with conventional imaging. When prostate cancer focused PET agents are used in the setting of biochemical failure after radiation therapy, more than 25% of men have evidence of recurrent tumor within the pelvis.8, 9, 10
One explanation offered for the lack of proven benefit from elective pelvic LN irradiation is that consensus guidelines for nodal target volumes may exclude areas at risk for failure.6, 11 Recently, incorporation of molecular imaging has been suggested as a possible way to improve planning for men undergoing primary radiation therapy for high-risk prostate cancer. In one series, when pretreatment [68 Ga]PSMA-11-PET was performed and registered with the treatment planning CT, the LN clinical target volume (CTV) was modified to extend beyond consensus guidelines in more than one third of cases.12 Another plausible explanation for negative trials of elective pelvic LN irradiation is that the doses commonly used for elective pelvic LN irradiation, on the order of 45 to 50 Gy, may not be adequate for larger tumor deposits, which still do not meet size criteria for detection with conventional imaging. Information obtained from molecular imaging may therefore also be leveraged to improve nodal irradiation by identifying LN targets that would benefit from focal dose escalation but are missed with conventional imaging.
The PET tracer [18F]fluciclovine is a nonnatural amino acid that targets increased amino acid transport in prostate cancer.13 This PET tracer was approved by the US Food and Drug Administration in 2016 for detection and localization of prostate cancer in men with biochemical recurrence after definitive therapy. Clinical trials have indicated that [18F]fluciclovine has high specificity and good detection rates for extraprostatic disease in the setting of biochemical recurrence,14, 15 and recent small studies suggest [18F]fluciclovine PET has utility in intermediate- and high-risk prostate cancer before therapy.16, 17, 18 However, the role of [18F]fluciclovine PET in primary prostate cancer remains to be defined, and there are very few data regarding the role of this agent in radiation therapy planning.
The purpose of this report was to assess the treatment planning feasibility and organ-at-risk (OAR) dosimetry of LN dose escalation for a case series of men with high-risk prostate cancer who underwent pretreatment [18F]fluciclovine PET and were noted to have suspicious LNs. We hypothesized that focal dose escalation would be achievable in these cases while respecting prespecified OAR constraints.
Methods and Materials
Case selection
We reviewed the records of 12 men enrolled in an ongoing clinical trial (NCT03264456) of [18F]fluciclovine PET/MRI for the upfront staging of men with histologically confirmed prostate adenocarcinoma and either Gleason score of 8 to 10 or prostate-specific antigen (PSA) > 20 ng/mL. Men were required to have conventional staging studies ([99mTc]MDP bone scintigram and tomographic pelvic imaging) that were without evidence of metastatic disease. The 4 available cases of men with evidence of [18F]fluciclovine-avid LNs who proceeded to definitive radiation therapy were selected for this treatment planning study (Table 1).
Table 1.
Patient characteristics
| Age (y) | PSA (ng/mL) | Gleason score | MRI findings | PET findings | |
|---|---|---|---|---|---|
| Case 1 | 59 | 76.5 | 4 + 5 = 9 |
Prostate: Seminal vesicle invasion identified with concern of bladder neck involvement. Pelvis: No suspicious LNs identified. |
|
| Case 2 | 57 | 57.68 | 3 + 4 = 7 |
Prostate: Bilateral T2 hypointense lesions. No evidence of extraprostatic extension. Pelvis: No suspicious LNs identified. |
|
| Case 3 | 63 | 11.1 | 4 + 5 = 9 |
Prostate: Left posterior peripheral zone lesion without capsular involvement. Pelvis: Enlarged LN (1 cm short axis) along the left external iliac vasculature. |
|
| Case 4 | 59 | 26.38 | 4 + 4 = 8 |
Prostate: Bilateral T2 hypointense lesions with broad capsular abutment suspicious for extraprostatic extension. No seminal vesicle invasion. Pelvis: No suspicious LNs identified. |
|
Abbreviations: CT = computed tomography; LN = lymph node; MRI = magnetic resonance imaging; PET = positron emission tomography.
PET-MRI acquisition
Whole-body and dedicated regional PET/MRI images of the prostate were acquired on a GE SIGNA 3.0 Tesla PET/MRI scanner. Whole-body magnetic resonance sequences included axial T1 fat-saturated, sagittal T1 non–fat saturated, and T2 weighted images. PET/MRI acquisition was simultaneous and included a total of 6 bed positions with 5-minute PET acquisition per bed position. The dedicated prostate PET/MRI protocol was performed per institutional protocol and included the essential components of multiparametric prostate MRI: diffusion-weighted images (up to b2000), small field-of-view T2-weighted images (axial, sagittal, and coronal), and dynamic contrast-enhanced images of the prostate gland. A delayed regional PET acquisition was performed during acquisition of the prostate MRI. The PET/MRI images were analyzed using MIM (Cleveland, OH), and the PET/MRI scans were extracted to DICOM files for integration into the Varian Eclipse software.
