Abstract
Purpose
Single-fraction radiation therapy (RT) is a convenient and cost-effective regimen for the palliation of painful bone metastases, but is still underused. Randomized controlled trials comparing single- versus multiple-fraction RT are limited by generalizability. We compared the pain response rates after single- versus multiple-fraction RT in nonrandomized studies.
Methods and Materials
We searched PubMed and Scopus from the inception of each database through August 2018. We sought to identify nonrandomized studies in which data on pain response rates could be extracted for single- and multiple-fraction RT. Our primary outcomes of interest were the overall and complete pain response rates in evaluable patients. The analysis was performed using a random-effects model with the Mantel-Haenszel method.
Results
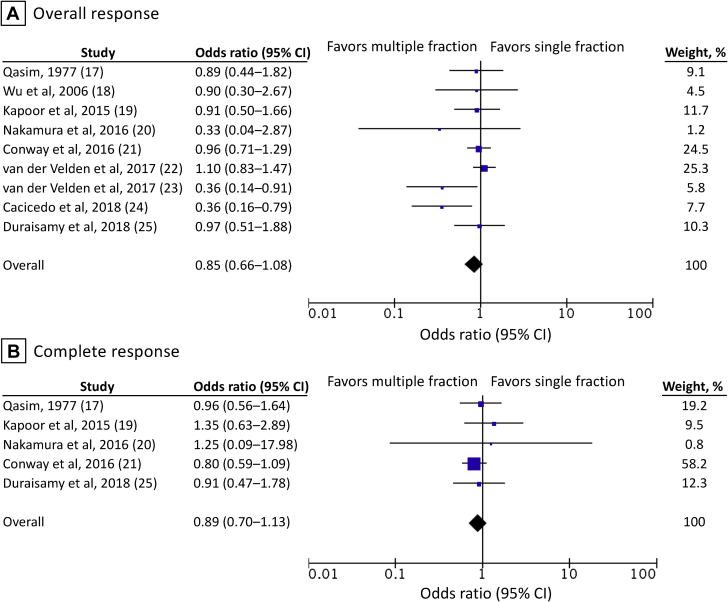
Of the 3933 articles identified through our search, 9 met our inclusion criteria. Five of 9 included studies did not exclude patients with features of complicated bone metastases. A 1 × 8 Gy radiation schedule was frequently used in single-fraction therapy, and schedules of 5 × 4 Gy and 10 × 3 Gy were frequently used in multiple-fraction therapy. In the 9 studies, the overall response rate was 67% (884 of 1321 patients) for patients in the single-fraction arm and 70% (953 of 1360 patients) for those in the multiple-fraction arm (pooled odds ratio [OR]: 0.85; 95% confidence interval [CI], 0.66-1.08). In 5 studies, the complete response rate was 26% (195 of 753 patients) for patients in the single-fraction arm and 35% (289 of 821 patients) for those in the multiple-fraction arm (pooled OR: 0.89; 95% CI, 0.70-1.13).
Conclusions
There were no significant differences in the overall and complete response rates between single- and multiple-fraction RT. The effectiveness of single-fraction regimens was shown in nonrandomized settings, which better reflect daily practice than randomized studies. The CIs for the pooled ORs included clinically meaningful differences, and the study results are inconclusive.
Summary.
Randomized controlled trials comparing single- versus multiple-fraction radiation therapy for painful bone metastases are limited by generalizability. This meta-analysis of nine studies showed that there were no significant differences in the overall and complete response rates between single- and multiple-fraction regimens in non-radomized studies. The effectiveness of single-fraction regimens was shown in non-randomized settings, which better reflect daily practice than randomized studies.
