Abstract
Purpose
To assess gastrointestinal (GI) and genitourinary (GU) adverse events (AEs) of 11C-choline-positron emission tomography (CholPET) guided lymph node (LN) radiation therapy (RT) in patients who experience biochemical failure after radical prostatectomy.
Methods and Materials
From 2013 to 2016, 107 patients experienced biochemical failure of prostate cancer, had CholPET-detected pelvic and/or paraortic LN recurrence, and were referred for RT. Patients received androgen suppression and CholPET guided LN RT (median dose, 45 Gy) with a simultaneous integrated boost to CholPET-avid sites (median dose, 56.25 Gy), all in 25 fractions. RT-naïve patients had the prostatic fossa included in the initial treatment volumes followed by a sequential boost (median dose, 68 Gy). GI and GU AEs were reported per Common Terminology Criteria for Adverse Events (version 4.0) with data gathered retrospectively. Differences in maximum GI and GU AEs at baseline, immediately post-RT, and at early (median, 4 months) and late (median, 14 months) follow-up were assessed.
Results
Median follow-up was 16 months (interquartile range [IQR], 11-25). Median prostate-specific antigen at time of positive CholPET was 2.3 ng/mL (IQR, 1.3-4.8), with a median of 2 (IQR, 1-4) choline-avid LNs per patient. Most recurrences were within the pelvis (53%) or pelvis + paraortic (40%). Baseline rates of grade 1 to 2 GI AEs were 8.4% compared with 51.9% (4.7% grade 2) of patients post-RT (P < .01). These differences resolved by 4-month (12.2%, P = .65) and 14-month AE assessments (9.1%, P = .87). There was no significant change in grade 1 to 2 GU AEs post-RT (64.1%) relative to baseline (56.0%, P = .21), although differences did arise at 4-month (72.2%, P = .01) and 14-month (74.3%, P = .01) AE assessments.
Conclusions
Salvage CholPET guided nodal RT has acceptably low rates of acute GI and GU AEs and no significant detriment in 14-month GI AEs. These data are of value in counseling patients and designing prospective trials evaluating the oncologic efficacy of this treatment strategy.
Introduction
Prostate cancer is one of the most common malignancies among men worldwide and is a common cause of cancer-related death.1 After initial treatment, prostate cancer recurrence is often heralded by a rising serum prostate-specific antigen (PSA). Advances in prostate cancer–specific positron emission tomography (pcPET), such as 11C-choline-PET/CT (CholPET), have permitted the localization of sites of prostate cancer recurrence in men who have experienced biochemical failure (BF) after radical prostatectomy (RP).2, 3, 4, 5, 6
These advances in pcPET allow for novel interventional strategies designed to improve survival or reduce recurrence rates in patients with isolated nodal recurrence or oligometastatic disease.7, 8, 9, 10 However, salvage pelvic or paraortic (PA) nodal radiation therapy (RT) may cause early and late gastrointestinal (GI) adverse events (AEs) given the potentially large RT fields.11 Historical studies evaluating the role of 2-dimensional or 3-dimensional conformal pelvic ± PA nodal RT in genitourinary (GU) or gynecologic malignancies have reported rates of grade 2 GI AEs as high as 40% and grade 3 or higher rates of 1% to 5% with pelvic RT or 1% to 10% with pelvic and PA RT (Table 1).12, 13, 14, 15, 16, 17, 18 However, with improved understanding of GI tissue tolerances and advancements in treatment techniques such as image guided intensity modulated RT, it is reasonable to expect that contemporary pelvic or PA nodal RT would be associated with lower rates of AEs compared with historical series.
Table 1.
Summary of select studies evaluating pelvic ± paraortic nodal irradiation
| Author (year reported) | Center/Clinical trial | N | Study arm | RT technique | Duration of AE follow-up (mo) | GI G2+ AEs | GI G3+ AEs | GI G4+ AEs |
|---|---|---|---|---|---|---|---|---|
| Jethwa et al (2019) | Present study | 110 | CholPET guided RT to pelvic ± PA lymph nodes | VMAT/IMRT | 14 | 1% | 0% | |
| Picchio et al (2014)9 | San Raffaele Scientific Institute, Milan, Italy | 83 | CholPET guided RT to pelvic ± PA lymph nodes | IMRT | 3 | 4% | ||
| Vaugier et al (2019)31 | GETUG P07 | 67 | CholPET guided RT to pelvic lymph nodes | IMRT | 12 | 6% | 0% | 0% |
| Pilepich et al (1986)13, 14 | RTOG 7506 | 523 | Prostate + pelvic ± PA RT | 2D | 51 | 5% vs 7%∗ | <1%∗ | |
| Asbell et al (1988)12, 14 | RTOG 7706 | 445 | Prostate ± pelvic RT | 3DCRT | 84 | 2% vs 7%∗ | 0% vs 1%∗ | |
| Roach et al (2003)16 | RTOG 9413 | 1323 | Prostate ± pelvic RT | 3DCRT | 60 | 1% vs 2% | ||
| Pommier et al (2007)15 | GETUG-01 | 444 | Prostate ± pelvic RT | 3DCRT | 42 | 37% vs 43% | 11% vs 11% | |
| Rotman et al (1990)17 | RTOG 7920 | 367 | Pelvic RT ± PA nodal RT for advanced cervical cancer | 3DCRT | 60 | 3% vs 7% | ||
| Morris et al (1999)18 | RTOG 9001 | 403 | Pelvic + PA RT arm for advanced cervical cancer | 3DCRT | 43 | 11% |
Abbreviations: 3DCRT = 3-dimensional conformal radiation therapy; AE = adverse effects; Chol-PET = 11C-choline-positron emission tomography; IMRT = image modulated radiation therapy; PA = paraortic; RT = radiation therapy; RTOG = Radiation Therapy Oncology Group; VMAT = volumetrically modulated arc therapy.
