Abstract
Purpose
Accurate contouring in head and neck cancer (HNC) is critical. International consensus guidelines recommend the 5 + 5 mm rule for expansions around the primary tumor, wherein high- and low-dose clinical target volumes (CTV-P1 and CTV-P2, respectively) are created using successive 5 mm expansions on the gross tumor volume. To our knowledge, the necessity of a low-dose CTV-P2 has never been assessed; therefore, we evaluated the dosimetric impact of adding a CTV-P2 expansion using the 5 + 5 mm rule compared with contouring with a high-dose CTV-P1 alone.
Methods and materials
A retrospective study of clinically delivered (chemo)radiation therapy treatment plans for HNC was conducted. All patients were treated with 70 Gy in 35 fractions using volumetric modulated arc therapy in a single phase. CTV-P2 was retrospectively contoured per guidelines as a 5 mm expansion on CTV-P1 from the clinical plan, carving off specified barriers to spread. We used a 5 mm planning target volume (PTV) expansion. Our primary outcome was whether 95% of the volume of the PTV for the CTV-P2 contour (ie, PTV-P2) received at least 56 Gy. To assess dose falloff, the coverage of a PTV ring structure was created by subtracting PTV-P1 from PTV-P2.
Results
Twenty-seven patients from 4 HNC subsites (base of tongue, tonsil, hypopharynx, and supraglottic larynx) were included. In all 108 treatment plans, at least 95% of the PTV-P2 structure received at least 56 Gy. The minimum volume of the PTV-P2 structure receiving at least 56 Gy was 97.4%. Eight of 108 treatment plans had borderline coverage of the PTV ring substructure alone.
Conclusions
Adding a CTV-P2 structure using the 5 + 5 mm rule had no dosimetric impact, adds contouring time, adds treatment planning complexity, and could potentially introduce errors. The 5 + 5 mm rule may have value in other settings, such as when smaller PTV margins are used, and warrants further evaluation with prospective or randomized studies.
Introduction
Intensity modulated radiation therapy (IMRT) decreases acute and late side effects in head and neck cancer (HNC) treatment,1 but accurate contouring is critical.2, 3, 4 Substantial interobserver variability has been observed in HNC contouring.5, 6 Guidelines can help to improve standardization. Recent international consensus guidelines for contouring primary tumors in HNC have recommended an approach called the 5 + 5 mm expansion,7 which requires defining a gross tumor volume (GTV) for the primary tumor, adding 5 mm for a high-dose clinical target volume (CTV; CTV-P1 in the guidelines) and a further 5 mm expansion for a lower-dose CTV (CTV-P2 in the guidelines). The addition of this second CTV might add contouring time because the CTV must be modified manually to remove air and natural barriers to spread.
The rationale for the 5 + 5 mm expansion is derived from histopathologic surgical series reporting microscopic disease extension from the primary tumor. However, studies have reported varying distances of microscopic disease extension. Campbell et al examined 10 oral tongue cancer cases in which 99% of microscopic disease would be contained within 5 mm of the GTV,8 whereas Yuen et al studied 50 patients in whom 96% of microscopic disease would be contained within 12 mm of the GTV in oral tongue cancer.9 Fleury et al reported on a mixed cohort of 21 patients (primarily larynx and oropharynx) in whom all microscopic disease was within 5 mm of the GTV after excluding patients pretreated with chemotherapy and/or radiation therapy.10
The physical properties of modern photon-based HNC radiotherapy plans may result in adequate coverage of all areas within 5 mm of the high-dose CTV with substantial dose, merely because of dose falloff. The concept of high-dose and low-dose CTV expansions around a primary tumor is not frequently used in other cancer sites, with few exceptions such as magnetic resonance image guided brachytherapy for cervical cancer.11 For other tumor sites, such as stage III lung cancer, recommended CTV expansions are not as large as the histopathologic distance of microscopic extension: A 5 mm CTV margin is commonly recommended,12 even after consideration of histopathologic studies suggesting that 95% of microscopic disease would be contained within 6 to 8 mm of the GTV.13
Although recommended in textbooks14 and used in some clinical trials protocols,15, 16 the 5 + 5 mm expansion rule is not universal in all clinical trial protocols in HNC.17, 18 To our knowledge, the necessity of contouring a low-dose CTV-P2 has never been evaluated clinically or dosimetrically. Although IMRT allows for rapid dose falloff near organs at risk,19 we hypothesized that the small distance between the CTV-P1 and CTV-P2 structures means that the CTV-P2 would already be adequately treated in radiation therapy treatment plans where this contour was not initially present. The purpose of this study was to evaluate the dosimetric impact of contouring an additional CTV-P2 structure per the 5 + 5 mm expansion rule in clinically delivered radiation therapy treatment plans created using only a GTV and CTV-P1.
