Abstract
Purpose
To compare health-related quality of life (HRQOL) of high-dose-rate brachytherapy (HDRB) versus low dose-rate brachytherapy (LDRB) for localized prostate cancer in a multi-institutional phase 2 randomized trial.
Methods and Materials
Men with favorable-risk prostate cancer were randomized between monotherapy brachytherapy with either Iodine-125 LDRB to 144 Gy or single-fraction Iridium-192 HDRB to 19 Gy. HRQOL and urinary toxicity were recorded at baseline and at 1, 3, 6, and 12 months using the Expanded Prostate Cancer Index Composite (EPIC)-26 scoring and the International Prostate Symptom Score (IPSS). Independent samples t test and mixed effects modeling were performed for continuous variables. Time to IPSS resolution, defined as return to its baseline score ±5 points, was calculated using Kaplan-Meier estimator curves with the log-rank test. A multiple-comparison adjusted P value of ≤.05 was considered significant.
Results
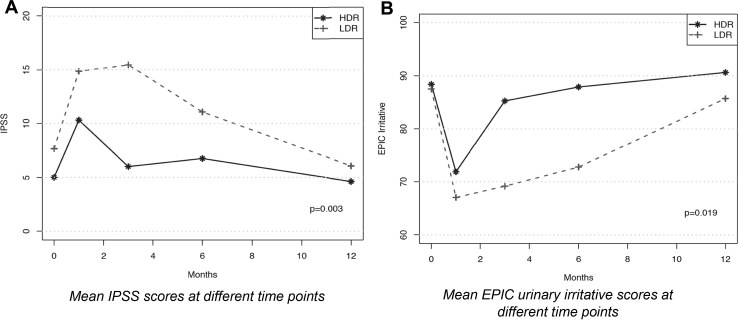
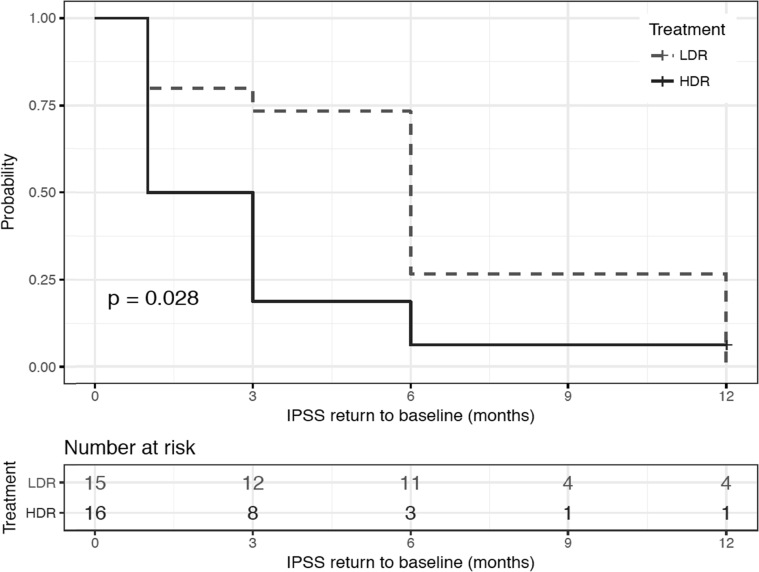
LDRB and HDRB were performed in 15 and 16 patients, respectively, for a total of 31 patients. At 3 months, patients treated with LDRB had a higher IPSS score (mean, 15.5 vs 6.0, respectively; P = .003) and lower EPIC urinary irritative score (mean, 69.2 vs 85.3, respectively; P = .037) compared with those who received HDRB. On repeated measures at 1, 3, 6, and 12 months, the IPSS (P = .003) and EPIC urinary irritative scores (P = .019) were significantly better in the HDR arm, translating into a lower urinary toxicity profile. There were no significant differences in the EPIC urinary incontinence, sexual, or bowel habit scores between the 2 groups at any measured time point. Time to IPSS resolution was significantly shorter in the HDRB group (mean, 2.0 months) compared with the LDRB group (mean, 6.0 months; P = .028).
Conclusions
HDRB monotherapy is a promising modality associated with a lower urinary toxicity profile and higher HRQOL in the first 12 months compared with LDRB.
Introduction
Low-dose-rate brachytherapy (LDRB) using a permanent seed implant is an effective definitive treatment for patients with localized low-risk and favorable intermediate-risk prostate cancer1, 2, 3, 4, 5, 6 with a prostate-specific antigen (PSA) progression-free survival (PFS) as high as 90% to 95% at 5 to 10 years.4, 5, 7 The results of the NRG Oncology/Radiation Therapy Oncology Group 0232 study were presented at the American Society for Radiation Oncology in 2016; the addition of external beam radiation therapy (EBRT) to LDRB did not result in improved PFS in a phase 3 randomized trial,8 suggesting that LDRB as monotherapy is an effective treatment in low-risk and favorable intermediate-risk prostate cancer.