Only reporting incidence of diarrhea.
Recently, several series evaluating the role of salvage pelvic or PA nodal RT for CholPET-detected pelvic or PA nodal recurrence have been reported.7, 8, 9, 10 Although they have small sample sizes and significant heterogeneity in treatment-related characteristics, these series have found low rates of grade 2 or 3 AEs for patients receiving RT, with promising oncologic efficacy. These data, along with others, have stimulated interest in evaluating this strategy in prospective studies.8, 19
The primary purpose of this study is to evaluate the rates of GI and GU AEs within a large observational series of patients who experienced BF after RP and received androgen suppression (AS) with pelvic and/or PA nodal RT using CholPET guided simultaneous integrated boost (SIB).
Methods and Materials
Patient population
From 2013 to 2016, 107 patients experienced BF (PSA >0.2 ng/mL) of prostate cancer after prostatectomy with or without previous RT to the prostatic fossa, had CholPET-detected prostate cancer recurrence limited to the pelvis and/or PA LNs, and were referred for radiotherapeutic management. Patients with osseous, visceral, or other distant metastases were excluded. When feasible, histologic confirmation of CholPET-avid disease was performed either through CT or ultrasound guided biopsy or lymph node dissection (LND). In circumstances in which a biopsy specimen was unobtainable or nondiagnostic, a diagnosis of nodal disease was made by interval CholPET assessment of treatment response to AS before delivery of RT. Clinical, biochemical, and pathologic features of the patients are summarized in Table 2.
Table 2.
Patient characteristics
| Variable, n = 107 | Value∗ | |
|---|---|---|
| Age | 68 (62-72) | |
| Initial disease characteristics | T2 | 40 (37.3%) |
| T3a | 29 (27.1%) | |
| T3b | 33 (30.8%) | |
| T4 | 2 (1.8%) | |
| Unknown | 3 (2.7%) | |
| N + | 13 (12.1%) | |
| PSA | 7.8 (5.4-11.2) | |
| Summed Gleason Score | 6 | 4 (3.7%) |
| 7 | 60 (56.1%) | |
| 8-10 | 42 (39.3%) | |
| Unknown | 1 (0.9%) | |
| Salvage prostatic fossa RT before Chol-PET detected nodal recurrence | 81 (75.7%) | |
| History of AS | 67 (62.6%) | |
| PSA at time of positive CholPET | 2.3 (1.3-4.8) | |
| No. recurrent sites/patient | 2 (1-4) | |
| Location of CholPET avid sites | Pelvic LN | 50 (46.7%) |
| Pelvic + PA LN | 35 (32.7%) | |
| PA LN | 7 (6.5%) | |
| Prostatic fossa + pelvic LN | 7 (6.5%) | |
| Prostatic fossa + PA LN | 4 (3.7%) | |
| Prostatic fossa + pelvic + PA LN | 4 (3.7%) | |
| Any histologic confirmation | 69 (64.5%) | |
| Method of Histologic confirmation | LN + at initial RP only | 6 (5.6%) |
| Biopsy | 40 (37.4%) | |
| Salvage LND | 23 (21.5%) | |
| Salvage LND after positive CholPET | 23 (21.5%) | |
| Chemotherapy after positive CholPET | 22 (20.6%) |
Abbreviations: AS = androgen suppression; Chol-PET = 11C-choline-positron emission tomography; LN = lymph node; LND = lymph node dissection; PA = paraortic; PSA = prostate-specific antigen; RP = radical prostatectomy; RT = radiation therapy.
Median values are reported as median (interquartile range). Other values are number of patients (%).
Treatment techniques
After 2 to 4 months of neoadjuvant AS, all patients underwent CT-based simulation, with the CholPET registered to the planning CT scan for accurate target delineation. Table 3 provides a detailed description of RT target volumes and treatment techniques. Dose-volume histogram (DVH) analysis was undertaken to limit radiation dose to organs at risk (OARs), including analyses of small and large bowel, rectum, urinary bladder, kidneys, femoral heads, and spinal cord/cauda equina with dose constraints modified from contemporary published guidelines.11 DVH constraints and plan statistics for small bowel, the chief dose-limiting organ, are also provided in Table 3.
Table 3.