Methods and Materials
This retrospective study was conducted at a single high-volume academic cancer center (approximately 400 HNC consultations per year by 4 radiation oncologists) after research ethics board approval (Western University Health Sciences Research Ethics Board #112441). Patients with HNC of the oropharynx, base of tongue, supraglottic larynx, or hypopharynx treated with definitive curative-intent (chemo)radiation therapy with 70 Gy in 35 fractions between 2013 and 2018 were eligible for inclusion. All plans were delivered in a single phase. Elective nodal volumes were treated with 56 Gy in 35 fractions, with a minority of patients receiving 63 Gy in 35 fractions to intermediate-risk volumes, discerned by the treating radiation oncologist. Synchronous primary HNCs were excluded.
Patients were identified through an HNC quality assurance rounds database. Staging was updated in accordance with the American Joint Committee on Cancer, 8th edition, where applicable. All patients were treated using volumetric modulated arc therapy.
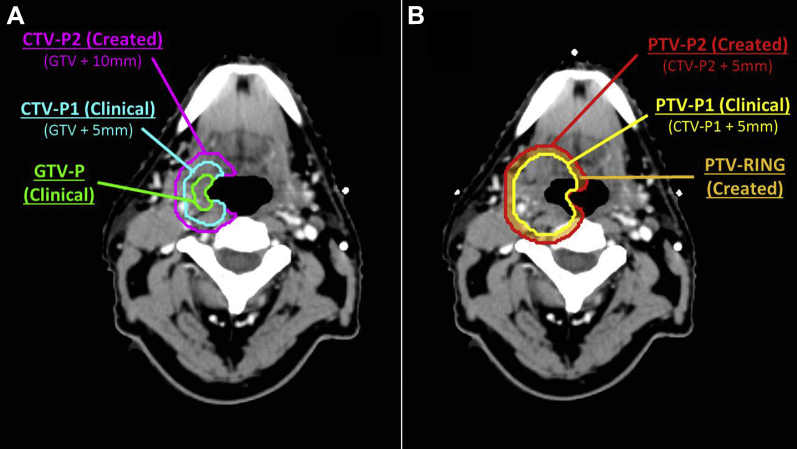
All radiation therapy treatment plans contained GTV and CTV-P1 contours used clinically. Our standard CTV-P1 margin was 5 mm but in select cases could have been expanded further at the discretion of the radiation oncologist if areas of uncertainty existed. We estimate that such further expansion occurred in <5% of cases. The standard PTV margin was 5 mm, excluding 5 mm of uninvolved skin during dosimetric evaluation. For this study, we retrospectively contoured an additional CTV-P2 structure per the recent international consensus guidelines,7 defined as CTV-P1 in the clinical plan plus 5 mm (carving off specified barriers to spread where specified in the guidelines). The guidelines suggested consideration of an additional 5 mm superoinferior margin beyond CTV-P2 for hypopharyngeal cancers, and this was contoured as CTV-P3 to evaluate that recommendation. A 5-mm PTV margin was added to all CTV structures to create PTV-P2 and PTV-P3, respectively, also excluding uninvolved skin (Fig 1A).
Figure 1.
Axial slice from a patient with a T2N1 p16 positive squamous cell carcinoma of the tonsil. (A) the 5 + 5 mm expansion rule, including GTV-P, CTV-P1 and CTV-P2. (B) PTV-P1 (yellow) from the clinically delivered plan, as well as the created PTV-P2 and PTV ring structures used for this study. Abbreviations: CTV = clinical target volume; GTV = gross tumor volume; P1 = high-dose; P2 = low-dose; PTV = planning target volume. (A color version of this figure is available at https://doi.org/10.1016/j.adro.2019.06.001.)
Our primary outcome was whether the PTV-P2 structure was adequately treated in the original treatment plan, defined a priori as V56 > 95% for PTV-P2, similar to coverage requirements in trial protocols. Secondary outcomes were whether PTV-P3 was adequately treated in hypopharyngeal cancers, also defined a priori as a V56 > 95%. To better understand the dose falloff between PTV-P1 and PTV-P2, we also assessed the coverage within a ring substructure (PTV-RING) created by subtracting PTV-P1 from PTV-P2 (Fig 1B) by at least 56 Gy as a secondary outcome.