High-dose-rate brachytherapy (HDRB) as a single modality is emerging as an alternative to LDRB with excellent outcomes as reported by retrospective and phase 2 studies.9, 10, 11, 12 Common problems associated with permanent seeds implant include discrepancy between planned and actual seed distribution, inability to correct seed position or to optimize the dose delivered once the seeds are in place,10 seed migration,13 and prostate volume changes during treatment, all of which are not relevant in HDRB. The main disadvantages of HDRB are monitoring and adjustment of catheters, inter- and intrafraction motion, and requirement for catheter and template fixation and locoregional anesthesia (spinal or epidural) if computed tomography (CT)-based planning is performed.10 Because a single radioactive source is used for many treatments, HDRB can be deployed in a cost-effective manner.10
Many studies have shown the feasibility and efficacy of HDRB as monotherapy in patients with intermediate risk-prostate cancer with 3- to 5-year PSA PFS as high as 88% to 100% for intermediate-risk prostate cancer at a median follow-up of 3 to 5 years.9, 10, 11, 14, 15, 16 Most of the longer-term efficacy data involve delivery of HDRB monotherapy in ≥4 fractions with more than 93% biochemical control at 5 years.11, 14, 17 To mimic the LDR experience by increasing patient convenience and avoiding hospitalization and immobilization, less-fractionated regimens with similar efficacy are emerging, although the follow-up is relatively short (1.6-4.4 years).9, 15, 18, 19, 20
HDRB as monotherapy was associated with decreased acute and late genitourinary toxicity rates compared with LDRB.9, 14, 21 Grills et al compared LDRB using Palladium-103 to HDR brachytherapy alone and found a significantly lower rate of acute grade 1 to 3 dysuria (67% vs 36%, P < .001) and urinary frequency/urgency (92% vs 54%, P < .001).21 Late grade 2 toxicity ranges between 2% and 10% and late grade 3 toxicity between 0% and 4%,9, 11, 15, 22 lower than rates reported in LDRB.4, 8, 23 A recent retrospective study compared HDRB monotherapy to LDRB with or without EBRT for localized prostate cancer. The authors reported similar PSA control rates at 5 years. As for toxicity, higher rate of grade ≥2 acute urinary toxicity was found in the LDRB (43%) group compared with the HDRB monotherapy group (12.3%, P < .0001). However, no difference was found in late grade ≥2 urinary toxicities between the 2 groups.12 A quality of life (QoL) study showed a return to baseline International Prostate Symptom Score (IPSS) levels at 12 weeks with HDRB,24 which is significantly quicker than with LDRB (for which return to baseline scores can take up to 12 months).
HDRB monotherapy is therefore a promising new treatment modality for patients with favorable-risk prostate cancer. It appears less toxic in the urinary domain and equivalent in terms of tumor control. To establish the role of HDRB as monotherapy, we conducted a pilot phase 2 randomized study evaluating the differences in health-related QoL (HRQOL) in the urinary domain between LDRB and HDRB as primary objective. Local tumor control and biochemical failure will be evaluated as secondary objectives with a repeat prostate biopsy at 36 months and serial PSA measurements, respectively, once adequate follow-up has been reached.
Methods and Materials
Study design
This is a phase 2, multi-institutional, randomized pilot study comparing LDRB using Iodine-125 seed implant to a total dose of 144 Gy against HDRB single-fraction 19-Gy monotherapy in patients with low- and favorable intermediate-risk prostate cancer. The clinical trial was registered on ClinicalTrials.gov (NCT02628041) and approved by the 3 participating institutions' research ethics boards.
Study objectives
The specific goals of the pilot study were the following:
-
1.
Accrual of 30 patients across 3 institutions in <12 months
-
2.
Complete follow-up in at least 90% of all accrued patients
-
3.
Less than 5% of major deviations on dose-volume constraints
-
4.
At least 80% compliance in filling out HRQOL questionnaires
The pilot study was deemed successful if all those criteria were met.
The primary objective was to evaluate the differences in HRQOL in the urinary domain between patients treated with LDRB and HDRB at 3 months using the Expanded Prostate Cancer Index Composite (EPIC)-26 short form.25 The 3-month cut-off was chosen as the primary endpoint based on several studies revealing peak urinary symptoms at 3 to 6 months.26, 27, 28, 29, 30, 31, 32 The EPIC score is the most used HRQOL instrument; it has been validated in men with prostate cancer33 and shown to be a robust tool with good psychometrics.34, 35, 36 The validated French version of the EPIC was used in French-Canadian patients.37 Secondary objectives were to compare HRQOL in the urinary, bowel, and sexual domains to evaluate differences in urinary function using the IPSS and to determine the time to IPSS resolution.
Selection criteria
Eligible patients had histologically confirmed adenocarcinoma of the prostate, clinical stage T1c-T2c, with a Gleason score ≤7 (3 + 4) and a PSA level <20 ng/mL. All patients had to be medically fit for brachytherapy with a prostate volume ≤60 mL as determined by ultrasound, CT, or magnetic resonance imaging and pretreatment IPSS ≤20. Informed consent was obtained from all patients. Exclusion criteria were clinical stage T3-T4, Gleason 7 (4 + 3), PSA >20 ng/mL, evidence of nodal or distant metastases, previous pelvic radiation therapy, previous transurethral resection of the prostate, use of androgen deprivation therapy, and connective tissue or inflammatory bowel disease. Subjects were randomized to 1 of the 2 treatment arms using block randomization. The use of α reductase inhibitors was not allowed within 2 weeks of randomization and use after the procedure was not regulated. A washout period of 2 weeks was required before randomization.
Baseline evaluation included a physical examination with digital rectal exam and performance status, pretreatment PSA, assessment of baseline toxicity using Common Terminology Criteria for Adverse Events (CTCAE) version 4.0, and completion of the IPSS and EPIC-26 questionnaires.25
Treatment
The implant procedure was done under spinal, epidural, or general anesthesia in the lithotomy position. The urethra was identified with a Foley catheter or aerated gel urethrogram.
