Abstract
Purpose
Current delineation of the gross tumor volume (GTV) in esophageal cancer relies on computed tomography (CT) and combination with 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET). There is increasing interest in integrating magnetic resonance imaging (MRI) in radiation treatment, which can potentially obviate CT- or FDG-PET/CT–based delineation. The aim of this study is to evaluate the feasibility of target delineation on T2-weighted (T2W) MRI and T2W including diffusion-weighted MRI (T2W + DW-MRI) compared with current-practice FDG-PET/CT.
Methods
Ten observers delineated primary esophageal tumor GTVs of 6 patients on FDG-PET/CT, T2W-MRI, and T2W + DW-MRI. GTVs, generalized conformity indices, in-slice delineation variation (root mean square), and standard deviations in the position of the most cranial and caudal delineated slice were calculated.
Results
Delineations on MRI showed smaller GTVs compared with FDG-PET/CT–based delineations. The main variation was seen at the cranial and caudal border. No differences were observed in conformity indices (FDG-PET/CT, 0.68; T2W-MRI, 0.66; T2W + DW-MRI, 0.68) and in-slice variation (root mean square, 0.13 cm on FDG-PET/CT; 0.10 cm on T2W-MRI; 0.14 cm on T2W + DW-MRI). In the 2 tumors involving the gastroesophageal junction, addition of DW-MRI to T2W-MRI significantly decreased caudal border variation.
Conclusions
MRI-based target delineation of the esophageal tumor is feasible with interobserver variability comparable to that with FDG-PET/CT, despite limited experience with delineation on MRI. Most variation was seen at cranial-caudal borders, and addition of DW-MRI to T2W-MRI may reduce caudal delineation variation of gastroesophageal junction tumors.
Introduction
Esophageal cancer is diagnosed in >450,000 patients per year worldwide and is the sixth most common cause of cancer-related death.1 Standard therapy with curative intent for patients with locally advanced esophageal cancer consists of neoadjuvant chemoradiation therapy followed by surgery, improving 5-year survival compared with surgery alone.2, 3 For patients who are unfit for major surgery, definitive chemoradiation therapy is preferred.4 Thus, radiation treatment plays a central role in the treatment of esophageal cancer.5 Accurate gross tumor volume (GTV) delineation of the primary tumor is essential when boost strategies are applied. Increasing evidence suggests that boosting gross primary disease may improve local tumor control.6 Implementation of simultaneous integrated boost techniques may offer the advantage of delivering a higher dose to the tumor while maintaining conventional doses to subclinical disease.7
Currently, delineation of esophageal tumors is performed on computed tomography (CT), and the added value of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has been explored.8, 9, 10 Even with FDG-PET/CT fusion, GTV interobserver variability remains high in some cases, especially at the cranial and caudal tumor borders.11
On-board online cone beam CT imaging contributed to the development of image guided radiation therapy, which improved precision of radiation therapy setup in esophageal cancer.12 However, cone beam CT offers suboptimal soft-tissue contrast, and imaging of moving organs can be difficult. Because of its superior differentiation of soft tissues, integration of magnetic resonance imaging (MRI) in radiation therapy is promising.13, 14 The clinical implementation of MRI before and during each fraction of radiation therapy is being explored with the introduction of the Unity MRI-linear accelerator (MR-Linac; Elekta AB, Stockholm, Sweden). The MR-Linac combines 1.5 T MRI with a state-of-the-art linear accelerator and an online adaptive workflow.15, 16 Continuous adaptation of treatment based on daily MRI scans has the potential to improve radiation precision.
MRI of the esophagus has been improved over the past years. Artefacts in esophageal MRI scans from movement of the esophagus have been reduced by technique optimizations, resulting in high-quality MRI. For instance, positioning a navigator on the diaphragm allows for image acquisition in expiration only, thereby reducing motion artefacts.17, 18 On MRI the individual layers of the esophageal wall can be clearly visualized,19, 20 and a good correlation of T-stage on MRI with histopathologic T-stage has been described.21 For anatomic visualization and staging of esophageal tumors, MRI may even be superior to other imaging strategies.22 MRI also provides the opportunity to perform functional imaging, such as diffusion-weighted (DW) MRI, a valuable cancer imaging biomarker measuring tumor physiology.23 DW-MRI depends on the reduction in diffusion within the water microenvironment.24, 25 An increase in cell density, secondary to fast cell proliferation in tumors, results in a high signal on DW images. A previous study showed that longitudinal tumor lengths of esophageal cancers are more accurately delineated using DW-MRI compared with CT or T2-weighted (T2W) MRI only.26
Delineation studies in other tumor subtypes (eg, head and neck, prostate, and pancreatic cancer) already showed promising results regarding delineation of the GTV on MRI.27, 28, 29, 30 However, delineation variation of the GTV in esophageal cancer on MRI compared with FDG-PET/CT is unknown.