Introduction
Randomized controlled trials (RCTs) have shown that single-fraction radiation therapy (RT) regimens demonstrate pain response rates that are similar to those of multiple-fraction regimens for painful bone metastases (BM).1, 2 Single-fraction RT is a convenient and cost-effective treatment for the palliation of painful BM.3, 4 However, the underutilization of single-fraction RT has been reported worldwide.5, 6, 7, 8, 9 An analysis of the National Cancer Data Base showed that among patients treated for nonspinal or vertebral metastases, 4.7% received 8 Gy in 1 fraction, whereas 95.3% received multiple-fraction treatment.10
Radiation oncologists’ continued reluctance to adopt single-fraction regimens may, at least in part, be attributable to the limitation of RCTs in terms of generalizability. RCTs have high internal validity and play a key role in comparing different interventions,11 but nonrandomized studies can provide additional evidence. RCTs and nonrandomized studies have different patient populations, and both types of studies may reach different conclusions, even after adjusting for known prognostic factors.11 In addition, patients with complicated BM (ie, impending or existing pathologic fracture, spinal cord compression, or cauda equina compression) were excluded from most RCTs on RT for BM.12 This exclusion of patients with complicated BM limits the generalizability of RCTs.
In this meta-analysis, we compared the pain response rates between single- and multiple-fraction RT for BM in nonrandomized studies, which better reflect daily practice than RCTs.
Methods and Materials
Data sources and study selection
We reported this review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.13 No formalized review protocol was created for the present review. A literature search was conducted in PubMed and Scopus, and the last search was performed on August 14, 2018. Search terms included synonyms of and words related to “bone”, “metastasis”, “radiation therapy”, “response”, and “pain.” The full search strategy is reported in Method E1 (available online at https://doi.org/10.1016/j.adro.2019.06.003). There were no restrictions regarding the date of publication. In this review, we only included articles in which data on pain response rates could be extracted for single- and multiple-fraction RT.
The exclusion criteria for abstract screening were as follows: Editorials or reviews; case reports (<10 patients); publications in languages other than English; radiopharmaceuticals; particle RT; brachytherapy; intraoperative RT; stereotactic body RT; intensity modulated RT; concurrent chemotherapy and RT; and half-body irradiation. Subsequently, we assessed the screened full-text papers for eligibility in accordance with the following exclusion criteria: randomized studies; studies in which the response rates were calculated on the basis of the number of painful irradiated lesions instead of the number of patients; and studies in which the number of patients with pain response was not reported and only the percentage of pain response was reported. At this stage, some studies were excluded on the basis of the exclusion criteria for abstract screening. Abstract screening was performed by 1 reviewer (T.S.). Full-text paper review was performed by 2 reviewers (T.S. and K.Y.) independently, and disagreements were addressed by discussion.
To avoid counting the same patients more than once, for studies in which the Rapid Response Radiotherapy Program14 database was used, we selected studies according to the following policy: For studies that shared, at least in part, the same enrollment period, only 1 study (the largest) was selected for this review.
Data extraction and risk of bias assessment
Our primary outcomes of interest were the overall and complete response rates in evaluable patients. Dropout rates were not reported in all studies; therefore, we could only analyze the response rates in evaluable patients. When more than 1 assessment of response was performed, the latest assessment performed within 3 months after RT was recorded. Secondary outcomes included retreatment rate, pathologic fracture rate, spinal cord compression rate, and acute toxicity. Additional information compiled included the name of the first author, publication year, journal name, study design, characteristics of BM, eligibility criteria, dose fractionation of RT, definition of pain response, and time at which pain response was measured. The risk of bias of the included studies was assessed by 1 author (T.S.) using the Risk of Bias in Nonrandomized Studies of Interventions tool.15
Statistical analysis
This meta-analysis was conducted using Review Manager, version 5.3 (Cochrane Collaboration, Oxford, United Kingdom) and StatsDirect, version 3.1.20 (StatsDirect Ltd, Cheshire, United Kingdom). The analysis was performed using a random-effects model with the Mantel-Haenszel method. Odds ratio (OR) and 95% confidence interval (CI) were calculated for each study and presented in a forest plot. The heterogeneity of the included studies was assessed with Cochran's Q test and the I2 index.16 Publication bias was assessed using a funnel plot and Egger's test. Subgroup analyses were performed on the basis of whether the response was characterized by pain intensity measures only or with both pain intensity measures and analgesic use. P < .05 was considered statistically significant.