Low-dose-rate brachytherapy
LDRB preplanning and intraoperative planning techniques were performed using Iodine-125 loose or stranded seeds to a total dose of 144 Gy under transrectal ultrasound (TRUS) as per institutional standards.38, 39, 40 The planning target volume (PTV) was defined as the prostate plus a 0 to 3 mm uniform margin. The optimized plan covered the PTV with a minimal peripheral dose of 144 Gy. The prostate volume receiving 150% (V150) and 200% (V200) of the prescription dose were to cover less than two-thirds and one-third of prostate volume, respectively. The urethra V150 was planned to be 0 mL. A CT scan was performed 30 days (D30) after the implant to evaluate implant quality and dosimetry. The prostate and rectum from the anal verge to the rectosigmoid junction were contoured and seed localization was performed. The dosimetry 30 days after the implant was considered satisfactory if the radiation dose delivered to 90% of the prostate volume (D90) was ≥130 Gy.41
High-dose-rate brachytherapy
Afterloading needles were placed under TRUS guidance. CT- or ultrasound-based brachytherapy treatment planning was performed as per institutional standards. The PTV was defined as the prostate gland. The rectum, bladder (for CT-based brachytherapy), and urethra were contoured. The prescription dose was 19 Gy in a single fraction. The dosimetry objectives were the following:
-
1.
Prostate D90 between 105% and 115%
-
2.
Prostate V150 ≤ 35%
-
3.
Prostate V200 ≤ 12%
-
4.
Urethra D10 < 115%
-
5.
Urethra maximum dose <120%
-
6.
Rectum V80 < 0.2 mL
-
7.
Rectum maximum dose <90%
Patient follow-up
Patients were assessed before treatment and after the procedure at 1, 3, 6, and 12 months. The EPIC-26, IPSS, and physician-reported toxicities using the CTCAE version 4 were obtained at each visit. PSA measurements were performed at baseline and every 3 months after the procedure.
Sample size
Given the lack of previous studies directly comparing LDRB and HDRB as monotherapy, power computations were completed by estimating expected effect sizes based on published data testing similar treatment protocols. Studies evaluating QoL for patients treated with EBRT and HDRB were used as a surrogate for sample size calculation. Six studies investigating the effect of LDRB26, 29, 32 and EBRT plus HDRB boost27, 30, 31 on urinary function, using the urinary domain of the EPIC instrument as an outcome, were selected. Raw change scores from baseline were calculated and converted to effect sizes (d), which were weighted using the study sample size and averaged by treatment modality (LDRB vs HDRB + EBRT). Results suggest that LDRB is associated with a large reduction in QoL related to urinary functioning (d = −0.81) at 3-month follow-up in comparison with HDR + EBRT (d = −0.19). Power computations using G*Power 3.1 software, a 2-tailed 5% α, and a standard 80% power level revealed that a total sample of 84 patients would be needed to detect these between-groups differences (−0.81 vs −0.19 = 0.62). With an anticipated attrition rate of 30% at 3 months (attrition rate range of 22%-33% in the 6 trials), our calculations indicate that a planned sample size of 84/(1 – 0.3) = 120 patients would be sufficient to detect the smaller toxicity of HDRB compared with LDRB on urinary functioning as assessed with EPIC at 3-month follow-up.
The study served as a pilot for the elaboration and conduct of the large ongoing Canadian Cancer Trials Group PR-19.
The primary endpoint of the large-scale trial is biochemical control; HRQOL is a secondary endpoint. The pilot trial was already enrolling when the decision was made to make local control the primary endpoint for the large-scale study.
Statistical analysis
Independent 2-sample t tests were performed for comparison of continuous variables (EPIC and IPSS) between the 2 treatment arms. The effects of treatment on repeated measurements of these continuous variables were assessed using mixed effects modeling. Estimator functions of time to IPSS resolution, defined as return to baseline score ±5, points was calculated using the Kaplan-Meier method and compared between covariate subgroups using the log-rank test. A multiple-comparison (FDR) adjusted P value of ≤.05 was considered significant. Treatment characteristics were also assessed using 2-sample t tests as appropriate. The associations between toxicities and treatment characteristics were evaluated using logistic regressions. CTCAE grade 0 or 1 was considered no toxicity, and toxicity was considered as grade ≥2. R version 3.3.2 2016 was used for the analyses.
Results
Between December 2015 and December 2016, 31 patients met the eligibility criteria and were randomized across 3 centers. The pilot study was deemed successful because all the specific pilot study goals (accrual, follow-up, major dose-volume histogram deviations, and compliance in filling out questionnaires) were met.
The patients' characteristics are described in Table 1. Median age at treatment was 64 years. LDRB was delivered to 15 patients and HDRB to 16 patients. The median baseline scores of IPSS and mean baseline scores of EPIC urinary incontinence, EPIC urinary irritative, EPIC bowel habits, and EPIC sexual function were not significantly different between groups. The prostate volume was significantly higher for the HDRB group compared to the LDRB group (median, 54.3 vs 40.7 mL; P = .016), whereas the number of needles was significantly lower (median, 17 vs 21; P = .0002). The treatment characteristics for LDRB and HDRB are detailed in Tables 2 and 3, respectively. TRUS-based treatment planning was exclusively used in all LDRB cases. In HDRB cases, TRUS-based treatment planning was used in 8 patients, and CT-based treatment planning was used in the remaining 8 patients as per institutional standard.
Table 1.