Because of the increasing interest in integrating MRI into radiation treatment, the aim of this study is to evaluate target delineation on T2W-MRI and T2W + DW-MRI compared with current-practice FDG-PET/CT.
Materials and Methods
Patients
Six patients, diagnosed with locally advanced esophageal cancer between December 2013 and December 2014, were prospectively included in a study evaluating response to neoadjuvant chemoradiation therapy of esophageal tumors by means of MRI and FDG-PET/CT examinations. This study was approved by the local medical ethics committee, and written informed consent was obtained from all patients (NCT 02125448). The present study concerns an ancillary study evaluating the feasibility of delineation on MRI compared with FDG-PET/CT before the start of neoadjuvant chemoradiation. Four patients were male, and 2 were female, with a mean age of 67 years (range, 54-74 years). We included squamous cell carcinomas (n = 3) and adenocarcinomas (n = 3). The patient characteristics are displayed in Table 1. All patients underwent FDG-PET/CT scan and endoscopy combined with endoscopic ultrasound (EUS) as standard of care. An additional MRI scan was acquired before treatment. After image acquisition, all patients were treated with chemoradiation therapy followed by esophagectomy.
Table 1.
Baseline characteristics of the 6 esophageal cancer cases
Image acquisition
FDG-PET/CT
The FDG-PET/CT scan was performed in radiation therapy treatment position on an integrated hybrid system combining multidetector CT and FDG-PET (Siemens, Erlangen, Germany). All patients were required to fast for at least 6 hours before the injection of 18F-FDG. Blood glucose levels were checked in every patient to exclude hyperglycemia. 18F-FDG (2.0 mega-Becquerels/kg) was intravenously injected 60 minutes before scanning. A CT scan was performed for attenuation correction purposes, and the PET was acquired 3 dimesionally with a scan time of 3 minutes per bed position. 18F-FDG-PET/CT reconstruction was performed with ordered-subsets expectation maximization for 21 subsets and 4 iterations (Gaussian filter).
MRI
MRI scans were performed on a 1.5 T MRI scanner (Philips Achieva or Ingenia, Best, the Netherlands) using TorsoXL (16 channels) or Anterior/Posterior (28 channel) receiver coils. MRI scanning consisted of T2W-MRI and DW-MRI in axial planes with a slice thickness of 6.5 mm. A respiratory navigator was positioned on the diaphragm to reduce motion artefacts, and scans were only acquired in expiration.18 The b = 800 s/mm2 images were used for delineation of the tumor. Apparent diffusion coefficient (ADC) maps are quantitative measurements of tissue diffusivity, calculated over different b-values, and can also be visually displayed.24, 25 These visual displayed ADC maps, calculated from b = 0, b = 200, and b = 800 s/mm2, were available during delineation.
Observers
The GTV of the primary tumor of the 6 cases was independently delineated by 10 observers from 2 centers in the Netherlands. First, they delineated the GTV on fused FDG-PET/CT only. After a minimum interval of 2 weeks, delineations of the GTV were repeated on T2W-MRI only. Thereafter, DW-MRI of b = 800 s/mm2 and ADC maps were added and delineations were adjusted to create a GTV based on a combination of T2W and DW-MRI.
Delineation guidelines
All clinical information of the cases (age, sex, histology, endoscopy report, EUS report, and FDG-PET/CT report) was provided, reflecting clinical practice. Observers delineated target volumes using Volumetool, a software tool developed at the University Medical Center Utrecht, The Netherlands. Before the start, 2 consensus meetings were organized to discuss magnetic resonance delineation guidelines for esophageal cancer based on the available literature. Gastrointestinal expert radiation oncologists, radiologists, and researchers in the field of esophageal cancer imaging from the 2 centers participated in these meetings. A digital manual was sent to the observers with the consensus guidelines for delineation on MRI. The observers were instructed to delineate the primary tumor GTV and to exclude potential suspicious locoregional lymph nodes on all delineation modalities. For delineation on MRI, observers were provided with the FDG-PET/CT report but were not allowed to review the noncoregistered PET/CT on a second screen.
FDG-PET/CT delineations
The mean activity in the liver served as the reference for physiologic uptake of FDG in a fasting patient,31 and a nuclear medicine physician standardized the window-level for all cases before FDG-PET/CT delineation. Observers were instructed to delineate the tumor on CT and adjust delineations after coregistration with FDG-PET. Observers were informed about the slice thickness of 3 mm.
T2W-MRI and T2W + DW-MRI delineations
An identical window level and grayscale per patient was preset for the T2W-MRI delineations. Observers were informed about the slice thickness of 6.5 mm. After addition of DW-MRI using b = 800 s/mm2 images, delineations were adjusted on T2W + DW-MRI. Observers received visually displayed ADC maps (calculated from b = 0, b = 200, and b = 800 s/mm2) to determine possible T2 shine-through effects, which result in a high signal intensity in the b = 800s/mm2 image but are not related to true diffusion restriction.