RT treatment techniques and field characteristics
| RT dose∗ | CTV1 | 45 Gy |
|---|---|---|
| CTV2 | 56.25 Gy (50-62.5 Gy) | |
| Volumes | ||
| CTV1 | Pelvic LN recurrence | Per Radiation Therapy Oncology Group (RTOG) contouring guidelines, with exceptions being that the superior margin was routinely at the level of the aortic bifurcation30 and presacral regions below the S2-3 level were included as clinically indicated |
| PA LN recurrence | Included pelvic LN volumes (per CTV1) plus 1.5-2 cm above the most superior CholPET-avid site of recurrence | |
| CTV2 | Gross CholPET-avid disease with an additional 5-10 mm anatomically constrained expansion (cropped out of bowel, bladder, and bone). | |
| Image guidance | Daily cone beam CT and/or kilovoltage orthogonal x-rays | |
| RT technique | Volumetrically modulated arc therapy | |
| Small bowel DVH constraints and achieved statistics† | Small bowel (bowel bag) | Max <52 Gy (50.8, 48.7, 52.4) |
| V50 Gy < 2 cm3 (0.1, 0, 1.0) | ||
| V45 Gy < 150 cm3 (27.9, 10.3, 44.3) | ||
| V30 Gy < 300 cm3 (213.4, 112.6, 272.2) | ||
| Superior RT field border‡ | L5/S1 | 12 (11.2%) |
| L4/L5 | 24 (22.4%) | |
| L3/L4 | 21 (19.6%) | |
| L2/L3 | 18 (16.8%) | |
| L1/L2 | 23 (21.5%) | |
| T12/L1 | 7 (6.5%) | |
| T11/T12 | 2 (1.9%) | |
Abbreviations: CholPET = 11C-choline-positron emission tomography; CT = computed tomography; CTV = clinical target volume; DVH = dose-volume histogram; LN = lymph node; PA = paraortic; RT = radiation therapy.
Values are reported as *median (range), †median (interquartile range), or as ‡number (%).
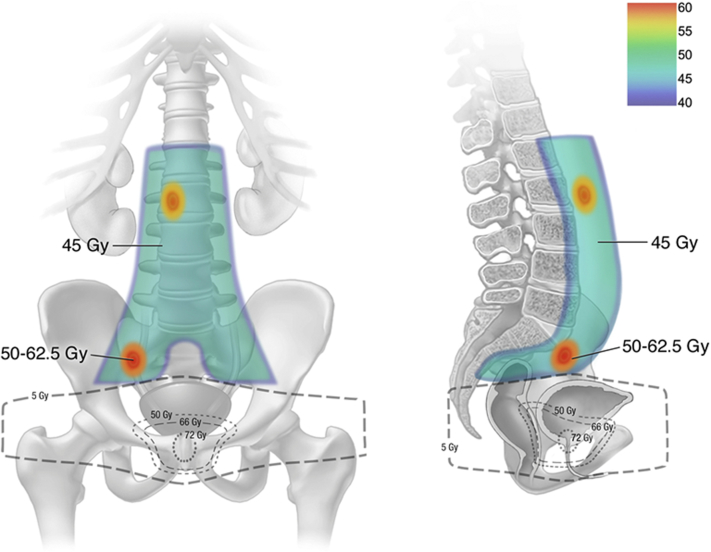
RT treatment fields were determined based on location of CholPET-detected nodal recurrence with consideration of previous RT treatment to minimize field overlap. Generally patients received elective pelvic nodal RT, or in the case of CholPET-detected PA nodal recurrence, pelvic + PA nodal RT. Elective target volumes were defined as clinical target volume 1 (CTV1). The gross CholPET-avid disease with a 5 to 10 mm expansion was targeted as CTV2. A 5-mm expansion was added to each CTV to generate a planning target volume (PTV). Median RT dose to PTV1 was 45 Gy, whereas PTV2 simultaneously received a median dose of 56.25 Gy (range, 50-62.5 Gy) all in 25 fractions (Fig 1). In those with previous RT to the prostatic fossa, the inferior RT field border was matched with previous RT fields. In RT-naïve patients, the prostatic fossa was included in CTV1, followed by a sequential boost to a median dose of 68 Gy.
Figure 1.
Representative image of our 11C-choline-positron emission tomography (CholPET) guided salvage pelvic and paraortic nodal radiation therapy technique matching with previous prostatic fossa radiation therapy fields and treating the elective pelvic and paraortic nodal regions, with a simultaneous integrated boost to CholPET-avid sites of disease using volumetrically modulated arc therapy and daily image guidance.
Patients received AS for 2 to 4 months before RT, concurrently with RT, and adjuvantly, with a minimum goal of 12 to 18 months in total duration. Longer-term AS was administered per clinical discretion and patient tolerance. Ninety-five percent of patients received gonadotropin-releasing-hormone receptor agonists or antagonists.