Assessment of [18F]fluciclovine PET for LN involvement
The optimal [18F]fluciclovine standardized uptake value (SUV) threshold for the detection of LNs harboring metastatic prostate cancer has not been fully established. Commonly used [18F]fluciclovine PET interpretation criteria suggest that LNs with uptake greater than blood pool are suspicious19; however, these interpretation criteria are derived from experience in men with biochemically recurrent prostate cancer and may not apply to a population of men with de novo prostate cancer. In this study we used both size and SUV criteria to characterize LNs. Small LNs (<1 cm) were classified as involved if the SUVmax was >1.3 times the blood pool reference, and LNs ≥1 cm were classified as involved if the SUVmax was more than the L3 vertebral body bone marrow reference. The lower SUV threshold for small LNs was used to improve sensitivity for smaller LN deposits, which are expected to have lower SUV measurements because of partial volume averaging related to the spatial resolution of PET.20
Radiation therapy simulation
CT simulation for radiation therapy planning was performed within 8 weeks of the initiation of neoadjuvant androgen suppression and within 10 weeks of the [18F]fluciclovine staging scan. Patients were asked to have an empty rectum and full bladder at time of simulation. A custom molded foam form was created to aid patient immobilization. A retrograde urethrogram was performed to improve visualization of the prostate apex.21 The CT scan extended from the L1/L2 to midfemur and had 2 to 3 mm slice thickness.
Radiation therapy target delineation
For all patients we initially defined traditional CTVs for the treatment of high-risk prostate cancer: CTVP+SV was defined as the entire prostate gland and portion of the seminal vesicles at risk for tumor involvement. At least the proximal 1 cm of the seminal vesicles was included. CTVeLN was defined as the elective LN target volumes, delineated in accordance with published consensus guidelines.22 Nodal regions included the obturator, internal iliac, external iliac, and distal common iliac chains.
Next the [18F]fluciclovine-PET scan was rigidly coregistered to the radiation simulation CT scan. Rigid coregistration was preferred for 2 specific reasons: (1) We recognize that 8 to 10 weeks of androgen suppression between [18F]fluciclovine and radiation therapy simulation may lead to changes in LN size that may lead to error in deformable registration, and (2) prior experience with [18F]fluciclovine coregistration in the biochemical recurrence setting indicates that rigid coregistration results in good anatomic agreement in most cases.23 Using the registered [18F]fluciclovine-PET/MRI, we delineated an additional CTV. The LNs were segmented on the T2 MRI sequence of the [18F]fluciclovine PET/MRI and superimposed on the coregistered CT simulation scan. The resulting structure was edited to create CTVLN BOOST by excluding bowel structures and barriers to tumor spread (eg, muscle or bone). We recognize that suspicious LNs are likely to be smaller on the CT simulation scan because of the initiation of androgen suppression, but CTVLN BOOST was not further reduced to account for residual microscopic tumor extension. This concept is analogous to involved node radiation therapy used for postchemotherapy consolidation of lymphoma.24 Planning target volume (PTV) expansions for CTVP+SV were 4 mm posteriorly and 7 mm in all other directions. PTV expansions for CTVeLN and CTVLN BOOST were 8 mm in all directions.
Radiation therapy planning and dosimetric goals
We used a moderately hypofractionated dose regimen, which is standard at our institution. Our institutional technique for elective pelvic irradiation with a simultaneous integrated boost to the prostate and proximal seminal vesicles has been described in multiple peer-reviewed publications,25, 26, 27, 28 and moderate hypofractionation is recognized by the National Comprehensive Cancer Network as an appropriate dose regimen for men with high-risk and node-positive prostate cancer.29 We prescribed 70 Gy to PTVP+SV, 60.2 Gy to PTVLN BOOST, and 50.4 Gy to PTVeLN simultaneously in 28 fractions of 2.5 Gy, 2.15 Gy, and 1.8 Gy, respectively. Specific dosimetric goals for PTV coverage and OAR constraints are given in Table 2. Radiation delivery techniques (eg, intensity modulated radiation therapy [IMRT] or volumetric modulated arc therapy) were identical to what was used for the original treatment plan.
Table 2.