Volumetric analysis
The mean and standard deviations (SDs) of the delineated GTVs were calculated per patient and compared between FDG-PET/CT, T2W-MRI, and T2W + DW-MRI delineations.
Contour analysis
Overlap analysis
To quantify the overlap among FDG-PET/CT–, T2W-MRI–, and T2W + DW-MRI–based delineations, we calculated the generalized conformity index (CIgen), defined as the sum of the common volumes between observer pairs divided by the sum of the encompassing volumes between each pair of observers. A CIgen of 1 indicates 100% agreement between observers, and a CIgen of 0 indicates no overlap in delineation. CIgens were calculated per patient and averaged over all patients per modality.32
Delineation variation of the central region: in-slice SD
The central delineated region was defined as the region that was delineated by all 10 observers. For this region, a surface distance variation was calculated. A reference contour for each patient was computed in 3 dimensions and denoted the median surface GTV.33 This median surface, encircling 50% coverage of the GTVs of all observers, was sampled using 8000 equally distributed points. For all points, the perpendicular distance to each delineated GTV surface was calculated. The variation of the different observers for each point on the median surface was expressed in a local observer variation (local SD). For the central delineated region, the overall observer variation was calculated for every patient as the quadratic mean of the local SD, demonstrating in-slice SD.
Cranial and caudal tumor border variation
The cranial and caudal variation of the esophageal tumor delineations was calculated as the SD of the most proximal and distal delineated slice. As a result of the difference in slice thickness between FDG-PET/CT (3 mm) and T2W + DW-MRI (6.5 mm), SDs of delineations in the most cranial and caudal delineated slice could only be compared between T2W-MRI and T2W + DW-MRI.34
Statistical methods
GTVs and generalized conformity indices were compared using a pairwise t test (paired 2-sided Student t test). To compare SDs, the 2-sided F-test was used.34 P values lower than .05 were considered statistically significant.
Results
Volumetric analysis
The 10 observers delineated significantly smaller absolute GTVs on T2W-MRI and T2W + DW-MRI compared with FDG-PET/CT (P = .07 for T2W-MRI vs FDG-PET/CT and P = .01 for T2W + DW-MRI vs FDG-PET/CT; Table 2). The FDG-PET/CT–based mean GTVs over all patients was 40.5 cm3 (SD-per-patient range, 0.8-9.7 cm3). The T2W-MRI–based mean GTV was 34.8 cm3 (SD-per-patient range, 1.4-16.1 cm3). On T2W + DW-MRI, the mean GTV decreased to 32.7 cm3 (SD-per-patient range, 1.7-8.2 cm3).
Table 2.
Volumetric index and interobserver variation of FDG-PET/CT–, T2W-MRI–, and T2W + DW-MRI–based GTV delineations
| Case | Mean volume (cm³)∗ |
Generalized conformity index |
Central overall SD (RMS, cm)† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PET/CT | T2W-MRI | T2W + DW-MRI | PET/CT | T2W-MRI | T2W + DW-MRI | PET/CT | T2W-MRI | T2W + DW-MRI | |
| 1 | 47.8 ± 7.0 | 40.6 ± 6.3 | 38.2 ± 6.1 | 0.68 | 0.72 | 0.72 | 0.13 | 0.06 | 0.07 |
| 2 | 46.3 ± 3.7 | 32.7 ± 4.2 | 32.4 ± 4.5 | 0.75 | 0.79 | 0.79 | 0.13 | 0.09 | 0.09 |
| 3 | 75.5 ± 9.7 | 63.8 ± 8.9 | 64.6 ± 6.1 | 0.74 | 0.67 | 0.67 | 0.11 | 0.14 | 0.14 |
| 4 | 39.6 ± 8.1 | 38.0 ± 16.1 | 33.1 ± 8.2 | 0.62 | 0.43 | 0.43 | 0.20 | 0.12 | 0.27 |
| 5 | 22.5 ± 8.6 | 21.9 ± 3.8 | 16.4 ± 3.6 | 0.53 | 0.62 | 0.62 | 0.08 | 0.08 | 0.10 |
| 6 | 11.2 ± 0.8 | 11.7 ± 1.4 | 11.5 ± 1.7 | 0.73 | 0.71 | 0.71 | 0.09 | 0.10 | 0.09 |
| Mean | 40.5 | 34.8 | 32.7 | 0.68 | 0.66 | 0.68 | 0.13 | 0.10 | 0.14 |
 | |||||||||
Abbreviations: DW = diffusion-weighted; FDG-PET/CT = fluorodeoxyglucose positron emission tomography/computed tomography; GTV = gross tumor volume; SD = standard deviation; T2W = T2-weighted; MRI = magnetic resonance imaging; RMS = root mean square.