Results
Characteristics of included studies
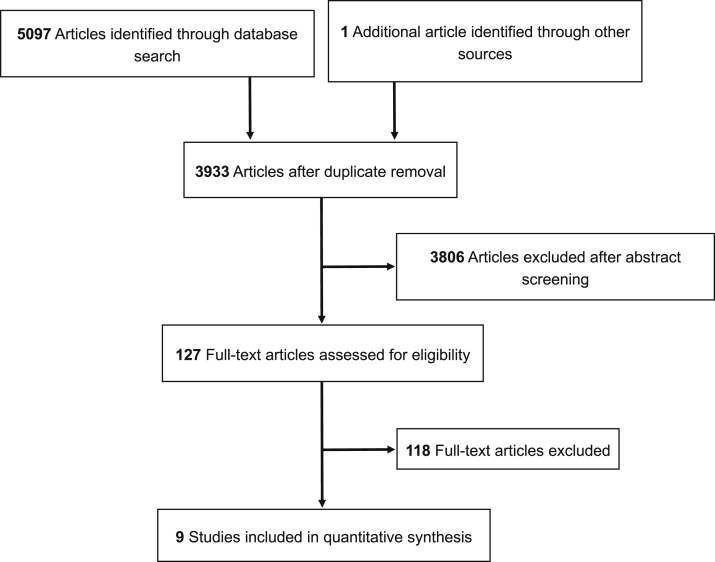
Of the 3933 articles identified through our search, 9 met our inclusion criteria (Fig 1). The characteristics of the included studies are presented in Table 1. Four studies were prospective in nature and 3 studies were based on prospectively collected data. Two studies had a retrospective design. In 4 studies, the main objective was the comparison of single- versus multiple-fraction RT.17, 19, 21, 25 In the other 5 studies, the response rates were extracted for each single- and multiple-fraction RT.18, 20, 22, 23, 24
Figure 1.
Flow diagram of study inclusion.
Table 1.
Study characteristics
| Study | Study design | No. of patients |
BM characteristics | Main study objective | Eligibility criteria for complicated BM | Radiation schedule |
Overall response definition | Complete response definition | When response was measured | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single fx | Multiple fx | Single fx | Multiple fx | ||||||||
| Qasim, 197717 | Retrospective | 69 | 246 | BM from lung or breast cancer | Compare single- vs multiple-fx RT | None | 800-1000 rads | 2000 rads/5 fx | Pain reduction with or without the need for mild analgesic drugs | When patients became completely free from pain | 3-4 wk after RT |
| Wu et al, 200618 | Based on prospectively collected data | 58 | 27 | BM mainly from breast or prostate cancer (patients who received treatment in 1 dominant area of bone pain) | Characterize effect of palliative RT | Excluded spinal cord compression; included bone lesions with neuropathic pain, established or high risk of pathologic fracture, and/or soft-tissue mass | 6-8 Gy (most received 8 Gy) | 18-30 Gy/4-10 fx | Reduction in worst pain score by ≥ 2/10 | NR | 4-6 wk after RT |
| Kapoor et al, 201519 | Single-center, prospective, observational | 116 | 71 | Spine metastases mainly from lung, breast, or prostate cancer | Compare single- vs multiple-fx RT | None | 8 Gy (100%) | 30 Gy/10 fx (100%) | Reduction in visual analog scale score by at least 2 points from baseline | Not specified | 30 d after completion of RT |
| Nakamura et al, 201620 | Single-institute, prospective | 5 | 12 | BM mainly from breast, lung, or prostate cancer (patients with neuropathic features) | Estimate prevalence of neuropathic pain features among patients who received palliative RT | None | 8 Gy (100%) | 30 Gy/10 fx, 20 Gy/5 fx | Pain score improvement ≥2 with no increase in analgesia or decrease in analgesia of ≥25% without increase in pain score∗ | An index pain score of 0 with no increase in analgesia∗ | 2 mo after start of RT |
| Conway et al, 201621 | Based on prospectively collected data | 509 | 395 | BM mainly from genitourinary, breast, or lung cancer | Compare single- vs multiple-fx RT | Included BM with pathologic fracture and/or neurologic compromise | 4-10 Gy (median, 8 Gy) | 4-50 Gy/5-25 fx (median, 20 Gy/5 fx) | Improvement in pain score by at least 1 point | Follow-up pain score of 0 | 3-4 wk after completion of RT |
| van der Velden et al, 201722 | Based on prospectively collected data | 382 | 389 | BM mainly from breast, prostate, or lung cancer | Develop and validate clinical risk score to predict pain response | None | 8 Gy | NR | Pain score improvement ≥2 with no increase in analgesia or decrease in analgesia of ≥25% without increase in pain score∗ | NR | Within 3 mo after RT |