Treatment planning goals for [18F]fluciclovine PET-MRI directed nodal dose escalation
| Structure | Dosimetric parameter | Per protocol | Variation acceptable |
|---|---|---|---|
| PTVP+SV | V70 Gy[%] | ≥95% | ≥90% |
| Maximum dose | <75 Gy | <77 Gy | |
| PTVLN BOOST | V60.2 Gy[%] | ≥90% | -∗ |
| Maximum dose | <66 Gy | <68 Gy | |
| PTVeLN | V50.4 Gy[%] | ≥95% | ≥90% |
| Rectum | V70 Gy[cc] | <3 cm3 | <5 cm3 |
| V60 Gy[%] | <10% | <15% | |
| V50 Gy[%] | <25% | <40% | |
| Small bowel | Maximum dose | <54 Gy | <58 Gy |
| V54 Gy[cc] | 0 cm3 | <20 cm3 | |
| V45 Gy[cc] | <120 cm3 | Not specified | |
| Bladder | V60 Gy[%] | <20% | <25% |
| V40 Gy[%] | <50% | <65% | |
| Femoral heads | V50 Gy[%] | <5% | <10% |
Abbreviations: CTV = clinical target volume; LN = lymph node; MRI = magnetic resonance imaging; P + SV = prostate and seminal vesicles; PET = positron emission tomography; PTV = planning target volume.
No minimum acceptable coverage of PTVLN BOOST is specified due to potential overlap between the target volume and bowel structures is may occur. In such instances effort should be made to ensure CTVLNBOOST V60.2 Gy[%] ≥90%.
Results
Case 1
Case 1 was a 59-year-old man with very high-risk prostate cancer (PSA, 76.5 ng/mL; Gleason 4 + 5 = 9; MRI suggestive of bladder neck invasion). Conventional staging with pelvic multiparameteric MRI and technetium 99m–methylene diphosphonate ([99mTc]MDP) bone scintigraphy did not show evidence of nodal or distant metastases. He underwent CT simulation after 6 weeks of neoadjuvant androgen suppression and received a dose of 70 Gy to the prostate and proximal seminal vesicles and 50.4 Gy to the elective pelvic LN regions, simultaneously in 28 fractions using a Varian TrueBeam STX linear accelerator, and a 9-field sliding window IMRT with 15 MV photons. Initial treatment planning was complicated by small bowel in close proximity to the seminal vesicles.
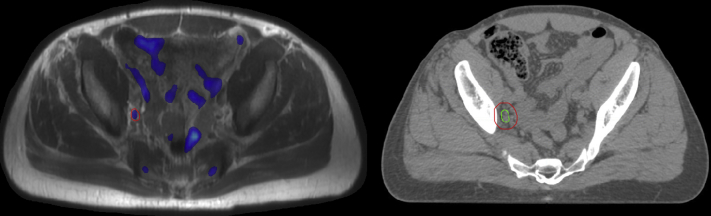
[18F]Fluciclovine PET/MRI (Fig 1) identified a right pelvic LN at the bifurcation of the iliac vessels, which was targeted in the boost replan. The resulting PET/MRI directed LN boost plan was feasible in this case, with the primary planning tradeoff being less favorable bladder dosimetry at the moderate dose levels (eg, bladder V40 Gy[%]). The PTVLN BOOST V60.2 Gy[%] = 79.4% was undercovered to achieve acceptable small bowel dosimetry; however, the CTVLN BOOST was entirely covered at the 60.2 Gy dose level. Dosimetric results of all replans, with comparison to the original treatment plans, are presented in Table 3.
Figure 1.
Imaging for case 1. PET-MRI (left) with suspicious lymph node delineated in red. CT simulation (right) scan with resulting clinical target volume in green and planning target volume in blue. Abbreviations: CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography.
Table 3.
Dosimetry comparison of the resulting [18F]fluciclovine PET-MRI directed nodal boost plans compared with the original treatment plan
| Structure | Dosimetric parameter | Case 1 |
Case 2 |
Case 3 |
Case 4 |
||||
|---|---|---|---|---|---|---|---|---|---|
| Original plan | PET-MRI–directed LN boost plan | Original plan | PET-MRI–directed LN boost plan | Original plan | PET-MRI–directed LN boost plan | Original plan | PET-MRI–directed LN boost plan | ||
| PTVP+SV | V70 Gy[%] | 90.9% | 92% | 94.6% | 95% | 95% | 95% | 95% | 98.2% |
| Maximum dose | 76.4 Gy | 76.9 Gy | 76.2 Gy | 75.9 Gy | 74.2 Gy | 75.6 Gy | 75.9 Gy | 76.5 Gy | |
| PTVLN BOOST | V60.2 Gy[%] | - | 79.4% | - | 92.6% | 95.7% | 93.3% | - | 90.1% |
| Maximum dose | - | 67.4 Gy | - | 67.0 Gy | 63.1 Gy | 65.7 Gy | - | 65.8 Gy | |
| PTVeLN | V50.4 Gy[%] | 96.8% | 92% | 93.5% | 95.6% | 94.1% | 97.2% | 92.2% | 93.2% |
| Rectum | V70 Gy[cc] | 2.8 cm3 | 3.7 cm3 | 4.8 cm3 | 2.1 cm3 | 2.8 cm3 | 1.6 cm3 | 2.6 cm3 | 3.8 cm3 |
| V60 Gy[%] | 12.7% | 13.6% | 6% | 8.9% | 7.0% | 8.5% | 12% | 13.2% | |
| V50 Gy[%] | 22.4% | 24.8% | 23.2% | 30.2% | 14.3% | 18.6% | 23.2% | 27% | |
| Small bowel | Maximum dose | 55.6 Gy | 54.9 Gy | 53.8 Gy | 56.1 Gy | 57.6 Gy | 57.0 Gy | 53.6 Gy | 57.9 Gy |
| V54 Gy[cc] | 1.3 cm3 | 0.5 cm3 | 0 cm3 | 8.2 cm3 | 0.5 cm3 | 0.5 cm3 | 0 cm3 | 0.7 cm3 | |
| V45 Gy[cc] | 329 cm3 | 344 cm3 | 97.5 cm3 | 147.9 cm3 | 275.3 cm3 | 389.8 cm3 | 199.7 cm3 | 226.5 cm3 | |
| Bladder | V60 Gy[%] | 12.3% | 13.9% | 13.5% | 17.7% | 9.5% | 9.7% | 7% | 10.2% |
| V40 Gy[%] | 45.1% | 61.3% | 44.9% | 58.7% | 46.5% | 39.2% | 43.1% | 53.8% | |
| Femoral heads | V50 Gy[%] | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
Abbreviations: CTV = clinical target volume; LN = lymph node; MRI = magnetic resonance imaging; P + SV = prostate and seminal vesicles; PET = positron emission tomography; PTV = planning target volume.
