Abstract
Purpose
Three-dimensional (3D) conformal radiation therapy is the standard technique used for adjuvant breast radiation. We report the clinical use of a novel 6-MV flattening-filter-free O-ring linear accelerator (6X-FFF ORL) for breast cancer that may improve upon 3D conformal radiation therapy with its higher dose rate and faster rotation and leaf speed than traditional C-arm gantries.
Methods and Materials
We retrospectively identified consecutive women with breast cancer who underwent surgery followed by radiation therapy to the breast or chest wall on Halcyon (Varian Medical Systems, Palo Alto, CA), a novel 6X-FFF ORL. We report their clinicopathologic information, radiation therapy details, acute toxicities, dose-volume histogram data, couch corrections, and treatment times.
Results
Thirty-four women were treated for breast cancer on a 6X-FFF ORL between February 2018 and September 2018. All patients underwent lumpectomy (92%) or mastectomy (8%). Tumors were left sided in 44% and bilateral in 9%, and 9% included comprehensive nodal radiation therapy. Twelve percent of patients were treated prone and 29% with deep-inspiration breath hold. Standard target and normal-tissue constraints were met in nearly all plans. The 3D vector couch correction average was 0.77 ± 0.05 cm. The mean beam-on time was 2.0 ± 0.3 minutes, and mean treatment time from start of imaging to beam-off was 4.4 ± 0.4 minutes. Grade 2 dermatitis, fatigue, and breast pain occurred in 18%, 9%, and 3% of patients, respectively.
Conclusions
In this first clinical report of breast radiation therapy with a 6X-FFF ORL, treatment was versatile and fast for complex setups and techniques, with acceptable toxicity and organ-at-risk doses. Thus, a 6X-FFF ORL can increase throughput or reduce length of day compared with a conventional C-arm linear accelerator in departments with a busy breast service.
Introduction
The multimodality management of breast cancer often involves surgery (lumpectomy or mastectomy) with or without adjuvant systemic therapy or radiation therapy. Since the initial reports of the use of radiation therapy to treat breast cancer in the 1930s,1, 2 numerous technological advances have been applied to breast radiation therapy to improve its clinical accuracy, efficacy, and tolerability. Computed tomography (CT) radiation treatment planning and 3-dimensional (3D) conformal radiation therapy have helped maximize target doses while minimizing organ-at-risk (OAR) doses.3, 4 Forward planning of field-in-field (FIF) segments has improved on the dose homogeneity and toxicity of simple 2-dimensional tangential radiation. Modern techniques, including deep-inspiration breath hold (DIBH)5 and prone positioning,6 have been used to further minimize OAR doses, particularly to the heart and lungs. Newer forms of radiation delivery including intensity modulated radiation therapy and proton-beam therapy have been used to improve treatment conformality in women with challenging anatomy.3 Volumetric modulated arc therapy, a subset of intensity modulated radiation therapy, delivers radiation in a continuous, dynamic arc, maximizing conformality while shortening treatment time.7, 8
A novel, commercially available linear accelerator (linac), Halcyon (Varian Medical Systems, Palo Alto, CA), has been designed to provide faster delivery and higher throughput with a single 6-MV flattening-filter-free (6X-FFF) beam energy, an O-ring gantry that rotates at higher speeds than a C-arm gantry, and fast-moving multileaf collimators compared with those of C-arm gantries.9 Flattening-filter-free (FFF) beams show increased dose rate, decreased radiation leakage, decreased head scatter, and decreased penumbra compared with flattening-filtered beams.10 Preclinical studies of 6X-FFF O-ring linac (ORL) imaging and treatments using thorax, lung, and breast phantoms have demonstrated accurate MV imaging dose calculation, treatment planning dose distribution,11 and degree of the interplay effect.12 In a previous study of a 6X-FFF ORL, 30 patients with head and neck cancer had dual-arc and triple-arc volumetric modulated arc therapy plans designed on a 6X-FFF ORL and dual-arc plans designed on a C-arm linac (CAL) (TrueBeam, Varian Medical Systems). Comparisons of the plans and treatments demonstrated decreased imaging times and plan delivery times for the 6X-FFF ORL plans compared with the CAL plans, with comparable plan quality and toxicity probabilities.9
In this study, we report our initial clinical experience treating patients with breast cancer on a 6X-FFF ORL. We hypothesized that breast radiation therapy on a 6X-FFF ORL would have acute toxicities, target, and OAR doses comparable to those with CAL and shorter treatment times.
Methods and Materials
We performed a retrospective review of women with breast cancer who underwent breast-conserving surgery or mastectomy followed by radiation therapy to the breast(s) or chest wall, with or without regional lymph nodes, on a 6X-FFF ORL between February 2018 and September 2018. Exclusion criteria were the receipt of breast, chest wall, or regional lymph node radiation therapy for palliative purposes or for metastatic disease. Clinical and radiation therapy planning data were retrospectively abstracted from patients’ electronic medical records with institutional review board approval.