| van der Velden et al, 201723 | Two-center, prospective, observational | 82 | 36 | Spine metastases mainly from breast, prostate, lung, or kidney cancer | Evaluate relationship between mechanical stability and response to palliative RT | Excluded BM with invalidating neurologic deficits (American Spinal Injury Association E or D without progression) | 8 Gy | 30 Gy/10 fx, 20 Gy/5 fx | Pain score improvement ≥2 with no increase in analgesia or decrease in analgesia of ≥25% without increase in pain score∗ | NR | 4-8 wk after RT |
| Cacicedo et al, 201824 | Multicenter, prospective observational (secondary analysis) | 37 | 88 | BM from lung, prostate, or breast cancer | Evaluate whether age is predictor of pain response | Excluded BM with pathologic fracture, spinal cord compression, or cauda equina syndrome | 8 Gy | 20 Gy/5 fx (87%), 20 Gy/4 fx (13%) | Pain score improvement ≥2 with no increase in analgesia or decrease in analgesia of ≥25% without increase in pain score† | NR | 4 wk after completion of RT |
| Duraisamy et al, 201825 | Single-center, retrospective | 63 | 96 | BM mainly from breast, lung, or prostate cancer | Compare single- vs multiple-fx RT | Excluded BM with spinal cord compression or pathologic fracture | 8 Gy, 10 Gy | 20 Gy/5 fx, 30 Gy/10 fx | Reduction in pain score by at least 1 (scale 1-4) at the treated site without analgesic intake or analgesic reduction by at least 25% from baseline without increase in pain | Pain score of 0 at the treated site with no increase in analgesic intake | 12 wk after RT |
Abbreviations: BM = bone metastases; fx = fraction; NR = not reported; RT = radiation therapy.
International Consensus Endpoint published in 2012.
International Consensus Endpoint published in 2002.
Five studies did not exclude patients with features of complicated BM. A 1 × 8 Gy radiation schedule was frequently used in single-fraction therapy, and schedules of 5 × 4 Gy and 10 × 3 Gy were frequently used in multiple-fraction therapy. In 4 studies, overall response was defined only on the basis of pain intensity.17, 18, 19, 21 In the other 5 studies, overall response was defined on the basis of pain intensity and analgesic use.20, 22, 23, 24, 25 One study24 used the International Consensus Endpoint, which was initially published in 2002,26 and 3 studies20, 22, 23 used the International Consensus Endpoint, which was updated in 2012.27
The 9 studies were adjudged as having a serious risk of bias in 3 to 5 domains and serious overall risk of bias (Table 2). Serious risk of bias was mainly observed in terms of confounding, selection of participants into the study, measurement of outcomes, and selection of the reported result.
Table 2.
Risk of bias assessment using the Risk of Bias in Nonrandomized Studies of Interventions tool
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Qasim, 197717 | S | S | M | S | NI | S | S | S |
| Wu et al, 200618 | S | S | L | S | NI | S | S | S |
| Kapoor et al, 201519 | S | S | L | S | NI | S | M | S |
| Nakamura et al, 201620 | S | S | L | L | M | S | M | S |
| Conway et al, 201621 | S | S | L | S | M | S | M | S |
| van der Velden et al, 201722 | S | S | L | L | L | S | S | S |
| van der Velden et al, 201723 | S | S | L | L | L | S | S | |
| Cacicedo et al, 201824 | S | S | L | L | NI | S | S | S |
| Duraisamy et al, 201825 | S | S | M | L | L | S | S | S |
Abbreviations: L = low risk of bias; M = moderate risk of bias; NI = no information; S = serious risk of bias.