AE assessment
Patients were assessed prospectively by their treating providers for GI AEs (diarrhea, fecal incontinence, proctitis, rectal hemorrhage, rectal stenosis, rectal ulceration, and small bowel obstruction) and nonerectile GU AEs (bladder spasm, noninfective cystitis, hematuria, urinary frequency, urinary incontinence, urinary retention, urinary tract obstruction, urinary tract pain, and urinary urgency) before initiation, during RT, and immediately after RT delivery, with grading of AEs per Common Terminology Criteria for Adverse Events (version 4.0). Patients were monitored in a prospective manner at 3- to 6-month intervals with data gathered retrospectively. Maximum GI and GU AE was recorded and used for analysis. Early AEs were defined as those that occurred between 1 to 8 months, with late AEs defined as those occurring between >8 and 36 months.
Statistical analysis
The analysis was performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA), with P < .05 denoting statistical significance. The Wilcoxon rank sum test and Wilcoxon signed rank test were used to test for a significant change in GI or GU AEs among baseline, immediately post-RT, and early (median, 4 months) and late (median, 14 months) follow-up and for a significant difference in the number of patients experiencing increased AEs at each follow-up relative to baseline. Patients with missing data at post-RT or early or late follow-up were excluded from these comparisons. Univariate methods were used to evaluate whether treatment characteristics including prior salvage LND, prior receipt of chemotherapy, or inclusion of the prostatic fossa at the time of CholPET were associated with GI or GU AEs. Inclusion of PA nodal fields and small bowel dosimetric parameters were assessed for an association with GI AEs. When comparing all other continuous variables among groups, the Student's t test was used.
Results
GI/GU AEs
Baseline patient characteristics are shown in Table 2. Median follow-up was 16 months (interquartile range [IQR], 11-25), with median early AE assessments and late AE assessments occurring at 4 months (IQR, 3-5) and 14 months (IQR, 12-17), respectively. Eighty-six patients remained (81%) on AS at the time of last follow-up after salvage nodal radiation, with a median duration of adjuvant AS of 16 months (IQR, 11-20).
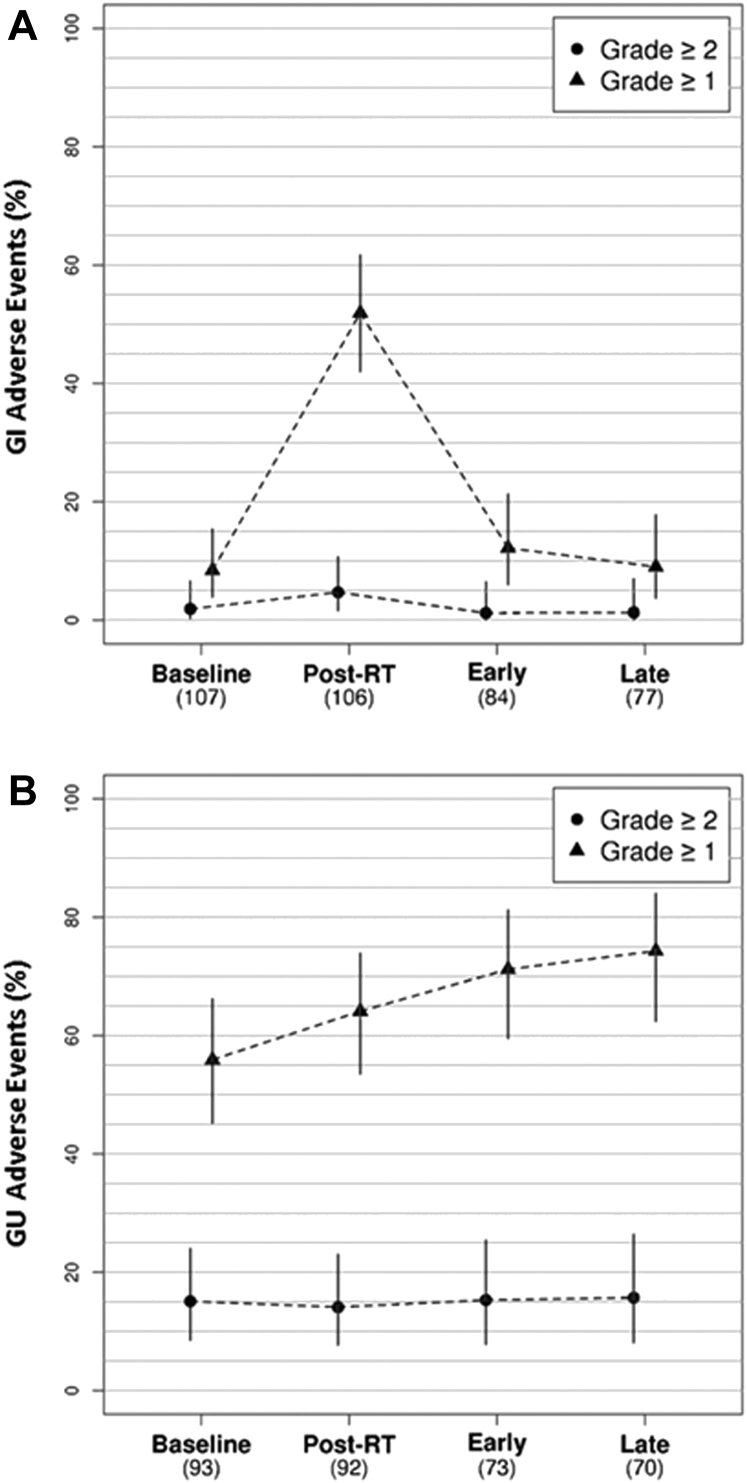
GI and GU AEs at baseline, immediately post-RT, and at early (median, 4 months) and late AE assessment (median, 14 months) are shown in Table 4 and Figure 2. Grade 1 to 2 GI AEs occurred in 51.9% of patients (4.7% of patients experienced grade 2) post-RT compared with 8.4% at baseline (P < .01). These differences resolved by 4-month (12.2%, P = .65) and 14-month AE assessment (9.1%, P = .87). Although there was no significant change in grade 1 to 2 GU AEs post-RT (64.1%) relative to baseline (56.0%, P = .21), differences did arise at 4-month (72.2%, P = .01) and 14-month (74.3%, P = .01) AE assessment. There were no grade 3 or higher GI or GU AEs.