Prescriptions and constraints
All patients underwent CT simulation either in supine position, with or without DIBH, or prone position. Target volumes were contoured per Radiation Therapy Oncology Group (RTOG) guidelines.13 Patients received either conventionally fractionated radiation therapy in 1.8 to 2.0 Gy per fraction to a total dose of 50 to 50.4 Gy or hypofractionated treatment in 2.66 Gy per fraction to a total dose of 42.56 Gy. When indicated at the discretion of the treating physician, a lumpectomy cavity boost was delivered in 2 Gy per fraction to a total dose of 10 Gy. When indicated at the discretion of the treating physician, an anterior oblique supraclavicular field with or without a matching posterior field was added and treated to 50 Gy. In cases in which a supraclavicular field was used, a separate supraclavicular isocenter was necessary owing to the field size limitation of the 6X-FFF ORL (28 cm superior to inferior). Target coverage parameters required that 95% of the breast planning target volume (PTV) receive at least 95% of the prescription dose. All treatment planning was performed in Eclipse (Varian Medical Systems; v15.1 for Halcyon 1.0 and v15.6 for Halcyon 2.0). All plans used 6X-FFF photon beams with opposed tangential fields. Dose homogeneity constraint goals stipulated that the volume of breast receiving more than 105% of the prescription dose be limited to less than 10% to 15% of the breast volume, with a maximum hotspot of <110%. OAR constraints included ipsilateral lung V20 < 15% (max 20%) for breast-only plans and V20 < 35% (max 40%) for breast and nodal plans, heart V20 < 5%, and mean heart dose <4 Gy.
Treatment planning
All treatment plans were generated with one of the following forward-planning techniques: an irregular surface compensator (ISC) or FIF with native FFF beam or dynamic beam flattening (DBF) enabled. The ISC technique uses dynamic leaf motion to generate fluence maps that produce a homogenous dose distribution at a prescribed depth.14, 15 In the regular FIF technique, the dose inhomogeneity caused by the variation in tissue depth is removed by manually adding multiple (<3) weighted segments. However, for FFF beams with nonuniform beam profiles, this manual process is unintuitive and challenging for planners. In the FIF with DBF technique, 1 layer of multileaf collimators is used to flatten the field dynamically while the other forms the aperture defining the field shape. Detailed information about DBF for breast irradiation was reported previously.16 The dynamically flattened beam profile makes it similar to flattening-filtered beams, enabling the planner to use the regular FIF technique without additional training.
Twenty-nine of the 34 patients in the study were treated with the ISC, 3 were treated with DBF-enabled FIF, and 2 were treated with FIF. The DBF technique was not available in the first version of the 6X-FFF ORL (v1.0), so all treatments on v1.0 used ISC when necessary to produce a homogeneous dose distribution in the target. After the second version of the 6X-FFF ORL (v2.0) was introduced, DBF was implemented for a number of patients. However, ISC was favored given its lower monitor units (143% increase in monitor units to deliver an identical dose with DBF at 5 cm depth for a 20 × 20 cm field) and shorter treatment times (average 9 minutes for DBF and 3-4 minutes for ISC).16 In 2 cases, a simple FIF technique used with unflattened fields was found to be sufficient to produce a homogenous dose distribution given the patient anatomy.
Image guidance
All patients received cone beam CT (CBCT) with every treatment fraction. This is a requirement for the 6X-FFF ORL, which does not perform port films and does not contain a field light to confirm skin markers or source-to-surface distance. Version 1.0 included only MV CBCT capability, using the same MV x-ray source used for treatment fields. The MV dose was accounted for and included in the delivered dose with the planning system. Version 2.0 was enhanced to include a kV CBCT system. Of the 34 patients in the study, 27 were treated entirely with MV CBCT, 2 started with MV CBCT and finished with kV CBCT with a brief transition period of 3 fractions on a CAL, and 5 were treated entirely with kV CBCT.
Data analysis
The primary objectives of this study are to report the versatility, toxicity, dose-volume histogram (DVH) data, and speed of radiation therapy with a 6X-FFF ORL for breast cancer. Versatility was assessed by reporting clinicopathologic features of the varied breast/chest wall cases treated on the machine. Acute toxicity data were graded in accordance with the Common Terminology Criteria for Adverse Events v4.03, using provider-reported outcomes assessed weekly on treatment and at standard follow-up visits. Target and OAR DVH data were recorded to assess the dosimetry of treatments. The average treatment couch corrections applied from skin-marker alignment after matching online CBCT with planning CT were measured to demonstrate the manual setup consistency and the added value of daily CBCT using a 6X-FFF ORL. Speed was assessed by average daily treatment time, imaging time, and total room usage time.