Mean per patient and modality ± standard deviation. P < .05 considered statistically significant; calculated between the means per modality, paired-sample t test (2-tailed).
Central overall SD: quadratic mean of the local SD weighted for surface (RMS) of the central part of the median surface where 100% of the observers agreed delineations.
Overlap analysis
The mean generalized CIgen over all patients was 0.68 on FDG-PET/CT, 0.66 on T2W-MRI, and 0.68 on T2W + DW-MRI (Table 2), which was not significantly different across modalities (P = .68 for FDG-PET/CT vs T2W-MRI; P = .83 for FDG-PET/CT vs T2W + DW-MRI; and P = .26 for T2W-MRI vs T2W + DW-MRI).
Delineation variation of the central region: in-slice SD
The in-slice SD of the central delineated region was small in the 6 included cases and did not differ between modalities (mean, 0.13 cm on FDG-PET/CT, 0.10 cm on T2W-MRI, and 0.14 cm on T2W + DW-MRI; Table 2).
Cranial and caudal tumor border variation
On all modalities, delineation variability mainly occurred at the cranial and caudal tumor borders. SDs in the position of the most cranial and caudal delineated slice are displayed in Table 3, with significance levels calculated to report delineation differences between T2W-MRI and T2W + DW-MRI.
Table 3.
Delineation variation on FDG-PET/CT, T2W-MRI, and T2W + DW-MRI at the cranial and caudal tumor borders
| Case | Cranial delineation variation 1 SD (cm)∗ |
P value† | Caudal delineation variation 1 SD (cm)∗ |
P value† | ||||
|---|---|---|---|---|---|---|---|---|
| PET/CT | T2W-MRI | T2W + DW-MRI | PET/CT | T2W-MRI | T2W + DW-MRI | |||
| 1 | 1.25 | 1.38 | 1.30 | .86 | 0.39 | 0.57 | 0.53 | .84 |
| 2 | 0.35 | 0.21 | 0.21 | 1.00 | 0.20 | 0.37 | 0.33 | .78 |
| 3 | 0.09 | 0.55 | 0.37 | .25 | 0.84 | 0.76 | 0.37 | .04 |
| 4 | 0.67 | 1.22 | 1.00 | .56 | 0.21 | 1.22 | 0.46 | .01 |
| 5 | 1.22 | 0.44 | 0.33 | .44 | 0.57 | 0.94 | 0.82 | .69 |
| 6 | 0.20 | 0.27 | 0.31 | .69 | 0.17 | 0.45 | 0.53 | .65 |
Abbreviations: DW = diffusion-weighted; FDG-PET/CT = fluorodeoxyglucose positron emission tomography/computed tomography; MRI = magnetic resonance imaging; T2W = T2-weighted.
Slice thickness on PET/CT = 3 mm; slice thickness on T2W-MRI and T2W + DW-MRI = 6.5 mm.
P < .05 considered statistically significant; 2-sided F-test, calculated between T2W- and T2W + DW-MRI.
Figure 1 visualizes slice variation at the proximal and distal border for delineations on FDG-PET/CT, T2W-MRI, and T2W + DW-MRI. Case 1 showed large delineation variation at the cranial border. In this case, satellite lesions were present cranially of the tumor, which were described in the endoscopic report. These lesions were included in the GTV by 4 of 10 physicians on FDG-PET/CT, T2W-MRI, and DW-MRI. In case 3 and 4 with gastroesophageal junction (GEJ) involvement, a significant decrease in caudal border variation was observed on T2W + DW-MRI compared with T2W-MRI (P = .04 in case 3, P = .01 in case 4; Table 3). Figure 2 shows an example of GTV delineation variation at the GEJ on T2W-MRI (Fig 2a) and T2W + DW-MRI (Fig 2c), displaying improved delineation agreement at the caudal border after addition of DW-MRI.
Figure 1.