Our sample size was calculated assuming that 15% of our treatment plans would have inadequate coverage of PTV-P2. We powered the study to restrict the 95% confidence interval around that hypothesized proportion (without continuity correction) to ±15% for each anatomic subsite, which required 27 patients for each subsite.15 We selected the most recently treated patients who met inclusion criteria for each subsite.
Results
A total of 108 treatment plans were analyzed, 27 per subsite (base of tongue, tonsil, hypopharynx, and supraglottic larynx). Demographic characteristics are presented in Table 1. The dose parameters of the clinical radiation therapy plans, including GTV volumes and dosimetric data of PTV coverage, are provided in Appendix EA (available online at https://doi.org/10.1016/j.adro.2019.06.001).
Table 1.
Demographic characteristics of study patients
| All patients n (%) | Base of tongue n (%) | Tonsil n (%) | Hypopharynx n (%) | Supraglottic larynx n (%) | |
|---|---|---|---|---|---|
| Age, y | |||||
| Median | 61.5 | 59 | 65 | 63 | 61 |
| Range | 39-80 | 39-80 | 42-80 | 40-78 | 47-77 |
| Sex | |||||
| Male | 95 (88.0%) | 24 (88.9%) | 25 (92.6%) | 23 (85.2%) | 23 (85.2%) |
| Female | 13 (12.0%) | 3 (11.1%) | 2 (7.4%) | 4 (14.8%) | 4 (14.8%) |
| T stage | |||||
| T1 | 9 (8.3%) | 2 (7.4%) | 1 (3.7%) | 5 (18.5%) | 1 (3.7%) |
| T2 | 47 (43.5%) | 14 (51.9%) | 14 (51.9%) | 6 (22.2%) | 13 (48.1%) |
| T3 | 32 (29.6%) | 3 (11.1%) | 6 (22.2%) | 11 (40.7%) | 12 (44.4%) |
| T4 | 20 (18.5%) | 8 (29.6%) | 6 (22.2%) | 5 (18.5%) | 1 (3.7%) |
| N stage | |||||
| N0 | 14 (13.0%) | 2 (7.4%) | 1 (3.7%) | 3 (11.1%) | 8 (29.6%) |
| N1 | 35 (33.3%) | 12 (44.4%) | 14 (51.9%) | 7 (25.9%) | 3 (11.1%) |
| N2∗ | 47 (43.5%) | 11 (40.7%) | 9 (33.3%) | 12 (44.4%) | 15 (55.6%) |
| N3 | 11 (10.2%) | 2 (7.4%) | 3 (11.1%) | 5 (18.5%) | 1 (3.7%) |
| p16 positive | N/A | 22 (81.5%) | 21 (77.8%) | N/A | N/A |
Abbreviation: N/A = not available.
N2 category includes both N2 in human papillomavirus (HPV) associated oropharyngeal cancer and N2a-c in HPV-negative or unknown oropharyngeal cancer.
The volumes of PTV-P2, PTV-P3, and PTV-RING structures covered by 56 Gy are presented in Table 2, along with the rates of inadequate coverage by subsite. In all patients, the dosimetric coverage of PTV-P2 and PTV-P3 met the prespecified threshold of V56 > 95% (inadequate coverage rate, 0%; 95% confidence interval, 0%-3.4%).
Table 2.
Characteristics of radiation treatment plans
| All patients | Base of tongue | Tonsil | Hypopharynx | Supraglottic larynx | |
|---|---|---|---|---|---|
| PTV-P2 (cm3) | |||||
| Median | 155.8 | 164.3 | 176.5 | 167.5 | 133.3 |
| Range | 57.8-616 | 57.8-616 | 70.6-400.1 | 61.2-389.1 | 63.6-279.3 |
| PTV-P2 V56∗ (%) | |||||
| Median | 99.8 | 99.6 | 99.3 | 100 | 100 |
| Minimum | 97.4 | 97.4 | 97.8 | 99.6 | 97.9 |
| PTV-P2 ring V56 (%) | |||||
| Median | 99.3 | 98.6 | 97.4 | 99.9 | 99.9 |
| Minimum | 90.6 | 90.6 | 91.2 | 98.4 | 93.8 |
| PTV-P3 (cm3) | |||||
| Median | 184.7 | ||||
| Range | 74.9-412.3 | ||||
| PTV-P3 V56 (%) | |||||
| Median | 99.0 | ||||
| Minimum | 95.7 | ||||
| PTV-P3 ring V56 (%) | |||||
| Median | 96.6 | ||||
| Minimum | 89.3 | ||||
| Inadequate coverage rate for PTV-P2 (%; 95% CI) | 0 (0-3.4) | 0 (0-12.5) | 0 (0-12.5) | 0 (0-12.5) | 0 (0-12.5) |
Abbreviations: CI = confidence interval; P1 = high-dose margin; P2 = low-dose margin; P3 = superoinferior margin; PTV = planning target volume.