Statistics
Data were reported using descriptive statistics (means, medians, ranges, and standard deviations when appropriate for continuous variables and percentages for categorical variables). Beam-on time was compared with a reference value using a 2-sided, 1-sample Student's t-test,17 whereas couch corrections were compared qualitatively. Data were analyzed using the MATLAB R2017a Statistics Toolbox software package (MathWorks Inc, Natick, MA).
Results
Types of patients treated on a 6X-FFF ORL
Our analysis included 34 consecutive patients analyzed at a median follow-up interval of 2.91 months (range, 0.76-7.23 months).
Most patients had unilateral disease (47%, n = 16 right sided; 44%, n = 15 left sided), whereas 9% of patients (n = 3) had bilateral disease. Tumor histology was primarily invasive ductal carcinoma (78%, n = 29), with 8% invasive lobular carcinoma (n = 3), 8% mixed invasive ductal and lobular carcinoma (n = 3), and 5% ductal carcinoma in situ (n = 2). Most patients had stage I disease (67%, n = 25), and all but 1 (97%, n = 36) had stage 0, I, or II disease. Most patients received lumpectomy (92%, n = 34), but 3 patients had mastectomy with reconstruction (8%). Chemotherapy was delivered in 41% of patients (n = 14), not concurrently with radiation therapy, although 9% of patients (n = 3) received systemic anti-HER2 therapy concurrently with radiation therapy.
Radiation therapy details are summarized in Table 1. Most patients were treated supine (88%, n = 30), whereas 12% (n = 4) were treated prone. DIBH was used in 29% of patients (n = 10). Right breast–only and left breast–only radiation therapy were the most common targets treated (38% [n = 13] and 35% [n = 12], respectively), with 1 patient (3%) receiving bilateral breast–only radiation therapy. Three patients (9%) received unilateral high-tangent fields to encompass the low axilla. One patient (3%) received bilateral breast radiation therapy, with the left side receiving a high tangent field. Two patients (6%) were treated to the left chest wall with comprehensive nodal irradiation (1 patient [3%] did not receive a supraclavicular field owing to patient refusal), and 1 patient (3%) was treated to the right chest wall with comprehensive nodal irradiation. Patients received a median total dose of 52.56 Gy (range, 42.56-52.70 Gy) in 21 fractions (range, 16-28). A tumor bed boost of 10 Gy in 5 fractions was delivered 78% of the time (n = 29) using electron fields (34%, n = 10) on a CAL (electrons are not delivered on the 6X-FFF ORL) or mini tangential photon fields (66%, n = 19) (2 mini tangents were not delivered on the 6X-FFF ORL owing to use of >6 MV energy or wedges). No patients were treated with bolus.
Table 1.
Details of radiation therapy course
| Variable | Value (%) |
|---|---|
| RT position | |
| Supine | 30 (88) |
| Prone | 4 (12) |
| Deep-inspiration breath hold | |
| Yes | 10 (29) |
| No | 24 (71) |
| RT field | |
| Left breast only | 12 (35) |
| Right breast only | 13 (38) |
| Bilateral breast only | 2 (6) |
| Left breast and low axilla | 1 (3) |
| Right breast and low axilla | 2 (6) |
| Left breast and low axilla, and right breast only | 1 (3) |
| Left chest wall and comprehensive nodal irradiation∗ | 2 (6) |
| Right chest wall and comprehensive nodal irradiation | 1 (3) |
| Delivered dose, Gy | |
| Median | 52.56 |
| Range | 42.56-52.70 |
| No. of fractions | |
| Median | 21 |
| Range | 16-28 |
| Tumor bed boost† | |
| Yes | 29 (78) |
| No | 8 (22) |
| Boost modality | |
| Electrons‡ | 10 (34) |
| Mini tangents‡ | 19 (66) |
| Boost dose in 5 fractions, Gy | |
| Median | 10 |
| Range | 10-10 |
Abbreviation: RT = radiation therapy.
One patient did not receive a supraclavicular field due to refusal.
Values out of number of breasts, not patients treated (3 patients with bilateral disease and RT).
Electrons are not delivered on the 6-MV flattening-filter-free O-ring linear accelerator. Two mini tangents were not delivered on the 6-MV flattening-filter-free O-ring linear accelerator.
Dosimetric parameters are reported in Table 2. All plans met constraints for target coverage. Two plans (5%) did not meet the heterogeneity constraint, breast PTVeval V105 < 10% to 15%, although the point max constraint of <V110 was met in both. Two patients (6%) received a total of 3 fractions on a CAL during the transition from MV CBCT to kV CBCT, using forward-planned FIF with dosimetric parameters comparable to the primary 6X-FFF ORL plans. All but 1 patient (97%) met the ipsilateral lung V20 constraint with a V20 of 25.2% for a breast-only treatment, and all but 1 patient (97%) met the mean heart constraint with a mean heart dose of 5.7 Gy. For left-sided-only treatments and right-sided-only treatments, the mean heart dose was 1.87 Gy (range, 1.21-5.69 Gy) and 1.12 Gy (range, 0.66-1.72 Gy), respectively. When excluding the 2 right-sided-only treatments that included nodal irradiation, the mean heart dose was 1.08 Gy (range, 0.66-1.42 Gy), with median V5, V10, and V20 of 0%.