Case 2
Case 2 was a 57-year-old man with high-risk prostate cancer (PSA, 57.68 ng/mL; Gleason 3 + 4 = 7; MRI showing bilateral T2 lesions but no extracapsular extension). Conventional staging with pelvic multiparameteric MRI and [99mTc]MDP bone scintigraphy did not show evidence of nodal or distant metastases. He underwent CT simulation after 6 weeks of neoadjuvant androgen suppression and received a dose of 70 Gy to the prostate and proximal seminal vesicles and 50.4 Gy to the elective pelvic LN regions, simultaneously in 28 fractions using a Varian Clinac IX linear accelerator, and a 9-field sliding window IMRT with 15 MV photons. Initial treatment planning was complicated by small bowel within an inguinal hernia and hardware in the superior right femur, where entrance dose was avoided.
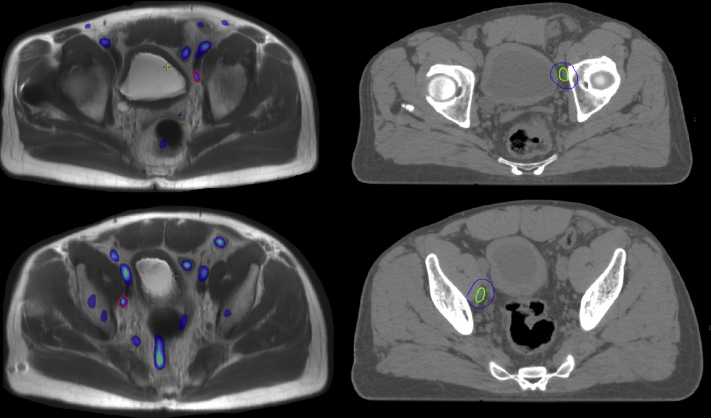
[18F]Fluciclovine PET/MRI (Fig 2) identified 2 pelvic LNs, 1 right and 1 left, both just posterior to the external iliac vessels near the superior aspect of the obturator fossa. The resulting PET-MRI directed LN boost plan was also feasible in this case, with the planning tradeoffs being increased moderate dose spill to the bladder and less favorable small bowel dosimetry (both small bowel maximum dose and V45 Gy).
Figure 2.
Imaging for case 2. PET-MRI (left) with suspicious lymph nodes delineated in red. CT simulation (right) scan with resulting clinical target volume in green and planning target volume in blue. Abbreviations: CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography.
Case 3
Case 3 was a 63-year-old man with high-risk prostate cancer (PSA, 11.1 ng/mL; Gleason 4 + 5 = 9; left posterior peripheral zone lesion without capsular involvement). [99mTc]MDP bone scintigraphy was initially concerning for uptake in the lumbar spine, but dedicated spine MRI revealed discogneic arthritic changes only. Multiparametric MRI was notable for an enlarged LN (1 cm short axis) along the left external iliac vasculature. The patient underwent CT simulation after 6 weeks of neoadjuvant androgen suppression and received a dose of 70 Gy to the prostate and proximal seminal vesicles, 60.2 to the enlarged left pelvic LN, and 50.4 Gy to the elective pelvic LN regions, simultaneously in 28 fractions on a Varian Clinac IX linear accelerator, and a 9-field sliding window IMRT with 6 MV photons. The PTVLN BOOST in the treated plan was located favorably in the left internal iliac region, which was well covered by the 60.2 Gy prescription dose.