V56 refers to the volume of the structure (%) receiving at least 56 Gy (see text for definition of planning target volume structures).
Only 8 of 108 treatment plans failed to meet a V56 for the PTV-P2-RING of 95%, with the minimum being 90.6%. Seven of 27 hypopharynx plans failed to meet a V56 for the PTV-P3-RING of 95%, with the minimum being 89.3%.
Discussion
In our study of clinically delivered radiation therapy plans for HNC, we found that creating an additional contour of CTV-P2 as recommended by international guidelines using the 5 + 5 mm expansion would not have altered our treatment plans because this area was already sufficiently covered by 56 Gy. We conclude that this extra 5 mm margin (CTV-P2) may be redundant and can be omitted when treating HNC under certain circumstances. Omitting the CTV-P2 may decrease errors in contouring and planning and would save time for both radiation oncologists and dosimetrists.
We speculate that the volume of CTV-P2 was already adequately treated due to the dose homogeneity requirements in HNC in addition to the small maximum distance between CTV-P1 and CTV-P2 of 5 mm. In addition, there are few other examples in radiation oncology in which a low-risk CTV around the primary tumor is used, such as magnetic resonance imagining guided brachytherapy for cervical cancer.11 More rapid dose falloff using photon external beam radiation therapy can be achieved by prescribing to lower isodose lines in stereotactic radiation therapy,20 but this results in maximum hot spots of 125% to 150% within the PTV which would be undesirable in HNC.
A small minority of plans did not meet V56 > 95% for the PTV-RING structures. PTV-RING may be used prospectively in treatment planning to optimize PTV coverage outside PTV-P1. However, there is no specified constraint for a similar substructure in clinical trial protocols,15, 16 and all plans would have been clinically acceptable using the 5 + 5 rule.
These findings must be considered within the context of the limitations of this study. First, the study results may not apply when PTV margins <5 mm are used. Margin reduction to 3 mm from 5 mm reduced the frequency, severity, and duration of toxicity from radiation therapy without compromising outcomes.21 Second, our CTV-P2 was added retrospectively, so there may have been slight differences compared with CTV-P2 added prospectively because CTVs can inherently represent areas of clinical uncertainty. Third, we did not evaluate the potential role of dose to elective nodal areas in the coverage of our 5 + 5 mm structures.
Fourth, our findings apply to photon-based plans and may not apply to proton radiation therapy. Fifth, we did not include glottic laryngeal cancers because our institutional protocol includes the entire larynx in the CTV contour. Sixth, we acknowledge that coverage of target structures could be a function of local dosimetry and ideally could be validated if there are other institutions that contour HNC in a similar manner. The potential impact of treatment with volumetric modulated arc therapy and simultaneous integrated boost was not evaluated, but we speculate that fixed-beam IMRT or a sequential boost treatment plans likely would have similar results. Further research on the impact of the 5 + 5 rule on contouring time and contouring/planning errors is warranted. Finally, this was not an outcomes-based study of local control; ideally, margins in HNC should be further evaluated in prospective or randomized studies.
Conclusions
In the setting of 5 mm PTV expansions, the addition of a CTV-P2 structure using the 5 + 5 mm expansion rule had no dosimetric impact on radiation therapy treatment planning and appears redundant. The 5 + 5 mm rule may have value in other settings, such as when smaller PTV margins are used or as a step-wise method of assessing potential patterns of microscopic spread for difficult cases.
Although we recognize the theoretical merit of delineating a CTV-P2 based on biologic principles, other factors must also be considered, including the potential for introducing error as a result of increased planning complexity and the additional time required for contouring. We encourage individual institutions to examine their radiation plans with and without a separate CTV-P2 to assess dosimetric coverage of the CTV-P2 area. We believe that further research is warranted in determining CTV margins and whether a CTV-P2 impacts clinical outcomes. Ultimately, randomized trials should be designed to address the optimal target volumes for HNC treatment.
Footnotes
Disclosures: The authors of this study declare they have no conflicts of interest.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2019.06.001.