Table 2.
Dosimetric parameters of targets and OARs
| Variable | Value, median (range), % |
|---|---|
| Breast PTVeval | |
| V95 | 98.9 (95.2-99.9) |
| V105 | 7.6 (0-26.1) |
| Boost PTVeval∗ | |
| V95 | 100 (98.6-100) |
| V105 | 3.6 (0-27.3) |
| Chest wall PTVeval | |
| V95 | 97.5 (96.3-98.7) |
| V105 | 8.2 (7.4-8.9) |
| Heart (all patients) | |
| V5 | 2.2 (0-85.6) |
| V10 | 0.2 (0-14.6) |
| V20 | 0 (0-5.2) |
| Mean, Gy | 1.38 (0.66-5.69) |
| Heart (Left-sided-only treatments) | |
| V5 | 3.4 (0.4-55.9) |
| V10 | 1.1 (0-14.6) |
| V20 | 0.3 (0-5.2) |
| Mean, Gy | 1.87 (1.21-5.69) |
| Heart (Right-sided-only treatments) | |
| V5 | 0 (0-15.0) |
| V10 | 0 (0-1.3) |
| V20 | 0 (0-0) |
| Mean, Gy | 1.12 (0.66-1.72) |
| Heart (Bilateral treatments) | |
| V5 | 6.0 (3.1-85.6) |
| V10 | 0.6 (0.1-9.4) |
| V20 | 0.1 (0-1.3) |
| Mean, Gy | 3.09 (2.96-3.59) |
| Heart (MV CBCT-only patients) | |
| V5 | 2.5 (0-85.6) |
| V10 | 0.1 (0-14.6) |
| V20 | 0 (0-5.2) |
| Mean, Gy | 1.60 (0.78-5.69) |
| Heart (kV CBCT-only patients) | |
| V5 | 0.4 (0-3.4) |
| V10 | 0 (0-2.0) |
| V20 | 0 (0-1.4) |
| Mean, Gy | 1.21 (0.66-1.97) |
| Ipsilateral lung | |
| V5 | 32.9 (0-85.0) |
| V10 | 16.9 (0-48.8) |
| V20 | 13.3 (0-35.6) |
| Mean, Gy | 7.19 (0.75-18.70) |
Abbreviations: CBCT = cone beam computed tomography; OAR = organ at risk; PTVeval = planning target volume for evaluation.
Non–6-MV flattening-filter-free O-ring linear accelerator boosts excluded.
Early toxicity summary
There were no grade 3 or higher acute toxicities. Fatigue and dermatitis were the most frequent grade 1 acute toxicities, each occurring in 65% (n = 22) of patients, with grade 1 breast pain (38%, n = 13), decreased range of motion (9%, n = 3), limb edema (9%, n = 3), and depression (6%, n = 2) also being reported. The most common grade 2 acute toxicity was dermatitis (18%, n = 6), followed by fatigue (9%, n = 3) and breast pain (3%, n = 1).
Patient setup uncertainty and image guided radiation therapy experience
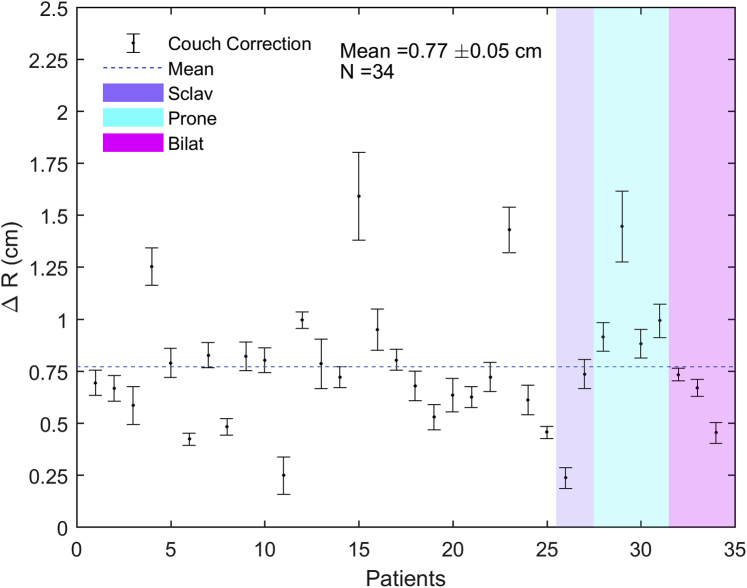
The average 3D vector couch corrections between skin-marker alignment and online CBCT-assisted positioning for all patients in this study and for all fractions of 6X-FFF ORL treatment was 0.77 ± 0.05 cm (Fig 1). The average 3D vector couch correction for patients treated with a 3-field plan (6%, n = 2), in prone position (12%, n = 4), or bilaterally (9%, n = 3) was 0.5 ± 0.2 cm, 1.1 ± 0.1 cm, and 0.62 ± 0.08 cm, respectively.