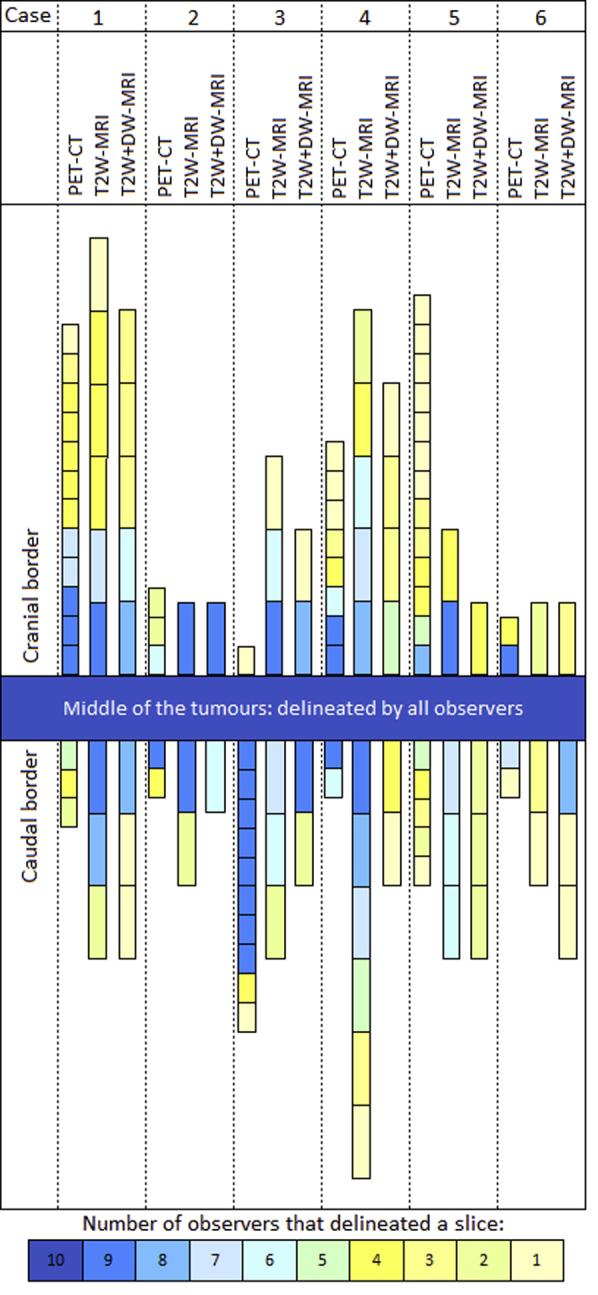
Slice variation on FDG-PET/CT, T2W-MRI, and T2W + DW-MRI at the cranial and caudal tumor borders. Each block represents a delineated slice. The number of observers delineating a slice is displayed under the figure. The length of the blocks varies according to the slice thickness (which was 3 mm on FDG-PET/CT and 6.5 mm on MRI). Abbreviations: FDG-PET/CT = fluorodeoxyglucose positron emission tomography computed tomography; T2W = T2-weighted; DW = diffusion-weighted; MRI = magnetic resonance imaging.
Figure 2.
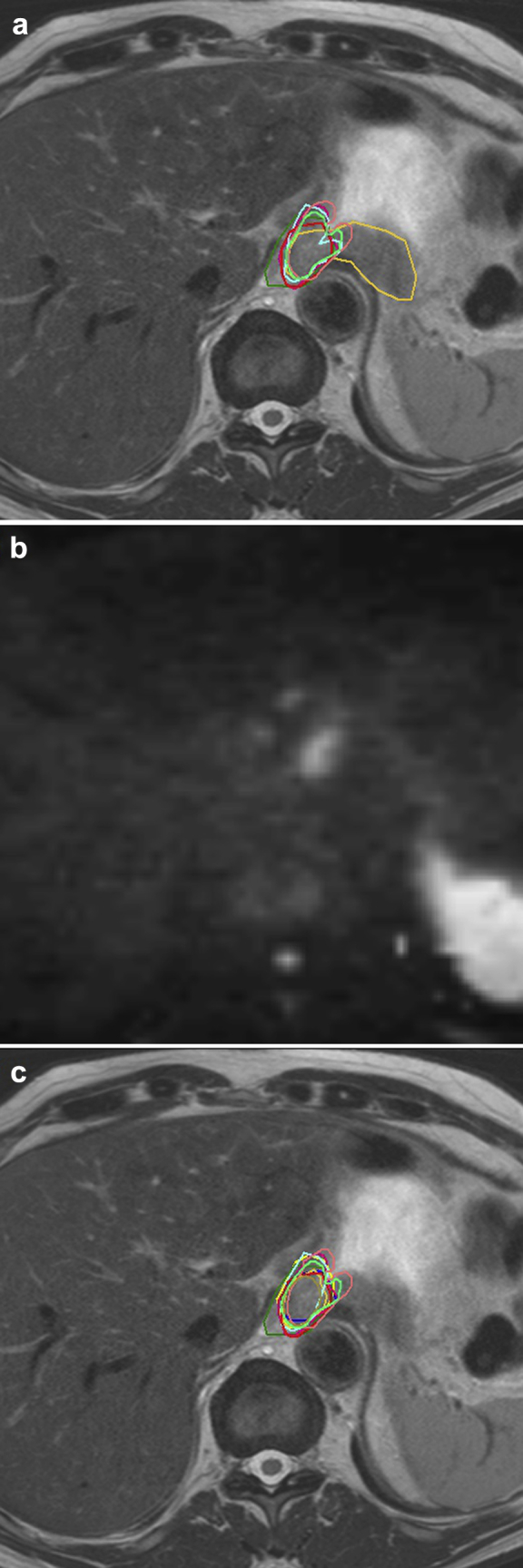
A 71-year-old male patient (case 3) with a tumor involving the gastroesophageal junction. (a) Delineations of the esophageal GTV of the 10 observers on T2-weighted MRI only. (b) The added diffusion-weighted MRI scan of the same slice, showing the differentiation between tumor and gastric wall thickening. (c) Delineations adjusted after the addition of diffusion-weighted MRI. Abbreviations: GTV = gross tumor volume; MRI = magnetic resonance imaging.