Table 4.
GI and GU AEs
| Organ system | AE grade | Baseline |
Post-XRT |
Early (1-8 mo) |
Late (>8-36 mo) |
|---|---|---|---|---|---|
| N = 107∗ | N = 107∗ | N = 106∗ | N = 94∗ | ||
| GI | Grade 0 | 98 (91.6%) | 51 (48.1%) | 72 (87.8%) | 70 (90.9%) |
| Grade 1 | 7 (6.5%) | 50 (47.2%) | 9 (11.0%) | 6 (7.8%) | |
| Grade 2 | 2 (1.9%) | 5 (4.7%) | 1 (1.2%) | 1 (1.3%) | |
| GU | Grade 0 | 41 (44.1%) | 33 (35.9%) | 20 (27.8%) | 18 (25.7%) |
| Grade 1 | 38 (40.9%) | 46 (50.0%) | 41 (56.9%) | 41 (58.6%) | |
| Grade 2 | 14 (15.1%) | 13 (14.1%) | 11 (15.3%) | 10 (14.3%) | |
| Grade 3 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.4%) |
Abbreviations: AE = adverse effects; GI = gastrointestinal; GU = genitourinary; RT = radiation therapy.
Number of patients with sufficient follow-up time for evaluation.
Figure 2.
Outcomes for gastrointestinal (A) and genitourinary (B) adverse events. Abbreviations: GI = gastrointestinal; GU = genitourinary; RT = radiation therapy.
Variables associated with AEs
There was no association between the receipt of prior chemotherapy (P = .49), prior LND (P = .89), prior PF RT (P = .16), inclusion of PA LN fields above L3/L4 (P = .38), or inclusion above L2/L3 (P = .92) and increased post-RT GI AEs. The median (goal planning parameter) achieved small bowel DVH parameters included a bowel maximum dose of 50.8 Gy (<52 Gy), V50 Gy of 0.1 cm3 (<2 cm3), V45 Gy of 27.9 cm3 (<150 cm3), and V30 Gy of 213.4 cm3 (<300 cm3). Deviations from goal planning parameters occurred for maximum dose (25.2%), V50 Gy (14.0%), V45 Gy (1.9%), and V30 Gy (7.5%), although these deviations were not associated with increased risk of post-RT GI AEs, with P = .86, .64, .30, and .33, respectively. Similarly, small bowel V45 Gy > 28 cm3 (median) was not associated with increased GI AEs (P = .37). None of these factors were associated with increased GI AEs at 4-month or 14-month assessment (all P > .05).
Variables associated with increased acute GU AEs were also assessed. Compared with patients who had previous prostatic fossa RT, receipt of prostatic fossa RT during CholPET guided RT (24%) was not associated with increased GU AEs immediately post-RT (P = .06), at 4-month assessment (P = .83), or at 14-month assessment (P = .55), although a trend toward significantly worse GU AEs immediately post-RT is evident in this limited patient subset. Receipt of prior chemotherapy was associated with increased GU AEs immediately post-RT (P = .02), but no differences persisted at 4-month (P = .36) or 14-month assessment (P = .71). Salvage LND before CholPET guided RT (P = .91) or inclusion of PA nodal fields was not associated with any further increases in GU AEs immediately post-RT or at 4-month or 14-month AE assessment (all P > .05).
Discussion
We report on a cohort of 107 patients who experienced BF of prostate cancer after RP and underwent salvage combined modality therapy involving AS with pelvic and/or PA lymph node irradiation using 11C-choline-PET guided SIB to sites of CholPET-avid recurrence. Patients were treated with AS consistent with current guidelines for patients who present with nodal metastases.20 Additionally, patients were treated to the contralateral and adjacent nodal regions even if only a single CholPET-avid site was found. The rationale for this approach is based on pathologic analysis of surgical LND specimens that identify the presence of more nodal micrometastases than are evident with pcPET techniques and is consistent with recent recommendations relating to radiotherapeutic management of prostate cancer nodal recurrence.21, 22 With a median follow-up of 16 months, the findings from this patient cohort indicate acceptably low rates of acute GI and GU AEs, particularly compared with historical series. Furthermore, no significant detriment in GI AEs was recorded at the 14-month late AE assessment.