Figure 1.
Couch corrections after initial setup based on image guided radiation therapy. Average 3-dimensional vector couch correction after initial setup based on image guided radiation therapy for all patients, for all fractions of 6-MV flattening-filter-free O-ring linear accelerator treatment. There were no observed differences in average couch corrections between patients treated with a supraclavicular field versus without a supraclavicular field, in prone versus supine position, or bilaterally versus unilaterally.
Treatment time and throughput analysis
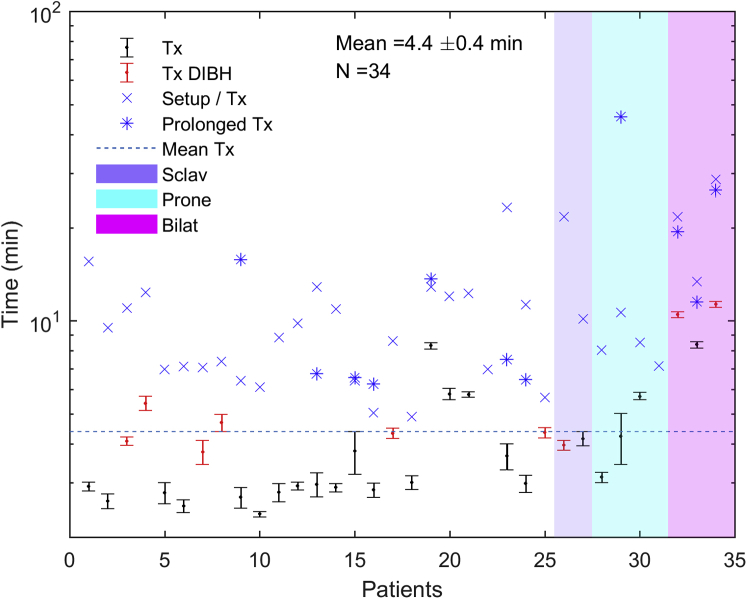
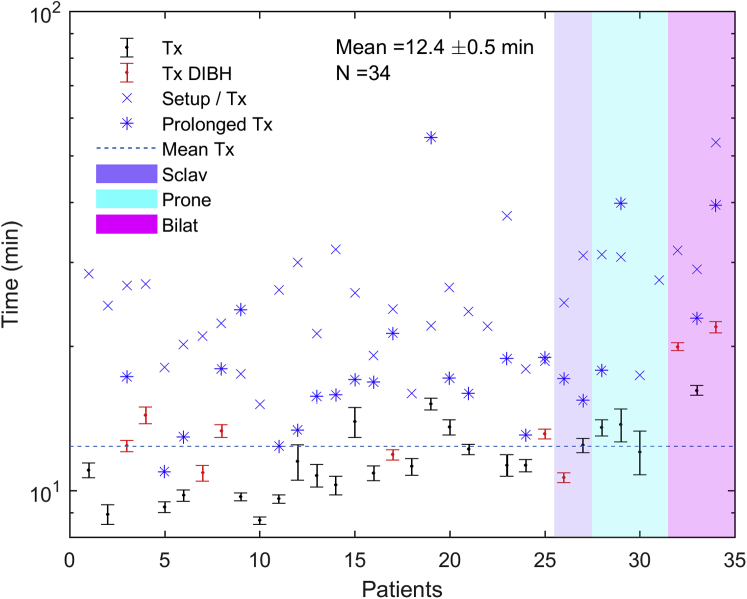
Figures 2 and 3 summarize the treatment times for a standard fraction, the initial fraction (including setup time), and fractions prolonged because of extenuating circumstances unrelated to the treating machine, including software faults, interlocks requiring physics override, equipment-related delays (eg, DIBH device problems), and waiting for setup approval. Outlier treatment times were excluded from average calculations but are depicted in the figures. All treatment times were recorded by the therapists immediately after treatment. Prolonged treatment times were denoted, along with explanations of what caused the prolonged treatments. These explanations were used to determine which prolonged times were for reasons unrelated to the linac itself and therefore should be excluded from the mean calculation as outliers. No times were excluded based on quantitative criteria. Figure 2 shows the treatment time defined as the time from the start of imaging to beam off. The average treatment time for all patients was 4.4 ± 0.4 minutes. The average treatment times for patients treated with DIBH and without DIBH were 5.8 ± 0.98 minutes and 3.9 ± 0.37 minutes, respectively. The average treatment times for patients treated with a 3-field plan, in prone position, or bilaterally were 4.1 ± 0.1 minutes, 4.4 ± 0.65 minutes, and 10.1 ± 0.88 minutes, respectively. The average total room time (ie, from leaving the gowned waiting area to return) for all patients was 12.4 ± 0.52 minutes (Fig 3). The average total room time for patients treated with and without DIBH was 14.3 ± 1.33 minutes and 11.6 ± 0.42 minutes, respectively. The average total room time for patients treated with a 3-field plan, in prone position, or bilaterally was 11.6 ± 0.89 minutes, 13.1 ± 0.46 minutes, and 19.4 ± 1.7 minutes, respectively. The average beam-on time for 28 of the 34 patients (82%) was 2.0 ± 0.3 minutes. A total of 24 values across 11 patients and 52 values across 25 patients were excluded as outliers from the mean treatment time and total room time calculations, respectively.