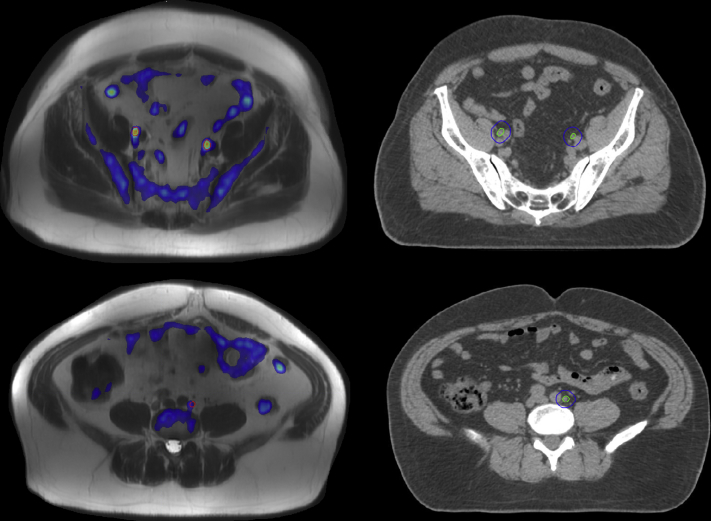
[18F]Fluciclovine PET/MRI (Fig 3) confirmed [18F]fluciclovine uptake in the enlarged LN and identified 4 additional LNs in the bilateral pelvis. The resulting PET/MRI directed LN boost plan was able to meet target coverage goals with the primary tradeoffs of increased small bowel Dmax (57.9 Gy) and bladder V40 Gy[%].
Figure 3.
Imaging for case 3. PET-MRI (left) with suspicious lymph nodes delineated in red. CT simulation (right) scan with resulting clinical target volume in green and planning target volume in blue. Abbreviations: CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography.
Case 4
Case 4 was a 59-year-old man with high-risk prostate cancer (PSA, 26.38 ng/mL; Gleason 4 + 4 = 8; MRI suggestive of bilateral tumor nodules with capsular abutment). He underwent CT simulation after 6 weeks of neoadjuvant androgen suppression and received a dose of 70 Gy to the prostate and proximal seminal vesicles and 50.4 Gy to the elective pelvic LN regions, simultaneously in 28 fractions using a Varian Clinac IX linear accelerator, and a 9-field sliding window IMRT with 6 MV photons.
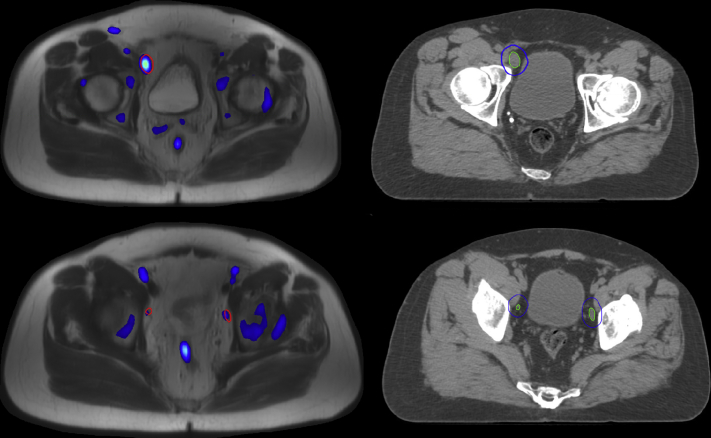
[18F]Fluciclovine PET/MRI (Fig 4) identified 4 pelvic LNs, one of which was located outside of the consensus guideline nodal target volume. The resulting PET/MRI directed LN boost plan met the OAR feasibility criteria. The resulting PET-MRI directed LN boost plan was able to meet target coverage goals, with the primary tradeoff being increased small bowel V45 Gy.
Figure 4.
Imaging for case 4. PET-MRI (left) with suspicious lymph nodes delineated in red. CT simulation (right) scan with resulting clinical target volume in green and planning target volume in blue. Abbreviations: CT = computed tomography; MRI = magnetic resonance imaging; PET = positron emission tomography.