Supplementary data
References
- 1.Nutting C.M., Morden J.P., Harrington K.J. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boero I.J., Paravati A.J., Xu B. Importance of radiation oncologist experience among patients with head-and-neck cancer treated with intensity-modulated radiation therapy. J Clin Oncol. 2016;34:684–690. doi: 10.1200/JCO.2015.63.9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters L.J., O’Sullivan B., Giralt J. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: Results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 4.Wuthrick E.J., Zhang Q., Machtay M. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol. 2015;33:156–164. doi: 10.1200/JCO.2014.56.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong T.S., Tomé W.A., Harari P.M. Heterogeneity in head and neck IMRT target design and clinical practice. Radiother Oncol. 2012;103:92–98. doi: 10.1016/j.radonc.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasch C., Steenbakkers R., Van Herk M. Target definition in prostate, head, and neck. Semin Radiat Oncol. 2005;15:136–145. doi: 10.1016/j.semradonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Grégoire V., Evans M., Le Q.T. Delineation of the primary tumour clinical target volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAG-KHT, LPRHHT, NCIC CTG, NCRI, NRG oncolog. Radiother Oncol. 2018;126:3–24. doi: 10.1016/j.radonc.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Campbell S., Poon I., Markel D. Evaluation of microscopic disease in oral tongue cancer using whole-mount histopathologic techniques: Implications for the management of head-and-neck cancers. Int J Radiat Oncol Biol Phys. 2012;82:574–581. doi: 10.1016/j.ijrobp.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Yuen P.W., Lam K.Y., Chan A.C.L., Wei W.I., Lam L.K. Clinicopathological analysis of local spread of carcinoma of the tongue. Am J Surg. 1998;175:242–244. doi: 10.1016/s0002-9610(97)00282-1. [DOI] [PubMed] [Google Scholar]
- 10.Fleury B., Thariat J., Barnoud R. Microscopic extensions of head and neck squamous cell carcinomas: Impact for clinical target volume definition. Cancer Radiother. 2014;18:666–671. doi: 10.1016/j.canrad.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Pötter R., Tanderup K., Kirisits C. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018;9:48–60. doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Ruysscher D., Faivre-Finn C., Moeller D. European Organization for Research and Treatment of Cancer (EORTC) recommendations for planning and delivery of high-dose, high precision radiotherapy for lung cancer. Radiother Oncol. 2017;124:1–10. doi: 10.1016/j.radonc.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Giraud P., Antoine M., Larrouy A. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys. 2000;48:1015–1024. doi: 10.1016/s0360-3016(00)00750-1. [DOI] [PubMed] [Google Scholar]
- 14.Garden A.S., Beadle B.M., Gunn G.B. 5th ed. Wolters Kluwer Health; Philadelphia, PA: 2018. Radiotherapy for head and neck cancers. Indications and techniques. [Google Scholar]
- 15.Siu L.L., Waldron J.N., Chen B.E. Effect of standard radiotherapy with cisplatin vs accelerated radiotherapy with panitumumab in locoregionally advanced squamous cell head and neck carcinoma. JAMA Oncol. 2017;3:220. doi: 10.1001/jamaoncol.2016.4510. [DOI] [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov. Radiation therapy with cisplatin or cetuximab in treating patients with oropharyngeal cancer (RTOG 1016). Available at: https://clinicaltrials.gov/ct2/show/NCT01302834. Accessed February 28, 2019.
- 17.Nichols A.C., Yoo J., Hammond J.A. Early-stage squamous cell carcinoma of the oropharynx: Radiotherapy vs. Trans-Oral Robotic Surgery (ORATOR) – study protocol for a randomized phase II trial. BMC Cancer. 2013;13:133. doi: 10.1186/1471-2407-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov. Randomized phase II trial of transoral endoscopic head and neck surgery followed by risk-based IMRT and weekly cisplatin versus IMRT and weekly cisplatin for HPV negative oropharynx cancer. Available at: https://www.clinicaltrials.gov/ct2/show/NCT01953952. Accessed February 28, 2019.
- 19.Lee N.Y., Terezakis S.A. Intensity-modulated radiation therapy. J Surg Oncol. 2008;97:691–696. doi: 10.1002/jso.21014. [DOI] [PubMed] [Google Scholar]
- 20.Guckenberger M., Andratschke N., Dieckmann K. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124:11–17. doi: 10.1016/j.radonc.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Navran A., Heemsbergen W., Janssen T. The impact of margin reduction on outcome and toxicity in head and neck cancer patients treated with image-guided volumetric modulated arc therapy (VMAT) Radiother Oncol. 2019;130:25–31. doi: 10.1016/j.radonc.2018.06.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.