Approximately one-quarter of men will experience BF within 5 years of RP for prostate cancer, which often prompts salvage RT to the prostatic fossa.23 Biochemical control of disease at 4 years thereafter occurs in 20% to 80%.24 pcPET has greater sensitivity in the localization of prostate cancer recurrence compared with conventional imaging modalities and has facilitated an improved understanding of the natural history of prostate cancer recurrence.3, 5, 6 Parker et al6 reported on CholPET-detected recurrence patterns in patients who experienced BF after RP and post-RP RT to the prostatic fossa. At a median PSA of 1.4 ng/mL, a maximal superior extent of disease was limited to the pelvic LNs (41%), whereas an additional 22% had common iliac LNs and 20% had PA LNs. The rate of osseous metastases was <5%. These data support the observation that a subset of patients may develop a locoregionally predominant pattern of LN spread as an intermediate stage of disease progression occurring between BF and development of more distant metastases. As such, clinicians have begun to reconsider locoregionally directed treatment strategies in select patients who experience BF after primary treatment.8, 19
Multiple cooperative group trials have evaluated the role of elective pelvic and/or PA LN irradiation in the management of patients with prostate cancer and a high risk of occult lymph node metastases and have not found a significant oncologic advantage with such treatment.12, 13, 15, 16 However, these trials do not provide guidance on how best to manage patients with clinical or pathologic LN recurrences of prostate cancer after initial treatment. Recent and large observational series using RT and/or surgery as initial management in patients with N1 disease have reported an apparent benefit with respect to various endpoints, including recurrence-free, cause-specific, and overall survival.25, 26, 27, 28 The use of pcPET has allowed for earlier and improved detection of LN recurrences2, 3, 5, 6 and provided opportunities for salvage treatment strategies using AS, salvage LND ± postoperative RT, salvage RT, or a combined-modality approach.7, 8, 9, 10, 21, 29, 30
As suggested by Parker et al,6 approximately 40% of patients will be identified as having LN recurrence above standard elective pelvic RT fields necessitating large salvage RT nodal treatment fields and raising concern for the morbidity of such a treatment. For instance, in our series, 71 patients (66%) underwent RT with a superior field border that included PA sites at or above the L3/L4 interspace. We identified low rates of GI/GU AEs, with an immediately post-RT grade 2 AE rate of 5%, which diminished to 1% by 14-month AE assessment, and no grade 3 GI AEs. There was a significant increase in GU AEs with time; however, it is notable that the majority of patients experienced grade 1 AEs, with a low rate of grade 2 + AEs. We did not identify any association between the inclusion of PA nodal fields or small bowel DVH parameters and an increase in GI toxicity. Inclusion of the PF within salvage RT fields was not associated with a significant increase in GU toxicities, although a trend was noted.
Importantly, these data represent a significant improvement in the AEs reported in historical series evaluating the utility of 2- or 3-dimensional conformal pelvic RT with or without PA nodal RT for GU or gynecologic malignancies (Table 4), for which grade 2 + GI AEs may range from 2% to 40%.12, 13, 15, 16, 17, 18 We contend these differences are attributable to an improved understanding of RT dose-volume relationships that predict for GI AEs, relatively strict planning parameters, and the use of volumetrically modulated arc therapy with daily image guidance, which allowed for reductions in RT exposure to GI OARs.11 Our institutional OAR planning parameters include small bowel maximum dose <52 Gy, V50 Gy < 2 cm3, V45 Gy < 150 cm3, and V30 Gy < 300 cm3, all of which tend to be more strict than consensus recommendations.11 Twenty-five percent of patients had deviations greater than our maximum dose of 52 Gy, although data would support acceptably low rates of GI toxicity if the small bowel maximum dose is kept to less than 55 Gy.31, 32 The greatest priority was placed on maintaining the V45 Gy < 150 cm3, evidenced by our median V45 Gy of 27.9 cm3. Two patients exceeded this constraint (151 cm3 and 175 cm3), although both were maintained at less than the 195 cm3 suggested in published guidelines.11 Taken together, we treated 66% of our patients with nodal fields extending above standard pelvic RT fields with an SIB to a dose of 56.25 Gy in 25 fractions to CholPET-avid sites of disease, but we did so carefully with strict adherence to small bowel dosimetric parameters as our greatest planning priority.
The most comparable reported series is that by Picchio et al,9 which included 83 patients with CholPET-detected LN-only recurrences. Patients received elective nodal RT to the entire at-risk nodal chain (mean dose, 52 Gy in 28 fractions) with an SIB to a mean dose of 65 Gy. Sites of CholPET-avid disease were predominately pelvic (52%), abdominal (16%), and pelvic/abdominal (19%). They identified a 4.3% rate of acute grade 2 GI AEs. More recently, GETUG-P07 has reported 1-year AEs of CholPET-directed salvage RT for prostate cancer pelvic LN recurrence. Acute grade 2 GU and GI AEs were reported in 13% and 15%, respectively.33 By 1 year, these rates dropped to 10% and 6%, respectively. Potential explanations for the higher AEs in this series is that they treated to higher RT doses of 54 Gy to the elective pelvis with an SIB to 66 Gy while also having robust AE assessments across time in the context of a prospective clinical trial. Similar to our series, they did not identify any increased acute or 1-year GI or GU AEs in the subset of patients who received RT to the prostatic fossa at the time of pelvic LN RT; however, these subset comparisons are likely underpowered. Albeit with significant patient cohort and treatment heterogeneity, the systematic reviews published by Ost et al7 and Ploussard et al10 and the recently published prospective phase 2 trial by Ost et al8 further corroborate the low rates of grade 2 GI/GU AEs and provide support for the therapeutic efficacy of this salvage strategy.