Patient characteristics
| Characteristics | LDRB group, n (%) | HDRB Group, n (%) |
Entire cohort, n (%) | P value |
|---|---|---|---|---|
| No. of patients | 15 (48.4) | 16 (51.6) | 31 | |
| Median age (IQR) | 63 (61-68) | 66 (61-72) | 64 (61-70) | .59∗ |
| ECOG performance status | 0 | 0 | 0 | 1∗ |
| Pretreatment PSA median (IQR) | 5.37 (4.9-7.8) | 6.35 (4.9-8.7) | 5.6 (4.8-8.3) | .4∗ |
| Gleason score | .6∗ | |||
| 6 | 5 (33.3) | 7 (43.8) | 12 (38.7) | |
| 7 | 10 (66.7) | 9 (56.3) | 19 (61.3) | |
| Clinical stage | .7∗ | |||
| T1c | 12 (80) | 12 (75) | 24 (77.4) | |
| T2a | 3 (20) | 4 (25) | 7 (22.6) | |
| Pretreatment IPSS, median (IQR) | 8 (4-10) | 5 (1-8) | 7 (2-9) | .12∗ |
| Pretreatment EPIC urinary incontinence, mean (range) | 93.6 | 97.3 | 95.3 | .35† |
| Pretreatment EPIC urinary Irritative, mean (range) |
89.3 | 90.6 | 89.9 | .7† |
| Pretreatment EPIC sexual, mean (range) | 70.4 | 63.6 | 67.1 | .5† |
| Pretreatment EPIC bowel, median (IQR) | 97.2 | 97.1 | 97.2 | .9† |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; EPIC = Expanded Prostate Cancer Index Composite; HDRB = high-dose-rate brachytherapy; IQR = interquartile range; LDRB = low-dose-rate brachytherapy; PSA = prostate specific antigen.
P value calculated using Kruskal-Wallis test.
P value calculated using Student t test.
Table 2.
Treatment characteristics for LDRB
| Characteristics | Median (range) |
|---|---|
| No. of needles | 21 (17-24) |
| No. of sources | 57 (40-76) |
| Prostate volume, mL | 40.7 (25-48) |
| Source activity, mCi | 0.59 (0.4-0.6) |
| Preplanning/intraoperative prostate D90, Gy∗ | 176.8 (159.5-198.7) |
| Preplanning/intraoperative prostate V100† | 99% (96.7%-100%) |
| Preplanning/intraoperative prostate V150 | 62.6% (44.6%-77.5%) |
| Preplanning/intraoperative prostate V200 | 28.9% (18.6%-44.7%) |
| Preplanning/intraoperative urethra D5 | 91.5% (84.4%-110%) |
| Preplanning/intraoperative urethra V150, mL | 0 (0-17.5) |
| Prostate D90 on day 30 postimplant CT, Gy | 160.5 (138-190) |
| Rectum V100 on day 30 postimplant CT, mL | 0.2 (0-0.3) |
Abbreviations: CT = computed tomography; LDRB = low-dose-rate brachytherapy.
D90: Radiation dose delivered to 90% of the prostate volume.
V100: Volume of the prostate receiving 100% of the prescription dose.
Table 3.
Treatment characteristics for HDRB
| Characteristics | Median (range) |
|---|---|
| No. of needles median | 17 (17-20) |
| Prostate volume median, mL | 54.3 (35.5-143) |
| Prostate D90 median | 107% (100.5%-158%) |
| Prostate V100 median | 96.3% (94.2%-98.4%) |
| Prostate V150 median | 30.3% (17.3%-42.5%) |
| Prostate V200 median | 9.1% (6.8%-14.6%) |
| Urethra D10 median | 112% (103%-114.7%) |
| Urethra maximum dose median | 115.7% (107.4%-120.5%) |
| Rectum V80 median, mL | 0.04 (0-0.5) |
| Rectum maximum dose median | 83.7% (69.5%-96.8%) |
Abbreviation: hDRB = high-dose-rate brachytherapy
At 3 months, patients treated with LDRB had a higher IPSS score (median, 14 vs 5 respectively; adjusted P = .001) and lower EPIC urinary irritative score (mean, 69.2 vs 85.3 respectively; adjusted P = .037) compared with patients who received HDRB. On repeated measures at 1, 3, 6, and 12 months, the IPSS (P = .003) and EPIC urinary irritative scores (P = .011) were significantly better in the HDR arm, translating into a lower urinary toxicity profile, as shown in Figure 1. There were no significant differences in the EPIC urinary incontinence, sexual function, or bowel habits scores between the 2 groups at any measured time point. Time to IPSS resolution was significantly shorter in the HDRB group (median, 2 months) compared with the LDRB group (median, 6 months; P = .028; Fig 2). As for the physician-reported grade ≥2 toxicities using CTCAE version 4, no significant difference was found between the 2 treatment arms in regard to urinary, bowel, and sexual toxicities. The grade ≥2 urinary toxicity rates of LDRB and HDRB at different time points are detailed in Table 4. No grade 3 toxicity was reported.
Figure 1.
Health-related quality of life differences between low dose-rate brachytherapy and high-dose rate brachytherapy.
Figure 2.
Time to International Prostate Symptom Score resolution illustrated using Kaplan-Meier estimator curves.
Table 4.
CTCAE version 4 urinary toxicity rates at different time points
| Grade ≥2 toxicity | At 1 mo (%) | At 3 mo (%) | At 6 mo (%) | At 12 mo (%) |
|---|---|---|---|---|
| Dysuria | ||||
| LDR | 12.5 | 6.2 | 0 | 0 |
| HDR | 0 | 0 | 0 | 0 |
| Increased frequency | ||||
| LDR | 25 | 0 | 0 | 6.2 |
| HDR | 13.3 | 7.7 | 0 | 0 |
| Urgency | ||||
| LDR | 0 | 6.2 | 0 | 0 |
| HDR | 0 | 0 | 6.7 | 0 |
| Urinary obstruction | ||||
| LDR | 18.8 | 12.5 | 0 | 0 |
| HDR | 13.3 | 0 | 0 | 0 |
Abbreviations: CTCAE = Common Terminology Criteria for Adverse Events; HDRB = high-dose rate brachytherapy; LDRB = low dose-rate brachytherapy.
Discussion
Increasing evidence supports the use of HDRB as monotherapy in localized prostate cancer. Indeed, HDRB offers many advantages over LDRB: It allows optimization of both source dwell times and positions, providing better target coverage while sparing the normal tissue, and limits radiation exposure for the patient and the staff.10 HDRB is generally considered to have less acute and late urinary toxicity compared to LDRB.9, 14, 21 Indeed, the results of our study revealed improved HRQOL scores with HDBR in the acute and long-term setting compared with LDRB.