Discussion
The recent introduction of new PET radiotracers has significantly improved the yield of imaging for men with prostate cancer. In the setting of rising PSA after local therapy, PET tracers including [11C]acetate, [18F]fluciclovine, [11C]choline, and PSMA ligands have been found to localize tumor significantly more often than the prior standard of bone scintigraphy and pelvic MRI. Given the success in the setting of biochemical recurrence, a natural next step is to investigate the utility of upfront PET for men with newly diagnosed prostate cancer. The goal of ongoing clinical trials aiming to characterize the role of PET for initial staging is to provide earlier identification of men with metastatic disease who will benefit from early initiation of aggressive systemic therapies.30 Preliminary results of these clinical trials also suggest that detection rates of suspicious pelvic LN metastases among men with high-risk features are significantly higher than those of conventional pelvic CT or MRI.31 At least 1 series with surgical-pathologic correlation has confirmed a high specificity of LNs with [18F]fluciclovine uptake.18
Given the compelling early results of trials of upfront PET for high-risk prostate cancer, we sought to assess the treatment planning feasibility of focal dose escalation to [18F]fluciclovine PET–positive LNs. We used the clinical information of the 4 men enrolled in our institutional trial of [18F]fluciclovine PET for initial staging who have thus far been identified as having pelvic lymphadenopathy. In each case the LN boost CTV was delineated by coregistering the [18F]fluciclovine PET-MRI with the CT simulation scan (emphasis of rigid registration was the bony pelvis). The suspicious LNs were identified using the [18F]fluciclovine PET. Our preference was to perform segmentation of the associated anatomic MRI scan, as opposed to the PET thresholding technique reported by Emory investigators,23 because this is more reflective of our routine clinical practice in other disease sites where PET data sets are commonly used. We did not reduce the size of the CTV if LNs were noted to have decreased in size after the initiation of androgen suppression. Overall, we found that delineation of the additional LN boost CTV was straightforward. We also noted 1 case in which a suspicious LN was located outside of consensus contouring guidelines, a phenomenon that has also been reported by others who have examined the potential utility of PET to assist with prostate cancer radiation therapy treatment planning.12
Before generating new treatment plans, we established a set of dosimetric goals and acceptable variations (Table 1) that were thought to represent a reasonable compromise in exchange for a dose delivered to macroscopic tumor. We chose a simultaneous integrated boost (dose painting) approach given our institutional experience using a 28-fraction regimen for men with high-risk prostate cancer. Other approaches, such as sequential volume reductions or a simultaneous integrated boost with conventionally fractionated pelvic RT followed by a sequential prostate boost, would likely yield similar results (and a full planning comparison across all commonly used prostate regimens is beyond the scope of this report). For each case, ranging from 1 to 5 additional LN boost targets, we were able generate treatment plans that respected our prespecified OAR constraints. Boost PTV coverage by the prescription dose met our goal in cases 2 to 4, but in case 1 the coverage was compromised to respect the small bowel dose limit, which suggests a likely need to reduce the PTV margin in select cases when this technique is used for a broader study population. The addition of the nodal boost also increased the volume of small bowel receiving 45 Gy in each case, ranging from 15 to 114.5 cm3, and 2 cases were associated with V45 Gy[cc] >300 cm3. We elected not to specify a small bowel V45 Gy[cc] threshold that would result in an unacceptable variation because this has not typically been included in national randomized trials of pelvic radiation for men with prostate cancer, such as RTOG 0924. Published reports also support that larger volumes of small bowel can receive 45 Gy without unacceptable risk of grade 3+ toxicity, particularly in the absence of concurrent radiosensitizing chemotherapy.32, 33 That said, the V45 Gy[cc] could likely be reduced simply by lowering the elective pelvic dose to 45 Gy while still maintaining escalated doses to suspicious LNs.
We acknowledge the inherent limitations of any treatment planning report, including a somewhat artificial treatment planning environment, limited case selection, and lack of clinical outcomes. We provided rationale for the dose and dose painting technique used in this report but note that similar results could likely be achieved with other approaches, such as conventional fractionation with cone down boost. We used straightforward image registration and target delineation techniques that are typical of many radiation oncology practices. [18F]fluciclovine PET was used in this study, but apart from the criteria used to define LN positivity, other PET agents could have been used with very similar methods. Finally, the dosimetric goals for the PET/MRI-directed LN boost plans may be criticized as too aggressive, but previously published experiences using simultaneous integrated boost of 60 to 65 Gy to enlarged LNs support safety,34, 35 with the caveat that most patients treated in this manner had only 1 enlarged LN. Series of extended field IMRT for the treatment of LN-positive cervical cancer have also tolerably delivered similar doses, even in the setting of concurrent radiosensitizing chemotherapy.36
Conclusions
Although we are careful not to overgeneralize from a 4-patient case series, we are encouraged that this approach may be applicable to a large portion of men with high-risk prostate cancer who are identified as having suspicious LNs by PET staging. We believe that this tailored approach will be tolerable to deliver and may be able to improve disease control for a select group of men with high-risk prostate cancer. We are currently developing a clinical trial to implement this technique as part of [18F]fluciclovine PET/MRI upfront staging and therapy for men with high-risk prostate cancer.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr McDonald: Varian Medical Systems (research funding). Dr Galgano reports Blue Earth Diagnostics (research funding). Dr McConathy: Blue Earth Diagnostics (research funding, consulting). Dr Yang: none. Dr Dobelbower: Varian Medical Systems (research funding). Dr Jacob: none. Dr Rais-Bahrami: Phillips/nVivo (consulting), Blue Earth Diagnostics (consulting). Dr Nix: Phillips/nVivo (consulting). Dr Popple: Varian Medical Systems (research funding, consulting). Dr Fiveash: Varian Medical Systems (research funding, consulting).