Pain response rates
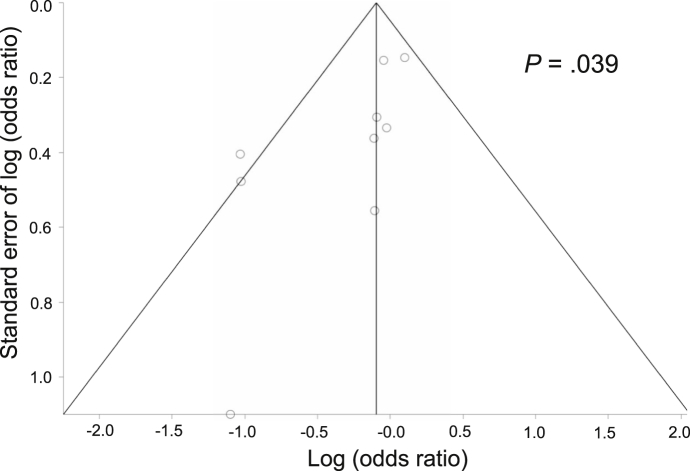
In the 9 studies included for the calculation of overall response rates, the single-fraction arm included 1321 evaluable patients, and the multiple-fraction arm included 1360 evaluable patients (Fig 2). The overall response rate was 67% (884 of 1321 patients) among patients in the single-fraction arm and 70% (953 of 1360 patients) among those in the multiple-fraction arm. The pooled OR was 0.85 (95% CI, 0.66-1.08). Although Cochran's Q test was not significant (P = .16), the I2 value (33%) indicated the possibility of moderate heterogeneity between the studies.16 A funnel plot and Egger's test (P = .039) indicated the potential presence of publication bias (Fig 3).
Figure 2.
Overall and complete response rates in the evaluable patients. Error bars indicate 95% confidence intervals.
Figure 3.
Funnel plot of studies that reported overall response rates with Egger's test results.
Smaller studies tended to show that multiple-fraction RT was associated with better response rates than single-fraction therapy. In the 5 studies that reported complete response rates, the complete response rate was 26% (195 of 753 patients) among patients in the single-fraction arm and 35% (289 of 821 patients) among those in the multiple-fraction arm (Fig 2). The pooled OR was 0.89 (95% CI, 0.70-1.13). In one study in which the denominators of the response rates were not presented, the denominators were calculated from the numerators and response rates in the present review.21
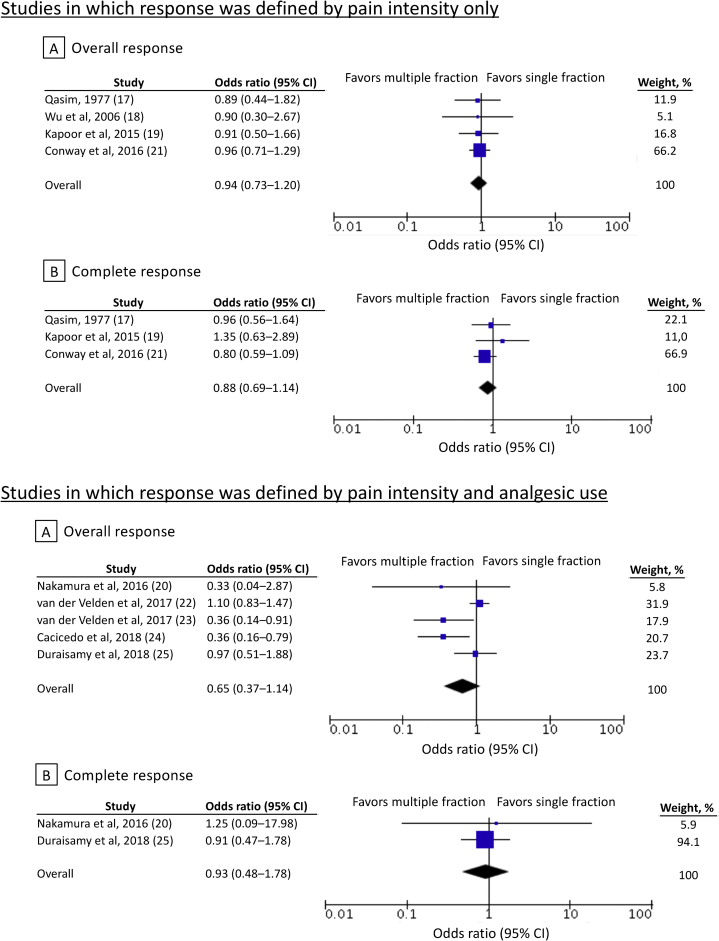
In another study that reported radiation schedules of 1 × 8 Gy and others, schedules other than 1 × 8 Gy were treated as multiple fractions in the present review.22 Subgroup analyses based on pain response definitions did not identify any specific patient subgroups that would derive more benefit from multiple-fraction RT (Fig 4).