Figure 2.
Average treatment time (from start of imaging to beam off) for all patients for all standard fractions of 6-MV flattening-filter-free O-ring linear accelerator treatment. The average treatment times for nonstandard fractions are displayed separately.
Figure 3.
Average total room time (time from leaving gowned waiting area to return) for all patients for all standard fractions of 6-MV flattening-filter-free O-ring linear accelerator treatment. The average total room times for nonstandard fractions are displayed separately.
Discussion
In this study, we report the first clinical experience treating patients with breast cancer with radiation therapy on a 6X-FFF ORL and demonstrated its versatility, acute toxicity, DVH data, and speed. The versatility was demonstrated by the wide variety of patients with breast cancer and of treatments, such as left or right sided, unilateral or bilateral, intact breast or postmastectomy, with or without regional nodal radiation, with or without DIBH, and supine or prone. In patients having tumor bed boosts, the previous standard preference at our institution for electron beam gradually shifted in the study period with clinical experience and successful plan comparisons so that a majority of our patients actually received mini tangent beam arrangements without difficulty. Thus, we demonstrated that a 6X-FFF ORL can be used across most clinical scenarios to treat patients with breast cancer.
Our results also demonstrated that even with only 6X-FFF energy, a 6X-FFF ORL has early toxicity similar to previously published data based on CALs with higher energies. Breast radiation therapy was well tolerated on a 6X-FFF ORL, with acute toxicity profiles comparable to published reports of standard breast radiation therapy regimens. In our cohort, there were no acute grade 3 toxicities and only 11 acute grade 2 toxicities: 6 (18%) for dermatitis, 3 (9%) for fatigue, and 1 (3%) for breast pain. The most common acute grade 1 toxicities were dermatitis (65%, n = 22), fatigue (65%, n = 22), and breast pain (38%, n = 13). All but 3 patients (92%) received hypofractionated whole-breast radiation therapy, delivered in 15 to 16 fractions with or without a 5-fraction boost. These numbers compare well with the MD Anderson randomized trial studying hypofractionated whole-breast radiation therapy (42.56 Gy in 16 fractions with 5- to 7-fraction boost), in which the rates of RTOG acute grade 1 and 2 dermatitis were 58% and 36%, respectively, in the hypofractionated cohort. For the same cohort, the rates of RTOG acute grade 1 and 2 fatigue were 70% and 9%, and acute grade 1 and 2 breast pain were 50% and 5%, respectively. There were no acute grade 3 toxicities.18
We have also demonstrated that 6X-FFF ORL plans met RTOG and institutional DVH constraints for target coverage and OAR sparing for nearly all plans, even with the inclusion of MV CBCT dose. Institutional goals, which are typically equally if not more stringent than those of seminal published randomized trials in breast radiation therapy, were met for target coverage in all patients; however, our heterogeneity constraint, Breast PTVeval V105 < 10% to 15%, was not met in 2 cases, although point max constraint of V110 was met in both. Both patients were treated with MV CBCT, which may have contributed to decreased homogeneity; this would likely be improved with kV CBCT. Only 1 patient (3%) exceeded our ipsilateral lung V20 constraint, with a V20 of 25.2% for breast-only radiation therapy. Her tumor location was in the right upper outer quadrant, and she received a lumpectomy cavity boost to a total dose of 52.56 Gy. Her breast anatomy was such that a sizable proportion of her right breast and lumpectomy cavity boost site rested posterolaterally along her chest wall, requiring a greater amount of ipsilateral lung to receive dose to achieve adequate target coverage at the discretion of the treating physician. All but 1 patient (97%) met our mean heart constraint, with a mean heart dose of 5.7 Gy. This patient was treated to the left chest wall and comprehensive regional nodes, including internal mammary lymph nodes, with MV CBCT and had unfavorable cardiac anatomy despite DIBH. This exceeded our institutional constraint at the discretion of the treating physician to improve target coverage.