References
- 1.Nabid A., Carrier N., Martin A.G. Duration of androgen deprivation therapy in high-risk prostate cancer: A randomized phase III trial. Eur Urol. 2018;74:432–441. doi: 10.1016/j.eururo.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Morris W.J., Tyldesley S., Rodda S. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Lawton C.A., DeSilvio M., Roach M., 3rd An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: Updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pommier P., Chabaud S., Lagrange J.L. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Update of the long-term survival results of the GETUG-01 randomized study. Int J Radiat Oncol Biol Phys. 2016;96:759–769. doi: 10.1016/j.ijrobp.2016.06.2455. [DOI] [PubMed] [Google Scholar]
- 5.Zumsteg Z.S., Spratt D.E., Romesser P.B. Anatomical patterns of recurrence following biochemical relapse in the dose escalation era of external beam radiotherapy for prostate cancer. J Urol. 2015;194:1624–1630. doi: 10.1016/j.juro.2015.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spratt D.E., Vargas H.A., Zumsteg Z.S. Patterns of lymph node failure after dose-escalated radiotherapy: Implications for extended pelvic lymph node coverage. Eur Urol. 2017;71:37–43. doi: 10.1016/j.eururo.2016.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beresford M.J., Gillatt D., Benson R.J., Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol. 2010;22:46–55. doi: 10.1016/j.clon.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Parker W.P., Davis B.J., Park S.S. Patterns of recurrence following primary radiation therapy for prostate cancer using C-11 choline positron emission tomography/computed tomography: Unique identification of sites of recurrence impacting clinical management. Int J Radiat Oncol Biol Phys. 2016;96:S112. [Google Scholar]
- 9.Akin-Akintayo O., Tade F., Mittal P. Prospective evaluation of fluciclovine ((18)F) PET-CT and MRI in detection of recurrent prostate cancer in non-prostatectomy patients. Eur J Radiol. 2018;102:1–8. doi: 10.1016/j.ejrad.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarzour J.G., Galgano S., McConathy J., Thomas J.V., Rais-Bahrami S. Lymph node imaging in initial staging of prostate cancer: An overview and update. World J Radiol. 2017;9:389–399. doi: 10.4329/wjr.v9.i10.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fodor A., Cozzarini C., Fiorino C., Di Muzio N.G. In Regard to Pommier et al. Int J Radiat Oncol Biol Phys. 2017;97:1109–1110. doi: 10.1016/j.ijrobp.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Calais J., Kishan A.U., Cao M. Potential Impact of (68)Ga-PSMA-11 PET/CT on the planning of definitive radiation therapy for prostate cancer. J Nucl Med. 2018;59:1714–1721. doi: 10.2967/jnumed.118.209387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savir-Baruch B., Banks K.P., McConathy J.E. ACR-ACNM practice parameter for the performance of fluorine-18 fluciclovine-PET/CT for recurrent prostate cancer. Clin Nucl Med. 2018;43:909–917. doi: 10.1097/RLU.0000000000002310. [DOI] [PubMed] [Google Scholar]
- 14.Parent E.E., Schuster D.M. Update on (18)F-fluciclovine PET for prostate cancer imaging. J Nucl Med. 2018;59:733–739. doi: 10.2967/jnumed.117.204032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bach-Gansmo T., Nanni C., Nieh P.T. Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine ((18)F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol. 2017;197:676–683. doi: 10.1016/j.juro.2016.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elschot M., Selnaes K.M., Sandsmark E. Combined (18)F-fluciclovine PET/MRI shows potential for detection and characterization of high-risk prostate cancer. J Nucl Med. 2018;59:762–768. doi: 10.2967/jnumed.117.198598. [DOI] [PubMed] [Google Scholar]
- 17.Jambor I., Kuisma A., Kahkonen E. Prospective evaluation of (18)F-FACBC PET/CT and PET/MRI versus multiparametric MRI in intermediate- to high-risk prostate cancer patients (FLUCIPRO trial) Eur J Nucl Med Mol Imaging. 2018;45:355–364. doi: 10.1007/s00259-017-3875-1. [DOI] [PubMed] [Google Scholar]
- 18.Selnaes K.M., Kruger-Stokke B., Elschot M. (18)F-Fluciclovine PET/MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur Radiol. 2018;28:3151–3159. doi: 10.1007/s00330-017-5213-1. [DOI] [PubMed] [Google Scholar]
- 19.Miller M.P., Kostakoglu L., Pryma D. Reader training for the restaging of biochemically recurrent prostate cancer using (18)F-fluciclovine PET/CT. J Nucl Med. 2017;58:1596–1602. doi: 10.2967/jnumed.116.188375. [DOI] [PubMed] [Google Scholar]