Figure 4.
Subgroup analyses based on pain response definitions. Error bars indicate 95% confidence intervals.
Secondary outcomes
Two studies reported the retreatment rates after RT. In one study, the retreatment rate was 10% (7 of 69 patients) among patients who received single-fraction RT and 14% (35 of 246 patients) among those who received multiple-fraction RT.17 In the other study, the retreatment rate was 25% (16 of 65 patients) for patients with single-fraction RT and 18% (17 of 97 patients) for those with multiple-fraction RT.25 Two studies reported the pathologic fracture rates after RT. In one study, the rate of pathologic fracture of the femoral neck was 30% (3 of 10 patients) among patients who received single-fraction RT and 24% (5 of 21 patients) among those with multiple-fraction RT.17 In the other study, the pathologic fracture rate was 3% (2 of 65 patients) among patients who received single-fraction RT and 2% (2 of 97 patients) in those with multiple-fraction RT.25
Only 1 study reported the spinal cord compression rate after RT.25 The spinal cord compression rate was 8% (5 of 65 patients) among patients who received single-fraction RT and 9% (9 of 97 patients) in those with multiple-fraction RT.25 Only 1 study reported on the toxicity of RT.17 This study reported that the radiation side effects were less marked in patients who received multiple-fraction RT (data not shown).17
Discussion
We found that there were no significant differences in the overall and complete response rates between single- and multiple-fraction RT. The effectiveness of single-fraction regimens was shown in nonrandomized studies, which better reflect daily practice than randomized settings. RCTs have strong internal validity but limited external validity and generalizability because RCTs often include patient populations that are not representative of the population with the disease.28 Evidence from nonrandomized studies can provide equivalent or potentially higher confidence in the evidence compared with RCT-based evidence, which does not adequately represent the population.29
Most RCTs comparing single- versus multiple-fraction regimens for painful BM excluded patients with complicated BM.12, 30 In a study of a population-based RT program, 34.4% of BM were complicated by fracture or neurologic compromise and less likely to receive single-fraction RT.31 Radiation oncologists’ knowledge of the exclusion of patients with complicated BM from RCTs may have contributed to their reluctance to use single-fraction regimens for complicated BM. In the present review, patients with complicated BM were not excluded in 5 of 9 studies, which benefited our study in terms of generalizability. One study included in the present review showed that for the patient subgroup with complicated BM, there were no differences in the overall pain response rates between the single-fraction and multiple-fraction groups.21 However, in the complicated BM subset, complete pain response and functional improvement occurred more commonly in the multiple-fraction group.21 The effectiveness of single-fraction regimens in patients with complicated BM should be investigated in future research.
We identified the potential presence of publication bias in the present meta-analysis. Smaller studies tended to show that multiple-fraction RT was associated with better response rates than single-fraction therapy. However, the present review included only 9 studies; thus, this finding may be inconclusive.
Response definitions influence the evaluation of palliative RT effects. According to the International Consensus Endpoint, patients with alleviated pain and increased opioid analgesic use would be diagnosed as nonresponders.27 However, these same patients would be diagnosed as responders if response was defined using only pain intensity measures. The response definitions may raise or lower the response rates.32 Our subgroup analyses based on pain response definitions did not identify any patient subgroups that might have benefitted more from multiple-fraction RT and highlights the overall robustness of our results.
The present review has some limitations. First, all 9 included studies were judged to have a serious overall risk of bias. Patients who received single-fraction therapy may not be comparable to those who received multiple-fraction therapy. The adjustment of patient characteristics between the treatment groups was not performed in any of the 9 studies. Future studies comparing single- and multiple-fraction RT in nonrandomized settings may benefit from the adjustment of baseline patient characteristics between the treatment groups.