Limitations of this study include the lack of patient-reported outcomes, reporting of AEs by the organ system maximum grade method rather than individual symptom indices over time, and the retrospective design without controlled comparisons of treatment with AS alone or other RT techniques. Treatments received before or after CholPET guided RT were heterogeneous. Reassuringly, no added GI AEs were identified in those who had inclusion of PA LN fields, previous RT to the prostatic fossa, previous chemotherapy, or previous salvage LND. Furthermore, despite favorable AE rates at 4-month and 14-month AE assessment, 14-month AE assessment was only available for 72% of patients, predominately because of patient loss to follow-up or limited follow-up time.
Conclusions
We report on a large series of patients who experienced BF of prostate cancer after RP with and without previous RT to the prostatic fossa and underwent salvage combined-modality therapy involving AS with pelvic and/or PA nodal RT using CholPET guided SIB. At 16 months’ median follow-up, we identified low rates of acute GI and GU AEs and no significant detriment in 14-month GI AEs. This study describes methods by which to treat pelvic and PA prostate cancer lymph node recurrences and may be of value in designing prospective trials evaluating this salvage treatment.
Footnotes
Sources of support: This work was made possible in part through a grant from the National Institutes of Health (NCIR01 CA200551).
Disclosures: none.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Evans J.D., Jethwa K.R., Ost P. Prostate cancer-specific PET radiotracers: A review on the clinical utility in recurrent disease. Pract Radiat Oncol. 2017;8:28–39. doi: 10.1016/j.prro.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Parker W.P., Davis B.J., Park S.S. Identification of site-specific recurrence following primary radiation therapy for prostate cancer using C-11 choline positron emission tomography/computed tomography: A nomogram for predicting extrapelvic disease. Eur Urol. 2017;71:340–348. doi: 10.1016/j.eururo.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanti S., Minozzi S., Castellucci P. PET/CT with (11)C-choline for evaluation of prostate cancer patients with biochemical recurrence: Meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging. 2016;43:55–69. doi: 10.1007/s00259-015-3202-7. [DOI] [PubMed] [Google Scholar]
- 5.Sobol I., Zaid H.B., Haloi R. Contemporary mapping of post-prostatectomy prostate cancer relapse with 11C-choline positron emission tomography and multiparametric magnetic resonance imaging. J Urol. 2017;197:129–134. doi: 10.1016/j.juro.2016.07.073. [DOI] [PubMed] [Google Scholar]
- 6.Parker W.P., Evans J.D., Stish B.J. Patterns of recurrence after postprostatectomy fossa radiation therapy identified by C-11 choline positron emission tomography/computed tomography. Int J Radiat Oncol Biol Phys. 2017;97:526–535. doi: 10.1016/j.ijrobp.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ost P., Bossi A., Decaestecker K. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: A systematic review of the literature. Eur Urol. 2015;67:852–863. doi: 10.1016/j.eururo.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Ost P., Reynders D., Decaestecker K. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 9.Picchio M., Berardi G., Fodor A. (11)C-Choline PET/CT as a guide to radiation treatment planning of lymph-node relapses in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2014;41:1270–1279. doi: 10.1007/s00259-014-2734-6. [DOI] [PubMed] [Google Scholar]
- 10.Ploussard G., Almeras C., Briganti A. Management of node only recurrence after primary local treatment for prostate cancer: A systematic review of the literature. J Urol. 2015;194:983–988. doi: 10.1016/j.juro.2015.04.103. [DOI] [PubMed] [Google Scholar]
- 11.Kavanagh B.D., Pan C.C., Dawson L.A. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S101–107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 12.Asbell S.O., Krall J.M., Pilepich M.V. Elective pelvic irradiation in stage A2, B carcinoma of the prostate: Analysis of RTOG 77-06. Int J Radiat Oncol Biol Phys. 1988;15:1307–1316. doi: 10.1016/0360-3016(88)90225-8. [DOI] [PubMed] [Google Scholar]
- 13.Pilepich M.V., Krall J.M., Johnson R.J. Extended field (periaortic) irradiation in carcinoma of the prostate—analysis of RTOG 75-06. Int J Radiat Oncol Biol Phys. 1986;12:345–351. doi: 10.1016/0360-3016(86)90349-4. [DOI] [PubMed] [Google Scholar]
- 14.Pilepich M.V., Pajak T., George F.W. Preliminary report on phase III RTOG studies of extended-field irradiation in carcinoma of the prostate. Am J Clin Oncol. 1983;6:485–491. doi: 10.1097/00000421-198308000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Pommier P., Chabaud S., Lagrange J.L. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25:5366–5373. doi: 10.1200/JCO.2006.10.5171. [DOI] [PubMed] [Google Scholar]