The mean beam-on, treatment, and total room times for a standard fraction were 2.0 ± 0.3 minutes, 4.4 ± 0.4 minutes, and 12.4 ± 0.5 minutes, respectively. Thus, the average beam-on time for the 6X-FFF ORL measured in this study represents a significant reduction (P < .001) in this parameter compared with a published value of average beam-on time of 3.0 minutes for breast 3D conformal radiation therapy on a 6X CAL.19 The treatment times remained comparable to a standard patient for even complex techniques such as prone, bilateral, or with DIBH. A potential operational concern that a clinical team might have for a 6X-FFF ORL is the ability to set up the patient well with only a gantry laser's guidance and without light field, crosshair, or shaded surface display verification. Our results showed that the average 3D vector couch correction based on daily CBCT matching from skin marker alignment for all patients was 0.77 ± 0.05 cm, which compares well with the average correction value of 0.6 ± 0.3 cm for CBCT with match to bony anatomy in tangential breast treatment with a conventional CAL.20 When considered separately, there were no observed differences in average couch corrections between patients treated with a supraclavicular field versus without a supraclavicular field, in prone versus supine position, or bilaterally versus unilaterally. However, it should be noted that given our limited sample size, we did not have the power to make a quantitative statistical comparison. In 4 patients (12%), consistent corrections greater than 1 cm were observed, with higher interfractional variation in corrections and without any clear distinction between these 4 patients and the rest of the cohort to account for the larger average corrections. If only skin mark alignment and shaded surface display check were used as daily positioning verification, these larger and more variable corrections would not be identified without daily image guided radiation therapy, demonstrating the added value of performing daily CBCT for select patients with breast cancer.
There is a paucity of published data regarding the use of DIBH for O-ring linacs. One published report discusses a case of DIBH treatment on a 6X-FFF ORL, where average treatment time for DIBH treatment using DBF was reduced from 23.0 minutes to 5.5 minutes when using ISC instead.16 In our study, 1 patient (3%) received DIBH with DBF, and given the long treatment time associated with this combination, all subsequent DIBH patients were treated only with ISC to reduce treatment time and decrease the burden on patients to hold their breath for longer amounts of time. A potential pitfall associated with DIBH treatment on a 6X-FFF ORL is that using ISC may require additional dosimetric training. There were, however, no issues related to collision or clearance when using DIBH on a 6X-FFF ORL.
The ability to reduce day length through faster treatments and improved throughput has a number of potential implications. Treatments on a 6X-FFF ORL are shorter than they would be on a CAL. Treatments in particular requiring a mask or uncomfortable position can be better tolerated. In our department, the use of a 6X-FFF ORL has been associated with a decrease in operational hours of up to 4 hours compared with when we only used CALs. This improves convenience for patients because we are able to provide more desirable treatment times than late evening hours. From a departmental standpoint, reducing day length decreases the staffing needed, which could allow valuable departmental resources to be allocated elsewhere. For departments that have long delays to starting treatment owing to linac availability, changing to a 6X-FFF ORL has the potential to reduce delays in starting patients on radiation therapy, which is highly important biologically in some cancers (eg, head and neck, cervix) and highly important psychologically for many patients.
There are several limitations to this study. Our follow-up interval is short, and longer-term follow-up will be necessary to assess late toxicity. Our dosimetric data may overestimate the doses received by various OARs for future patients treated on a 6X-FFF ORL, given that many of our patients were treated initially with MV CBCT image guidance with increased dose from imaging. Further study with longer follow-up interval, more boosts treated on a 6X-FFF ORL, and more patients treated with KV CBCT imaging are warranted to better speak to these limitations. Given the short beam-on time for 6X-FFF ORL treatments, another area of future study may be comparing setup shifts in patients with poorer performance status, who may struggle to maintain reproducible positioning for an extended period of time, treated on 6X-FFF ORL compared with CALs.
Conclusions
Herein, we provide the first clinical report of breast radiation therapy delivered on a 6X-FFF ORL. We found that the 6X-FFF ORL was versatile in terms of the variety of patients treated; acceptable in terms of acute toxicity, dosimetry, and setup corrections; and at least as quick as treatment on a CAL. A 6X-FFF ORL can increase throughput or reduce length of day in departments with a busy breast service, with acute toxicity and dosimetry comparable to those with treatment on a CAL.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: This work was partially supported by Varian Medical Systems: Dr Metz is on the advisory board of, obtained personal fee from, and received grant funding unrelated to this work from Varian Medical Systems; Dr Dong received grant funding and personal fees unrelated to this work from Varian Medical Systems; and Dr Kennedy received honoraria for speaking engagements from Varian Medical Systems.