The second limitation is that the CIs for the pooled ORs included clinically meaningful differences, rendering our study results inconclusive. Larger data may be necessary for the acquisition of more reliable conclusions. The third limitation of the present review is that only few studies reported data on retreatment rates, pathologic fracture rates, spinal cord compression rates, or acute toxicity. Limited data are available regarding these important outcomes, other than response rates, in nonrandomized studies comparing single- and multiple-fraction RT.
Conclusions
We compared the pain response rates after single- versus multiple-fraction RT for BM in nonrandomized studies. There were no significant differences in the response rates between single- and multiple-fraction RT. Considering the serious risk of bias in the effectiveness estimates for interventions in the included nonrandomized studies, the results of the present meta-analysis should be interpreted with caution. The present study indicated the effectiveness of single-fraction RT in nonrandomized studies, which are more representative of real-world settings than RCTs.
To date, this study seems to be the first meta-analysis of nonrandomized studies to compare different dose fractionation regimens in RT for painful BM. In the future, the performance of nonrandomized studies comparing single- versus multiple-fraction regimens, after adjustment for confounding factors, may be beneficial in drawing more valid comparison results. In addition, data on retreatment rates, pathologic fracture rates, spinal cord compression rates, and acute toxicity are worth recording in such studies.
Footnotes
Disclosures: The authors have no sources of support/funding to report.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2019.06.003.
Supplementary data
References
- 1.Rich S.E., Chow R., Raman S. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol. 2018;126:547–557. doi: 10.1016/j.radonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Pin Y., Paix A., Le Fevre C., Antoni D., Blondet C., Noel G. A systematic review of palliative bone radiotherapy based on pain relief and retreatment rates. Crit Rev Oncol Hematol. 2018;123:132–137. doi: 10.1016/j.critrevonc.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60:1373–1378. doi: 10.1016/j.ijrobp.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 4.van den Hout W.B., van der Linden Y.M., Steenland E. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: Cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95:222–229. doi: 10.1093/jnci/95.3.222. [DOI] [PubMed] [Google Scholar]
- 5.Bekelman J.E., Epstein A.J., Emanuel E.J. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310:1501–1502. doi: 10.1001/jama.2013.277081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrushevski A.N., Gabriel G.S., Hanna T.P., Allen S., Allison R.W., Barton M.B. Factors affecting the use of single-fraction radiotherapy for the palliation of bone metastases in Australia. Clin Oncol (R Coll Radiol) 2015;27:205–212. doi: 10.1016/j.clon.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Jeremic B., Vanderpuye V., Abdel-Wahab S. Patterns of practice in palliative radiotherapy in Africa - case revisited. Clin Oncol (R Coll Radiol) 2014;26:333–343. doi: 10.1016/j.clon.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura N., Shikama N., Wada H. Patterns of practice in palliative radiotherapy for painful bone metastases: A survey in Japan. Int J Radiat Oncol Biol Phys. 2012;83:e117–e120. doi: 10.1016/j.ijrobp.2011.11.075. [DOI] [PubMed] [Google Scholar]
- 9.Popovic M., den Hartogh M., Zhang L. Review of international patterns of practice for the treatment of painful bone metastases with palliative radiotherapy from 1993 to 2013. Radiother Oncol. 2014;111:11–17. doi: 10.1016/j.radonc.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Rutter C.E., Yu J.B., Wilson L.D., Park H.S. Assessment of national practice for palliative radiation therapy for bone metastases suggests marked underutilization of single-fraction regimens in the United States. Int J Radiat Oncol Biol Phys. 2015;91:548–555. doi: 10.1016/j.ijrobp.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Antman K., Amato D., Wood W. Selection bias in clinical trials. J Clin Oncol. 1985;3:1142–1147. doi: 10.1200/JCO.1985.3.8.1142. [DOI] [PubMed] [Google Scholar]