- 16.Roach M., 3rd, DeSilvio M., Lawton C. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21:1904–1911. doi: 10.1200/JCO.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Rotman M., Choi K., Guse C., Marcial V., Hornback N., John M. Prophylactic irradiation of the para-aortic lymph node chain in stage IIB and bulky stage IB carcinoma of the cervix, initial treatment results of RTOG 7920. Int J Radiat Oncol Biol Phys. 1990;19:513–521. doi: 10.1016/0360-3016(90)90475-y. [DOI] [PubMed] [Google Scholar]
- 18.Morris M., Eifel P.J., Lu J. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 19.Supiot S., Rio E., Pacteau V., Mauboussin M.H., Campion L., Pein F. OLIGOPELVIS- GETUG P07: A multicentre phase II trial of combined salvage radiotherapy and hormone therapy in oligometastatic pelvic node relapses of prostate cancer. BMC Cancer. 2015;15:646. doi: 10.1186/s12885-015-1579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohler J.L., Armstrong A.J., Bahnson R.R. Prostate cancer, version 1.2016. J Natl Compr Cancer Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 21.Zattoni F., Nehra A., Murphy C.R. Mid-term outcomes following salvage lymph node dissection for prostate cancer nodal recurrence status post-radical prostatectomy. Eur Urol Focus. 2016;2:522–531. doi: 10.1016/j.euf.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Lieng H., Hayden A.J., Christie D.R.H. Radiotherapy for recurrent prostate cancer: 2018 recommendations of the Australian and New Zealand radiation Oncology Genito-urinary group. Radiother Oncol. 2018;129:377–386. doi: 10.1016/j.radonc.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 23.Amling C.L., Blute M.L., Bergstralh E.J., Seay T.M., Slezak J., Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: Continued risk of biochemical failure after 5 years. J Urol. 2000;164:101–105. [PubMed] [Google Scholar]
- 24.Tendulkar R.D., Agrawal S., Gao T. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34:3648–3654. doi: 10.1200/JCO.2016.67.9647. [DOI] [PubMed] [Google Scholar]
- 25.Abdollah F., Karnes R.J., Suardi N. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. 2014;32:3939–3947. doi: 10.1200/JCO.2013.54.7893. [DOI] [PubMed] [Google Scholar]
- 26.James N.D., Spears M.R., Clarke N.W. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: Data from patients in the control arm of the STAMPEDE trial. JAMA Oncol. 2016;2:348–357. doi: 10.1001/jamaoncol.2015.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briganti A., Karnes R.J., Da Pozzo L.F. Combination of adjuvant hormonal and radiation therapy significantly prolongs survival of patients with pT2-4 pN+ prostate cancer: Results of a matched analysis. Eur Urol. 2011;59:832–840. doi: 10.1016/j.eururo.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Van Hemelryk A., De Meerleer G., Ost P. The outcome for patients with pathologic node-positive prostate cancer treated with intensity modulated radiation therapy and androgen deprivation therapy: A case-matched analysis of pN1 and pN0 patients. Int J Radiat Oncol Biol Phys. 2016;96:323–332. doi: 10.1016/j.ijrobp.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Rigatti P., Suardi N., Briganti A. Pelvic/retroperitoneal salvage lymph node dissection for patients treated with radical prostatectomy with biochemical recurrence and nodal recurrence detected by [11C]choline positron emission tomography/computed tomography. Eur Urol. 2011;60:935–943. doi: 10.1016/j.eururo.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 30.Suardi N., Gandaglia G., Gallina A. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: Results of a single-institution series with a minimum follow-up of 5 years. Eur Urol. 2015;67:299–309. doi: 10.1016/j.eururo.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Stanic S., Mayadev J.S. Tolerance of the small bowel to therapeutic irradiation: A focus on late toxicity in patients receiving para-aortic nodal irradiation for gynecologic malignancies. Int J Gynecol Cancer. 2013;23:592–597. doi: 10.1097/IGC.0b013e318286aa68. [DOI] [PubMed] [Google Scholar]
- 32.Verma J., Sulman E.P., Jhingran A. Dosimetric predictors of duodenal toxicity after intensity modulated radiation therapy for treatment of the para-aortic nodes in gynecologic cancer. Int J Radiat Oncol Biol Phys. 2014;88:357–362. doi: 10.1016/j.ijrobp.2013.09.053. [DOI] [PubMed] [Google Scholar]
- 33.Vaugier L., Palpacuer C., Rio E. Early toxicity of a phase 2 trial of combined salvage radiation therapy and hormone therapy in oligometastatic pelvic node relapses of prostate cancer (OLIGOPELVIS GETUG P07) Int J Radiat Oncol Biol Phys. 2019;103:1061–1067. doi: 10.1016/j.ijrobp.2018.12.020. [DOI] [PubMed] [Google Scholar]