A total of 31 patients were enrolled and treated with either LDRB or single-fraction 19-Gy HDRB. No differences in patients' characteristics were reported in the 2 arms. The prostate volume at the time of brachytherapy was significantly higher in the HDRB group (median, 54.3 vs 40.7 mL; P = .016), which could be explained by the heterogeneity in the brachytherapy imaging modality used considering prostate volume is better defined on TRUS.42 TRUS-based treatment planning was exclusively used in all LDRB cases, whereas both TRUS- and CT-based treatment planning was used for HDRB as per institutional standard. In HDRB cases, TRUS-based treatment planning was used in 8 patients, and CT-based treatment planning was used in the remaining 8 patients as per institutional standard. In HDRB, prostate volume is not a limiting factor as long as the dosimetric constraints are achieved.43 The number of needles used for LDRB was higher than in HDRB, which is inherent to the LDRB technique.
HDRB was associated with improved EPIC urinary irritative and IPSS scores at 3 months (P = .037 and P = .003, respectively) and on repeated measures at 1, 3, 6, and 12 months (P = .019 and P = .003, respectively). There were no significant differences in the EPIC urinary incontinence, sexual function, or bowel habits scores between the 2 groups at any measured time point. Time to IPSS resolution was significantly shorter in the HDRB group (mean, 2.0 months) compared with the LDRB group (mean, 6.0 months; P = .028). Acute urinary toxicity is not trivial; presence of acute urinary toxicity in patients treated with LDRB has been correlated with late urinary toxicity44 and was associated with decreased QoL, leading to increased psychologic distress.45 Acute urinary retention after prostate brachytherapy can lead to several side effects, such as prolonged catheterization, urethral and suprapubic pain, bleeding, loss of dignity, loss of job or absence from school, lack of sexual intercourse, pericatheter leakage of urine, and recurrent urinary tract infection.46 The management of urinary obstruction ranges from α-blockade to prolonged catheterization and surgical intervention, including transurethral resection of the prostate, the latter resulting in diminished QoL.47, 48 The psychologic and emotional burden of acute urinary retention and catheterization significantly influenced health-related QoL.49
When evaluating the dosimetric characteristics of LDRB compared with HDRB, dose distributions were much more homogeneous, and both urethra and rectum received significantly less dose in patients treated with the HDR technique in a randomized controlled trial.50 Furthermore, the dose to the bladder neck, which has been shown to correlate with grade ≥2 acute and late urinary toxicity in patients treated with LDRB,23 could be significantly reduced with HDRB. In addition, the dose and duration of radiation delivery have a significant impact on toxicity. Indeed, the higher dose delivered with LDRB and the long duration of treatment (5 half-lives of Iodine-125 is 300 days) could explain why the return to baseline urinary toxicity can take up to 12 months.44
A phase 2 randomized study was presented at the European Society for Radiotherapy and Oncology 2018, reporting on the acute toxicity of LDRB vs HDRB.51 A total of 100 patients were randomized between LDRB to 145 Gy and single-fraction HDRB of 19 Gy and 21 Gy in 48 and 2 patients, respectively. After a median follow-up of 23 months, 4 patients had a biochemical relapse with local failure, 2 patients in each arm. Acute grade 2 and grade 3 genitourinary toxicity occurred in 26% and 72% (P < .001) and 2% and 4% of patients treated with HDRB and LDRB, respectively.51 In contrast, grade 2 urinary, bowel, and sexual toxicities were lower in both arms; no difference was found between the 2 treatment arms, and no grade 3 or 4 toxicity was reported in our present study. As for the mean IPSS at 3 months, it was significantly lower in the HDRB arm (8.9 vs 15.9, P = .0004), although this difference disappeared at 12 months in the latter study.51 In our study, the IPSS was significantly lower in the HDRB group at all time points, including the 12-month mark.
Morton et al reported the clinical outcomes of 13.5 × 2 fractions and 19-Gy single-fraction HDRB.52 During the first year, the 2-fraction arm had a higher occurrence of grade 2 erectile dysfunction and higher IPSS scores. The mean EPIC urinary and sexual scores were significantly lower in the 2-fraction arm, suggesting that 19-Gy single-fraction treatment is associated with reduced urinary and sexual toxicities in the first year. Eight of 87 patients developed biopsy-proven local recurrence with minimal treatment effect at a median time of 36 months, and most patients had more aggressive (Gleason 4 pattern) disease on repeat posttreatment biopsy. The mean dose at the recurrence site was 29.1 Gy, and the dose to 98% and to 90% of the recurrence was 21.6 Gy and 23.2 Gy, respectively.
The 5-year outcomes of 19-Gy single-fraction HDRB were recently presented at the American Brachytherapy Society. A total of 68 patients with low- and intermediate-risk prostate cancer were treated with 19-Gy HDRB at a single institution. After a median follow-up of 3.9 years, acute and late grade 2 genitourinary toxicity rates were 11.8% and 14.7% with no grade 3 toxicity. The 5-year biochemical control and cause-specific survival rates were 73.9% and 100%, respectively. Local failure proven by biopsy was 19%, occurring at a median interval of 3.9 years.53
The most mature data on the efficacy of 19-Gy single-fraction HDRB were reported by Prada et al. After a median follow-up of 6 years, the overall survival, tumor-free survival, and actuarial biochemical control were 90%, 88%, and 66%, respectively.54
The rationale behind using a single-fraction treatment is to avoid the HDRB drawbacks of hospitalization and needle displacement and to promote patient convenience. As for the optimal single-fraction dose, 19 Gy seems to be well tolerated, and doses ≥20 Gy have been shown to significantly increase toxicity.55 The biological effective dose using an α/β ratio of 1.5 of 19-Gy single-fraction HDRB is 260 Gy,56 which was shown to produce at least the same benefit as that given by the 4 × 9.5 Gy and 2 × 13.5 Gy schemes57, 58 and to correspond biologically to approximately 90 Gy administered at 2 Gy/fraction.15
Despite the low toxicity rates, the biochemical and local failure rates are concerning and call into question the efficacy of 19-Gy single-fraction HDRB. These troubling results represent the experience of single institutions with a relatively small number of patients. As for the survival outcomes of this study, local and biochemical control will be assessed with a repeat biopsy at 36 months and serial PSA measurements once adequate follow-up is reached.