- 12.Cheon P.M., Wong E., Thavarajah N. A definition of "uncomplicated bone metastases" based on previous bone metastases radiation trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4:13–17. doi: 10.1016/j.jbo.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairchild A., Pituskin E., Rose B. The rapid access palliative radiotherapy program: Blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17:163–170. doi: 10.1007/s00520-008-0468-3. [DOI] [PubMed] [Google Scholar]
- 15.Sterne J.A., Hernan M.A., Reeves B.C. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qasim M.M. Single dose palliative irradiation for bony metastasis. Strahlentherapie. 1977;153:531–532. [PubMed] [Google Scholar]
- 18.Wu J.S., Monk G., Clark T., Robinson J., Eigl B.J., Hagen N. Palliative radiotherapy improves pain and reduces functional interference in patients with painful bone metastases: A quality assurance study. Clin Oncol (R Coll Radiol) 2006;18:539–544. doi: 10.1016/j.clon.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor A., Singhal M.K., Bagri P.K. Comparison of single versus multiple fractions for palliative treatment of painful bone metastasis: First study from north west India. Indian J Palliat Care. 2015;21:45–48. doi: 10.4103/0973-1075.150178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura N., Takahashi O., Zenda S. Neuropathic pain features in patients with bone metastases. Clin Oncol (R Coll Radiol) 2016;28:204–208. doi: 10.1016/j.clon.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Conway J.L., Yurkowski E., Glazier J. Comparison of patient-reported outcomes with single versus multiple fraction palliative radiotherapy for bone metastasis in a population-based cohort. Radiother Oncol. 2016;119:202–207. doi: 10.1016/j.radonc.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 22.van der Velden J.M., Peters M., Verlaan J.J. Development and internal validation of a clinical risk score to predict pain response after palliative radiation therapy in patients with bone metastases. Int J Radiat Oncol Biol Phys. 2017;99:859–866. doi: 10.1016/j.ijrobp.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 23.van der Velden J.M., Versteeg A.L., Verkooijen H.M. Prospective evaluation of the relationship between mechanical stability and response to palliative radiotherapy for symptomatic spinal metastases. Oncologist. 2017;22:972–978. doi: 10.1634/theoncologist.2016-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cacicedo J., Gomez-Iturriaga A., Navarro A. Analysis of predictors of pain response in patients with bone metastasis undergoing palliative radiotherapy: Does age matter? J Med Imaging Radiat Oncol. 2018 doi: 10.1111/1754-9485.12749. [DOI] [PubMed] [Google Scholar]
- 25.Duraisamy I.S., Saad M., Alip A. Single vs multiple fraction palliative radiotherapy for uncomplicated painful bone metastases treated at University of Malaya Medical Centre: A single institutional Malaysian experience. Aging Med. 2018;1:133–140. doi: 10.1002/agm2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow E., Wu J.S., Hoskin P., Coia L.R., Bentzen S.M., Blitzer P.H. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol. 2002;64:275–280. doi: 10.1016/s0167-8140(02)00170-6. [DOI] [PubMed] [Google Scholar]
- 27.Chow E., Hoskin P., Mitera G. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82:1730–1737. doi: 10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Visvanathan K., Levit L.A., Raghavan D. Untapped potential of observational research to inform clinical decision making: American Society of clinical Oncology research statement. J Clin Oncol. 2017;35:1845–1854. doi: 10.1200/JCO.2017.72.6414. [DOI] [PubMed] [Google Scholar]
- 29.Schunemann H.J., Tugwell P., Reeves B.C. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods. 2013;4:49–62. doi: 10.1002/jrsm.1078. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura N., Shikama N. The importance of defining ‘uncomplicated bone metastases’. Clin Oncol (R Coll Radiol) 2012;24:e193. doi: 10.1016/j.clon.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Tiwana M.S., Barnes M., Yurkowski E., Roden K., Olson R.A. Incidence and treatment patterns of complicated bone metastases in a population-based radiotherapy program. Radiother Oncol. 2016;118:552–556. doi: 10.1016/j.radonc.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Saito T., Toya R.Oya N. Pain response rates after conventional radiation therapy for bone metastases in prospective nonrandomized studies: A systematic review. Pract Radiat Oncol. 2019;9:81–88. doi: 10.1016/j.prro.2018.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.