- 20.Khalaf M., Abdel-Nabi H., Baker J., Shao Y., Lamonica D., Gona J. Relation between nodule size and (18)F-FDG-PET SUV for malignant and benign pulmonary nodules. J Hematol Oncol. 2008;1:13. doi: 10.1186/1756-8722-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Algan O., Hanks G.E., Shaer A.H. Localization of the prostatic apex for radiation treatment planning. Int J Radiat Oncol Biol Phys. 1995;33:925–930. doi: 10.1016/0360-3016(95)00226-4. [DOI] [PubMed] [Google Scholar]
- 22.Harris V.A., Staffurth J., Naismith O. Consensus guidelines and contouring atlas for pelvic node delineation in prostate and pelvic node intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:874–883. doi: 10.1016/j.ijrobp.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Schreibmann E., Schuster D.M., Rossi P.J., Shelton J., Cooper S., Jani A.B. Image guided planning for prostate carcinomas with incorporation of anti-3-[18F]FACBC (Fluciclovine) positron emission tomography: Workflow and initial findings from a randomized trial. Int J Radiat Oncol Biol Phys. 2016;96:206–213. doi: 10.1016/j.ijrobp.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Specht L., Yahalom J., Illidge T. Modern radiation therapy for Hodgkin lymphoma: Field and dose guidelines from the international lymphoma radiation oncology group (ILROG) Int J Radiat Oncol Biol Phys. 2014;89:854–862. doi: 10.1016/j.ijrobp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 25.McDonald A.M., Bishop J.M., Jacob R. Hypofractionated prostate radiotherapy with or without conventionally fractionated nodal irradiation: Clinical toxicity observations and retrospective daily dosimetry. Prostate Cancer. 2012;2012:546794. doi: 10.1155/2012/546794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald A.M., Jacob R., Dobelbower M.C., Kim R.Y., Fiveash J.B. Efficacy and toxicity of conventionally fractionated pelvic radiation with a hypofractionated simultaneous versus conventionally fractionated sequential boost for patients with high-risk prostate cancer. Acta Oncol (Stockholm, Sweden) 2013;52:1181–1188. doi: 10.3109/0284186X.2012.748987. [DOI] [PubMed] [Google Scholar]
- 27.McDonald A.M., Baker C.B., Popple R.A. Different rectal toxicity tolerance with and without simultaneous conventionally-fractionated pelvic lymph node treatment in patients receiving hypofractionated prostate radiotherapy. Radiat Oncol (London, England) 2014;9:129. doi: 10.1186/1748-717X-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker C.B., McDonald A.M., Yang E.S. Pelvic Radiotherapy versus radical prostatectomy with limited lymph node sampling for high-grade prostate adenocarcinoma. Prostate Cancer. 2016;2016:2674954. doi: 10.1155/2016/2674954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohler J.L., Armstrong A.J., Bahnson R.R. Prostate cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 30.Xu L., Pachynski R.K. Contemporary management of the newly diagnosed prostate cancer patient with metastatic disease at presentation. Curr Urol Rep. 2018;19:79. doi: 10.1007/s11934-018-0835-7. [DOI] [PubMed] [Google Scholar]
- 31.Corfield J., Perera M., Bolton D., Lawrentschuk N. 68)Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: A systematic review. World J Urol. 2018;36:519–527. doi: 10.1007/s00345-018-2182-1. [DOI] [PubMed] [Google Scholar]
- 32.Ling A., Furhang E., Ryemon S.N., Ennis R.D. Late small bowel toxicity after aggressive abdominopelvic intensity modulated radiation therapy. Adv Radiat Oncol. 2017;2:615–623. doi: 10.1016/j.adro.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green G., Williams R., Zhang J., Azawi S. Dose-volume relationship of acute and late small bowel toxicity from radiation therapy for prostate cancer: A veteran affairs study. Int J Radiat Oncol Biol Phys. 2014;90:S456. [Google Scholar]
- 34.Engels B., Soete G., Tournel K. Helical tomotherapy with simultaneous integrated boost for high-risk and lymph node-positive prostate cancer: Early report on acute and late toxicity. Technol Cancer Res Treat. 2009;8:353–359. doi: 10.1177/153303460900800505. [DOI] [PubMed] [Google Scholar]
- 35.Fonteyne V., Lumen N., Ost P. Hypofractionated intensity-modulated arc therapy for lymph node metastasized prostate cancer: Early late toxicity and 3-year clinical outcome. Radiother Oncol. 2013;109:229–234. doi: 10.1016/j.radonc.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Vargo J.A., Kim H., Choi S. Extended field intensity _modulated radiation therapy with concomitant boost for lymph _node-positive cervical cancer: Analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int J Radiat Oncol Biol Phys. 2014;90:1091–1098. doi: 10.1016/j.ijrobp.2014.08.013. [DOI] [PubMed] [Google Scholar]