Discussion
This multiobserver study shows that delineation of the primary esophageal tumor on MRI is feasible, with interobserver variability for GTV delineation on MRI comparable to that with FDG-PET/CT. The main variation is seen at the cranial and caudal tumor border, whereas centrally, differences in tumor delineations were small on T2W and T2W + DW-MRI. The addition of DW-MRI to T2W-MRI in the 2 GEJ-involving tumors significantly reduced the SD of the most caudal delineated slice, showing the potential value of DW-MRI for delineation of the caudal border in GEJ-involving cases.
To our knowledge, this is the first study comparing GTV delineation variability on T2W-MRI and T2W + DW-MRI to FDG-PET/CT for esophageal cancer. Currently, the gold standard for delineation of esophageal cancer is CT (with fused PET if available) and correlation with endoscopy/EUS findings. Delineation on CT only is challenging, mainly in differentiating tumor from normal tissue at the cranial and caudal tumor borders. Delineation on FDG-PET/CT may help in determining these tumor borders; however, FDG uptake does not differentiate between tumor and inflammation, which can be difficult especially in tumors involving the GEJ.35, 36 Furthermore, on FDG-PET/CT the target volume can depend on the threshold chosen and may increase or decrease depending on the windowing. On the contrary, this study showed promising results for delineation on MRI in patients with esophageal cancer. Especially for cases with GEJ involvement, the addition of DW imaging to T2W-MRI showed additional value for T2W-MRI only.
In this study, GTVs were significantly smaller on MRI compared with FDG-PET/CT. This may partly be caused by the acquisition of MRI at the end of the expiration, whereas for FDG-PET/CT no motion-specific scanning techniques were used, showing the extent of malignancy throughout the breathing cycle. Furthermore, the finding of a reduced GTV on esophageal MRI is in line with a study by Hou et al.26 They compared longitudinal length accuracy of esophageal cancer cases on T2W-MRI and DW-MRI to CT and found that DW-MRI resulted in the smallest tumor lengths. Another study showed a good correlation between DW-MRI tumor lengths and histopathologic tumor lengths.26 In prostate and cervical cancer, delineations on DW-MRI resulted in slightly smaller GTVs compared with histopathology.37, 38 An important matter is that smaller GTVs may translate into smaller clinical target volumes when automatic expansion is used, which theoretically could lead to underdosage of microscopic disease. The optimal approach to investigate the accuracy of delineation on MRI would be a direct correlation between a preoperative MRI in patients who are planned to undergo direct esophagectomy and histopathology. Because patients with locally advanced esophageal cancer are treated with neoadjuvant therapy (chemoradiation therapy or chemotherapy) before surgery, direct correlation of imaging to pathology cannot be performed. An alternative approach is to investigate local control rates, focusing on potential rim recurrences, when delineation on MRI is integrated in a magnetic resonance–based workflow.
A limitation of this study is the mean interval of 14 days between FDG-PET/CT and MRI acquisition, which limits the comparability between the different modalities in terms of tumor volume in light of potential tumor growth. Therefore, the main focus was the comparison of interobserver variability between modalities (demonstrated as CIgens and craniocaudal delineation variation). Second, MRI scans were acquired in the axial plane only (with sagittal reconstructions available), and addition of sagittal plane imaging of the tumors might further optimize delineation accuracy, especially in determining the cranial and caudal tumor extension. Third, the slice thickness of the MRI scans was 6.5 mm, and reducing this slice thickness may lead to further improvement in the accuracy in GTV delineations. Lastly, the number of patients in this study was limited to 6. Future studies should include a larger number of patients to validate our results.
The importance of delineation guidelines in radiation therapy is well known and has been studied for CT and FDG-PET/CT in different tumor sites.34, 39, 40 For delineation on MRI, the importance of guidelines has been shown for head and neck, pancreatic, and prostate cancer studies.27, 28, 29, 30 Consensus guidelines for esophageal cancer have been developed for CT and FDG-PET/CT contouring.41 Although GTV delineation was comparable on MRI and FDG-PET/CT, this study emphasizes the need to expand clinical experience on magnetic resonance delineation for esophageal cancer and to implement robust delineation guidelines. GTVs on MRI were overall smaller compared with those on FDG-PET/CT, and the impact on clinical practice is unknown. To ensure accurate GTV delineation of the macroscopic tumor and mirror clinical practice, we recommend further delineation studies on anatomic T2W-MRI combined with DW-MRI.