References
- 1.Rayter Z. History of breast cancer. In: Raytor Z., Mansi J., editors. Medical Therapy of Breast Cancer. Cambridge University Press; Cambridge, UK: 2003. pp. 1–36. [Google Scholar]
- 2.Slater J.M. From x-rays to ion beams: A short history of radiation therapy. In: Linz U., editor. Ion Beam Therapy: Fundamentals, Technology, Clinical Applications. Springer; Berlin: 2012. pp. 3–6. [Google Scholar]
- 3.Bradley J.A., Mendenhall N.P. Novel radiotherapy techniques for breast cancer. Annu Rev Med. 2018;69:277–288. doi: 10.1146/annurev-med-042716-103422. [DOI] [PubMed] [Google Scholar]
- 4.Bentel G., Marks L.B., Hardenbergh P., Prosnitz L. Variability of the location of internal mammary vessels and glandular breast tissue in breast cancer patients undergoing routine CT-based treatment planning. Int J Radiat Oncol Biol Phys. 1999;44:1017–1025. doi: 10.1016/s0360-3016(99)00123-6. [DOI] [PubMed] [Google Scholar]
- 5.Hjelstuen M.H., Mjaaland I., Vikström J., Dybvik K.I. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular- and internal mammary lymph nodes without compromising target coverage or dose restrictions to organs at risk. Acta Oncol. 2012;51:333–344. doi: 10.3109/0284186X.2011.618510. [DOI] [PubMed] [Google Scholar]
- 6.Lymberis S.C., deWyngaert J.K., Parhar P. Prospective assessment of optimal individual position (prone versus supine) for breast radiotherapy: Volumetric and dosimetric correlations in 100 patients. Int J Radiat Oncol Biol Phys. 2012;84:902–909. doi: 10.1016/j.ijrobp.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 7.Popescu C.C., Olivotto I.A., Beckham W.A. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010;76:287–295. doi: 10.1016/j.ijrobp.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Webb S. Medical Physics Web. Volumetric-modulated arc therapy: Its role in radiation therapy. http://medicalphysicsweb.org/cws/article/opinion/39542 Available at:
- 9.Michiels S., Poels K., Crijns W. Volumetric modulated arc therapy of head-and-neck cancer on a fast-rotating O-ring linac: Plan quality and delivery time comparison with a C-arm linac. Radiother Oncol. 2018;S0167-8140:30212–30213. doi: 10.1016/j.radonc.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Cashmore J. The characterization of unflattened photon beams from a 6 MV linear accelerator. Phys Med Biol. 2008;53:1933–1946. doi: 10.1088/0031-9155/53/7/009. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Netherton T., Nitsch P.L., Balter P.A., Gao S., Klopp A.H. Normal tissue doses from MV image-guided radiation therapy (IGRT) using orthogonal MV and MV-CBCT. J Appl Clin Med Phys. 2018;19:52–57. doi: 10.1002/acm2.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netherton T., Li Y., Nitsch P. Interplay effect on a 6-MV flattening-filter-free linear accelerator with high dose rate and fast multi-leaf collimator motion treating breast and lung phantoms. Med Phys. 2018;45:2369–2376. doi: 10.1002/mp.12899. [DOI] [PubMed] [Google Scholar]
- 13.White J., Tai A., Arthur D. Breast cancer atlas for radiation therapy planning: Consensus definitions. https://www.rtog.org/LinkClick.aspx?fileticket=vzJFhPaBipE%3d&tabid=236 Available at:
- 14.Hideki F., Nao K., Hiroyuki H., Hiroshi K., Haruyuki F. Improvement of dose distribution with irregular surface compensator in whole breast radiotherapy. J Med Phys. 2013;38:115–119. doi: 10.4103/0971-6203.116361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwahata N., Fujita H., Yamanishi H., Okazaki E., Fukuda H. Dosimetric comparison of irregular surface compensator and field-in-field for whole breast radiotherapy. J Med Phys. 2018;43:79–84. doi: 10.4103/jmp.JMP_73_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy C., Freedman G., Taunk N. Whole breast irradiation with Halcyon™ 2.0: Workflow and efficiency of field-in-field treatment with dynamic beam flattening technique and kV cone beam computed tomography. Cureus. 2018;10:e3510. doi: 10.7759/cureus.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey R.M. Commonly used t-tests in medical research. J Pract Cardiovasc Sci. 2015;1:185–188. [Google Scholar]
- 18.Shaitelman S.F., Schlembach P.J., Arzu I. Acute and short-term toxicities of conventionally fractionated versus hypofractionated whole breast irradiation in a prospective, randomized trial. JAMA Oncol. 2015;1:931–941. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyran M., Mailleux H., Tallet A. Volumetric-modulated arc therapy for left-sided breast cancer and all regional nodes improves target volumes coverage and reduces treatment time and doses to the heart and left coronary artery, compared with a field-in-field technique. J Radiat Res. 2015;56:927–937. doi: 10.1093/jrr/rrv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batumalai V., Phan P., Choong C., Holloway L., Delaney G.P. Comparison of setup accuracy of three different image assessment methods for tangential breast radiotherapy. J Med Radiat Sci. 2016;63:224–231. doi: 10.1002/jmrs.180. [DOI] [PMC free article] [PubMed] [Google Scholar]