This study served as the pilot study for the CCTG phase 2 trial evaluating prostate cancer control defined as absolute PSA nadir at 48 months of LDRB and HDRB (NCT02960087). To overcome the poor clinical outcomes so far reported with 19-Gy single-fraction HDR, the CCTG trial required a mandatory dose escalation to 120% to 150% of the prescription dose to the dominant intraprostatic lesion defined on magnetic resonance imaging.58 The trial is currently enrolling patients across numerous Canadian institutions and will provide robust evidence on the clinical outcomes of 19-Gy single-fraction HDRB.
Conclusions
Single-fraction 19-Gy HDRB is less toxic in the first year with better HRQOL compared with LDRB in men with favorable-risk prostate cancer. While waiting for the results of the CCTG phase 2 trial, this study provides preliminary data on the toxicity and HRQOL of 19-Gy single-fraction HDRB. Repeat biopsy at 36 months and absolute PSA nadir will be reported after adequate follow-up, providing short-term tumor control outcomes. The authors recommend abstaining from delivering 19-Gy single-fraction HDRB outside the context of a clinical trial.
Acknowledgments
The authors would like to express their deepest appreciation to all the nursing and research staff of Centre hospitalier de l’Université de Montréal, CHU de Quebec, and Sunnybrook. This project would not have been possible without their support.
Footnotes
Sources of support: This work was supported by AbbVie- CARO Uro-Oncologic Radiation Awards 2015, Canada. The funding source had no role in study design, data collection, data interpretation, data analysis, or writing of this report.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Bowes D., Crook J. A critical analysis of the long-term impact of brachytherapy for prostate cancer: A review of the recent literature. Curr Opin Urol. 2011;21:219–224. doi: 10.1097/MOU.0b013e3283449d52. [DOI] [PubMed] [Google Scholar]
- 2.Hinnen K.A., Battermann J.J., van Roermund J.G. Long-term biochemical and survival outcome of 921 patients treated with I-125 permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2010;76:1433–1438. doi: 10.1016/j.ijrobp.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky M.J., Kuban D.A., Levy L.B. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys. 2007;67:327–333. doi: 10.1016/j.ijrobp.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 4.Kittel J.A., Reddy C.A., Smith K.L. Long-term efficacy and toxicity of low-dose-rate (1)(2)(5)i prostate brachytherapy as monotherapy in low-, intermediate-, and high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2015;92:884–893. doi: 10.1016/j.ijrobp.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 5.Martinez E., Daidone A., Gutierrez C. Permanent seed brachytherapy for clinically localized prostate cancer: Long-term outcomes in a 700 patient cohort. Brachytherapy. 2015;14:166–172. doi: 10.1016/j.brachy.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester J.E., Grimm P.D., Wong J. Fifteen-year biochemical relapse-free survival, cause-specific survival, and overall survival following I(125) prostate brachytherapy in clinically localized prostate cancer: Seattle experience. Int J Radiat Oncol Biol Phys. 2011;81:376–381. doi: 10.1016/j.ijrobp.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Keyes M., Crook J., Morris W.J. Canadian prostate brachytherapy in 2012. Can Urol Assoc J. 2013;7:51–58. doi: 10.5489/cuaj.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prestidge B.R., Winter K., Sanda M. Initial report of NRG Oncology/RTOG 0232: A phase 3 study comparing combined external beam radiation and transperineal interstitial permanent brachytherapy with brachytherapy alone for selected patients with intermediate-risk prostatic carcinoma. Int J Radiat Oncol Biol Phys. 2016;96:S4. [Google Scholar]
- 9.Barkati M., Williams S.G., Foroudi F. High-dose-rate brachytherapy as a monotherapy for favorable-risk prostate cancer: A phase II trial. Int J Radiat Oncol Biol Phys. 2012;82:1889–1896. doi: 10.1016/j.ijrobp.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Demanes D.J., Ghilezan M.I. High-dose-rate brachytherapy as monotherapy for prostate cancer. Brachytherapy. 2014;13:529–541. doi: 10.1016/j.brachy.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Demanes D.J., Martinez A.A., Ghilezan M. High-dose-rate monotherapy: Safe and effective brachytherapy for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:1286–1292. doi: 10.1016/j.ijrobp.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki H., Masui K., Suzuki G. High-dose-rate brachytherapy monotherapy versus low-dose-rate brachytherapy with or without external beam radiotherapy for clinically localized prostate cancer. Radiother Oncol. 2019;132:162–170. doi: 10.1016/j.radonc.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Hathout L., Donath D., Moumdjian C. Analysis of seed loss and pulmonary seed migration in patients treated with virtual needle guidance and robotic seed delivery. Am J Clin Oncol. 2011;34:449–453. doi: 10.1097/COC.0b013e3181ec63c5. [DOI] [PubMed] [Google Scholar]
- 14.Martinez A.A., Demanes J., Vargas C. High-dose-rate prostate brachytherapy: An excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am J Clin Oncol. 2010;33:481–488. doi: 10.1097/COC.0b013e3181b9cd2f. [DOI] [PubMed] [Google Scholar]