Conclusions
MRI-based delineation of the esophageal GTV shows target delineation variability comparable to that with FDG-PET/CT, although overall GTVs were smaller on MRI compared with FDG-PET/CT. The addition of DW-MRI to T2W-MRI potentially facilitates delineation of the caudal border in GEJ tumors. Future research should focus on refinement of esophageal MRI acquisition and the development of internationally validated delineation guidelines to implement MRI delineation in esophageal radiation therapy clinics.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.van Hagen P., Hulshof M.C., van Lanschot J.J. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro J., van Lanschot J.J.B., Hulshof M.C.C.M. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 4.Bedenne L., Michel P., Bouche O. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 5.Lordick F., Mariette C., Haustermans K. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50–v57. doi: 10.1093/annonc/mdw329. [DOI] [PubMed] [Google Scholar]
- 6.Welsh J.W., Seyedin S.N., Allen P.K. Local control and toxicity of a simultaneous integrated boost for dose escalation in locally advanced esophageal cancer: Interim results from a prospective phase I/II trial. J Thorac Oncol. 2017;12:375–382. doi: 10.1016/j.jtho.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Deng W., Lin S.H. Advances in radiotherapy for esophageal cancer. Ann Transl Med. 2018;6:79. doi: 10.21037/atm.2017.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vesprini D., Ung Y., Dinniwell R. Improving observer variability in target delineation for gastro-oesophageal cancer: The role of (18F)fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography. Clin Oncol (R Coll Radiol) 2008;20:631–638. doi: 10.1016/j.clon.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Muijs C.T., Schreurs L.M., Busz D.M. Consequences of additional use of PET information for target volume delineation and radiotherapy dose distribution for esophageal cancer. Radiother Oncol. 2009;93:447–453. doi: 10.1016/j.radonc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Muijs C.T., Beukema J.C., Woutersen D. Clinical validation of FDG-PET/CT in the radiation treatment planning for patients with oesophageal cancer. Radiother Oncol. 2014;113:188–192. doi: 10.1016/j.radonc.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Nowee M.E., Voncken F.E.M., Kotte A.N.T.J. Dutch National Platform for Radiotherapy of Gastrointestinal Tumours (LPRGE) group. Gross tumour delineation on computed tomography and positron emission tomography-computed tomography in oesophageal cancer: A nationwide study. Clin Transl Radiat Oncol. 2018;14:33–39. doi: 10.1016/j.ctro.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Nunen A., van der Sangen M.J.C., van Boxtel M., van Haaren P.M.A. Cone-beam CT-based position verification for oesophageal cancer: Evaluation of registration methods and anatomical changes during radiotherapy. Tech InnovPatient Support Radiat Oncol. 2017;3-4:30–36. doi: 10.1016/j.tipsro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menard C., van der Heide U. Introduction: Systems for magnetic resonance image guided radiation therapy. Semin Radiat Oncol. 2014;24:192. doi: 10.1016/j.semradonc.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Pollard J.M., Wen Z., Sadagopan R. The future of image-guided radiotherapy will be MR guided. Br J Radiol. 2017;90:20160667. doi: 10.1259/bjr.20160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerkmeijer L.G., Fuller C.D., Verkooijen H.M. The MRI-linear accelerator consortium: Evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development. Front Oncol. 2016;6:215. doi: 10.3389/fonc.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz C. MR-linac: Clinical introduction. Front Oncol. 2016;6:215. doi: 10.3389/fonc.2016.00215. [Abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehman R.L., Felmlee J.P. Adaptive technique for high-definition MR imaging of moving structures. Radiol. 1989;173:255–263. doi: 10.1148/radiology.173.1.2781017. [DOI] [PubMed] [Google Scholar]
- 18.Lever F.M., Lips I.M., Crijns S.P. Quantification of esophageal tumor motion on cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2014;88:419–424. doi: 10.1016/j.ijrobp.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Riddell A.M., Davies D.C., Allum W.H. High-resolution MRI in evaluation of the surgical anatomy of the esophagus and posterior mediastinum. AJR Am J Roentgenol. 2007;188:W37–W43. doi: 10.2214/AJR.05.1795. [DOI] [PubMed] [Google Scholar]
- 20.Riddell A.M., Richardson C., Scurr E. The development and optimization of high spatial resolution MRI for imaging the oesophagus using an external surface coil. Br J Radiol. 2006;79:873–879. doi: 10.1259/bjr/36989440. [DOI] [PubMed] [Google Scholar]
- 21.Yamada I., Miyasaka N., Hikishima K. Ultra-high-resolution MR imaging of esophageal carcinoma at ultra-high field strength (7.0T) ex vivo: Correlation with histopathologic findings. Magn Reson Imaging. 2015;33:413–419. doi: 10.1016/j.mri.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 22.van Rossum P.S., van Hillegersberg R., Lever F.M. Imaging strategies in the management of oesophageal cancer: what's the role of MRI? Eur Radiol. 2013;23:1753–1765. doi: 10.1007/s00330-013-2773-6. [DOI] [PubMed] [Google Scholar]
- 23.Padhani A.R., Liu G., Mu-Koh D. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Bihan D., Poupon C., Amadon A. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging. 2006;24:478–488. doi: 10.1002/jmri.20683. [DOI] [PubMed] [Google Scholar]
- 25.Koh D.M., Collins D.J. Diffusion-weighted MRI in the body: Applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–1635. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 26.Hou D.L., Shi G.F., Gao X.S. Improved longitudinal length accuracy of gross tumor volume delineation with diffusion weighted magnetic resonance imaging for esophageal squamous cell carcinoma. Radiat Oncol. 2013;8:169. doi: 10.1186/1748-717X-8-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rud E., Klotz D., Rennesund K. Detection of the index tumour and tumour volume in prostate cancer using T2-weighted and diffusion-weighted magnetic resonance imaging (MRI) alone. BJU Int. 2014;114:E32–E42. doi: 10.1111/bju.12637. [DOI] [PubMed] [Google Scholar]
- 28.Dinh C.V., Steenbergen P., Ghobadi G. Magnetic resonance imaging for prostate cancer radiotherapy. Physica Medica. 2016;32:446–451. doi: 10.1016/j.ejmp.2016.01.484. [DOI] [PubMed] [Google Scholar]
- 29.Jager E.A., Ligtenberg H., Caldas-Magalhaes J. Validated guidelines for tumor delineation on magnetic resonance imaging for laryngeal and hypopharyngeal cancer. Acta Oncol. 2016;55:1305–1312. doi: 10.1080/0284186X.2016.1219048. [DOI] [PubMed] [Google Scholar]
- 30.Heerkens H.D., Hall W.A., Li X.A. Recommendations for MRI-based contouring of gross tumor volume and organs at risk for radiation therapy of pancreatic cancer. Pract Radiat Oncol. 2017;7:126–136. doi: 10.1016/j.prro.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Chirindel A., Alluri K.C., Tahari A.K. Liver standardized uptake value corrected for lean body mass at FDG PET/CT: Effect of FDG uptake time. Clin Nucl Med. 2015;40:e17–e22. doi: 10.1097/RLU.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kouwenhoven E., Giezen M., Struikmans H. Measuring the similarity of target volume delineations independent of the number of observers. Phys Med Biol. 2009;54:2863–2873. doi: 10.1088/0031-9155/54/9/018. [DOI] [PubMed] [Google Scholar]
- 33.Deurloo K.E., Steenbakkers R.J., Zijp L.J. Quantification of shape variation of prostate and seminal vesicles during external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:228–238. doi: 10.1016/j.ijrobp.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Nijkamp J., de Haas-Kock D.F., Beukema J.C. Target volume delineation variation in radiotherapy for early stage rectal cancer in the Netherlands. Radiother Oncol. 2012;102:14–21. doi: 10.1016/j.radonc.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Horton K.M., Fishman E.K. Current role of CT in imaging of the stomach. RadioGraphics. 2003;23:75–87. doi: 10.1148/rg.231025071. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi S., Ogura M., Suzawa N. 18F-FDG uptake in the stomach on screening PET/CT: Value for predicting Helicobacter pylori infection and chronic atrophic gastritis. BMC Medical Imaging. 2016;16:58. doi: 10.1186/s12880-016-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Boer P., Bleeker M.C., Spijkerboer A.M. Craniocaudal tumour extension in uterine cervical cancer on MRI compared to histopathology. Eur J Radiol Open. 2015;2:111–117. doi: 10.1016/j.ejro.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Nobin J., Orczyk C., Deng F.M. Prostate tumour volumes: Evaluation of the agreement between magnetic resonance imaging and histology using novel co-registration software. BJU Int. 2014;114:E105–E112. doi: 10.1111/bju.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim K., Small W., Portelance L. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79:348–355. doi: 10.1016/j.ijrobp.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 40.Michalski J.M., Lawton C., El Naqa I. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76:361–368. doi: 10.1016/j.ijrobp.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu A.J., Bosch W.R., Chang D.T. Expert consensus contouring guidelines for intensity modulated radiation therapy in esophageal and gastroesophageal junction cancer. Int J Radiat Oncol Biol Phys. 2015;92:911–920. doi: 10.1016/j.ijrobp.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice T.W., Blackstone E.H., Rusch V.W. 7th edition of the AJCC Cancer Staging Manual: Esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]