- 15.Prada P.J., Jimenez I., Gonzalez-Suarez H. High-dose-rate interstitial brachytherapy as monotherapy in one fraction and transperineal hyaluronic acid injection into the perirectal fat for the treatment of favorable stage prostate cancer: Treatment description and preliminary results. Brachytherapy. 2012;11:105–110. doi: 10.1016/j.brachy.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Ghadjar P., Keller T., Rentsch C.A. Toxicity and early treatment outcomes in low- and intermediate-risk prostate cancer managed by high-dose-rate brachytherapy as a monotherapy. Brachytherapy. 2009;8:45–51. doi: 10.1016/j.brachy.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Yoshioka Y., Konishi K., Sumida I. Monotherapeutic high-dose-rate brachytherapy for prostate cancer: Five-year results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int J Radiat Oncol Biol Phys. 2011;80:469–475. doi: 10.1016/j.ijrobp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Ghilezan M., Martinez A., Gustason G. High-dose-rate brachytherapy as monotherapy delivered in two fractions within one day for favorable/intermediate-risk prostate cancer: Preliminary toxicity data. Int J Radiat Oncol Biol Phys. 2012;83:927–932. doi: 10.1016/j.ijrobp.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Zamboglou N., Tselis N., Baltas D. High-dose-rate interstitial brachytherapy as monotherapy for clinically localized prostate cancer: Treatment evolution and mature results. Int J Radiat Oncol Biol Phys. 2013;85:672–678. doi: 10.1016/j.ijrobp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Hoskin P., Rojas A., Lowe G. High-dose-rate brachytherapy alone for localized prostate cancer in patients at moderate or high risk of biochemical recurrence. Int J Radiat Oncol Biol Phys. 2012;82:1376–1384. doi: 10.1016/j.ijrobp.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Grills I.S., Martinez A.A., Hollander M. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol. 2004;171:1098–1104. doi: 10.1097/01.ju.0000113299.34404.22. [DOI] [PubMed] [Google Scholar]
- 22.Rogers C.L., Alder S.C., Rogers R.L. High dose brachytherapy as monotherapy for intermediate risk prostate cancer. J Urol. 2012;187:109–116. doi: 10.1016/j.juro.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 23.Hathout L., Folkert M.R., Kollmeier M.A. Dose to the bladder neck is the most important predictor for acute and late toxicity after low-dose-rate prostate brachytherapy: Implications for establishing new dose constraints for treatment planning. Int J Radiat Oncol Biol Phys. 2014;90:312–319. doi: 10.1016/j.ijrobp.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komiya A., Fujiuchi Y., Ito T. Early quality of life outcomes in patients with prostate cancer managed by high-dose-rate brachytherapy as monotherapy. Int J Urol. 2013;20:185–192. doi: 10.1111/j.1442-2042.2012.03125.x. [DOI] [PubMed] [Google Scholar]
- 25.Szymanski K.M., Wei J.T., Dunn R.L. Development and validation of an abbreviated version of the Expanded Prostate Cancer Index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urol. 2010;76:1245–1250. doi: 10.1016/j.urology.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ash D., Bottomley D., Al-Qaisieh B. A prospective analysis of long-term quality of life after permanent I-125 brachytherapy for localised prostate cancer. Radiother Oncol. 2007;84:135–139. doi: 10.1016/j.radonc.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Choudhury A., Arthur C., Malik J. Patient-reported outcomes and health-related quality of life in prostate cancer treated with a single fraction of high dose rate brachytherapy combined with hypofractionated external beam radiotherapy. Clin Oncol (R Coll Radiol) 2014;26:661–667. doi: 10.1016/j.clon.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Vigneault E., Foster W., Aubin S. Comparison of permanent seed implant monotherapy with combined EBRT plus HDR boost in intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2014;90:S432. [Google Scholar]
- 29.Ferrer M., Suarez J.F., Guedea F. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:421–432. doi: 10.1016/j.ijrobp.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Morton G.C., Loblaw D.A., Sankreacha R. Single-fraction high-dose-rate brachytherapy and hypofractionated external beam radiotherapy for men with intermediate-risk prostate cancer: Analysis of short- and medium-term toxicity and quality of life. Int J Radiat Oncol Biol Phys. 2010;77:811–817. doi: 10.1016/j.ijrobp.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 31.Pinkawa M., Fischedick K., Treusacher P. Dose-volume impact in high-dose-rate Iridium-192 brachytherapy as a boost to external beam radiotherapy for localized prostate cancer—A phase II study. Radiother Oncol. 2006;78:41–46. doi: 10.1016/j.radonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Wei J.T., Dunn R.L., Sandler H.M. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 33.Wei J.T., Dunn R.L., Litwin M.S. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urol. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 34.Hamoen E.H., De Rooij M., Witjes J.A. Measuring health-related quality of life in men with prostate cancer: A systematic review of the most used questionnaires and their validity. Urol Oncol. 2015;33 doi: 10.1016/j.urolonc.2013.10.005. 69 e19-69.e28. [DOI] [PubMed] [Google Scholar]
- 35.Rnic K., Linden W., Tudor I. Measuring symptoms in localized prostate cancer: A systematic review of assessment instruments. Prostate Cancer Prostatic Dis. 2013;16:111–122. doi: 10.1038/pcan.2013.1. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt S., Garin O., Pardo Y. Assessing quality of life in patients with prostate cancer: A systematic and standardized comparison of available instruments. Qual Life Res. 2014;23:2169–2181. doi: 10.1007/s11136-014-0678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigneault E., Savard J., Savard M.H. Validation of the French-Canadian version of the Expanded Prostate Cancer Index Composite (EPIC) in a French-Canadian population. Can Urol Assoc J. 2017;11:404–410. doi: 10.5489/cuaj.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chira C., Delouya G., Larrivee S. Prostate volume changes during permanent seed brachytherapy: An analysis of intra-operative variations, predictive factors and clinical implication. Radiat Oncol. 2013;8:177. doi: 10.1186/1748-717X-8-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaudet M., Vigneault E., Aubin S. Dose escalation to the dominant intraprostatic lesion defined by sextant biopsy in a permanent prostate I-125 implant: A prospective comparative toxicity analysis. Int J Radiat Oncol Biol Phys. 2010;77:153–159. doi: 10.1016/j.ijrobp.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 40.Lawton C.A., Hunt D., Lee W.R. Long-term results of a phase II trial of ultrasound-guided radioactive implantation of the prostate for definitive management of localized adenocarcinoma of the prostate (RTOG 98-05) Int J Radiat Oncol Biol Phys. 2011;81:1–7. doi: 10.1016/j.ijrobp.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis B.J., Horwitz E.M., Lee W.R. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012;11:6–19. doi: 10.1016/j.brachy.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Morton G.C. Prostate high-dose-rate brachytherapy: Transrectal ultrasound based planning, a technical note. Pract Radiat Oncol. 2015;5:238–240. doi: 10.1016/j.prro.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Vigneault E., Mbodji K., Beaudet M.E. Does prostate volume has an impact on biochemical failure in patients with localized prostate cancer treated with HDR boost? Radiother Oncol. 2016;121:304–309. doi: 10.1016/j.radonc.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Keyes M., Miller S., Moravan V. Predictive factors for acute and late urinary toxicity after permanent prostate brachytherapy: Long-term outcome in 712 consecutive patients. Int J Radiat Oncol Biol Phys. 2009;73:1023–1032. doi: 10.1016/j.ijrobp.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Hervouet S., Savard J., Simard S. Psychological functioning associated with prostate cancer: Cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30:474–484. doi: 10.1016/j.jpainsymman.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Ikuerowo S.O., Ogunade A.A., Ogunlowo T.O. The burden of prolonged indwelling catheter after acute urinary retention in Ikeja-Lagos, Nigeria. BMC Urol. 2007;7:16. doi: 10.1186/1471-2490-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kollmeier M.A., Stock R.G., Cesaretti J. Urinary morbidity and incontinence following transurethral resection of the prostate after brachytherapy. J Urol. 2005;173:808–812. doi: 10.1097/01.ju.0000152698.20487.0e. [DOI] [PubMed] [Google Scholar]
- 48.Merrick G.S., Butler W.M., Wallner K.E. Effect of transurethral resection on urinary quality of life after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2004;58:81–88. doi: 10.1016/s0360-3016(03)00776-4. [DOI] [PubMed] [Google Scholar]
- 49.Roeloffzen E.M., Hinnen K.A., Battermann J.J. The impact of acute urinary retention after iodine-125 prostate brachytherapy on health-related quality of life. Int J Radiat Oncol Biol Phys. 2010;77:1322–1328. doi: 10.1016/j.ijrobp.2009.06.083. [DOI] [PubMed] [Google Scholar]
- 50.Major T., Polgar C., Jorgo K. Dosimetric comparison between treatment plans of patients treated with low-dose-rate vs high-dose-rate interstitial prostate brachytherapy as monotherapy: Initial findings of a randomized clinical trial. Brachytherapy. 2017;16:608–615. doi: 10.1016/j.brachy.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Agoston P., Jorgo K., Fröhlich G. HDR brachytherapy in one fraction vs LDR brachytherapy in the treatment of localized prostate cancer. Early results. Radiother Oncol. 2018;127(Suppl 1):S182–S183. [Google Scholar]
- 52.Morton G., Chung H.T., McGuffin M. Prostate high dose-rate brachytherapy as monotherapy for low and intermediate risk prostate cancer: Early toxicity and quality-of life results from a randomized phase II clinical trial of one fraction of 19Gy or two fractions of 13.5Gy. Radiother Oncol. 2017;122:87–92. doi: 10.1016/j.radonc.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Krauss D., Ye H., Martinez A. 5-year outcomes of a single institution prospective trial of 19 Gy single fraction high dose rate brachytherapy for low and intermediate risk prostate cancer. Brachytherapy. 2018;17:S41. doi: 10.1016/j.ijrobp.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Prada P.J., Cardenal J., Blanco A.G. High-dose-rate interstitial brachytherapy as monotherapy in one fraction for the treatment of favorable stage prostate cancer: Toxicity and long-term biochemical results. Radiother Oncol. 2016;119:411–416. doi: 10.1016/j.radonc.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Hoskin P., Rojas A., Ostler P. High-dose-rate brachytherapy alone given as two or one fraction to patients for locally advanced prostate cancer: Acute toxicity. Radiother Oncol. 2014;110:268–271. doi: 10.1016/j.radonc.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 56.Yoshioka Y., Suzuki O., Otani Y. High-dose-rate brachytherapy as monotherapy for prostate cancer: Technique, rationale and perspective. J Contemp Brachytherapy. 2014;6:91–98. doi: 10.5114/jcb.2014.42026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mavroidis P., Milickovic N., Cruz W.F. Comparison of different fractionation schedules toward a single fraction in high-dose-rate brachytherapy as monotherapy for low-risk prostate cancer using 3-dimensional radiobiological models. Int J Radiat Oncol Biol Phys. 2014;88:216–223. doi: 10.1016/j.ijrobp.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Mendez L.C., Ravi A., Chung H. Pattern of relapse and dose received by the recurrent intraprostatic nodule in low- to intermediate-risk prostate cancer treated with single fraction 19 Gy high-dose-rate brachytherapy. Brachytherapy. 2018;17:291–297. doi: 10.1016/j.brachy.2017.10.001. [DOI] [PubMed] [Google Scholar]