Abstract
Purpose
Surgical spacer placement (SSP) is useful in particle therapy (PT) for patients with abdominal or pelvic tumors located adjacent to normal organs. We developed a nonwoven fabric bioabsorbable spacer made of polyglycolic acid (PGA) sutures that degrades via hydrolysis. We then conducted this first-in-human phase 1 study of the combination of SSP and PT using the PGA spacer, which we termed space-making PT (SMPT). This study aimed to evaluate the safety and efficacy of SMPT in patients with unresectable malignant tumor located adjacent to normal organs.
Methods and Materials
The eligibility criteria included histologically proven malignant abdominal or pelvic tumor adjacent to the intestines, no metastasis, and no previous radiation therapy. Periodic computed tomography (CT) images were obtained before SSP and before, during, and after PT until the spacer disappeared. Treatment planning was performed for each CT image set until the end of PT, and doses for the planning target volume and organs at risk were analyzed. The thickness and volume of the PGA spacer were measured in each CT image set. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0.
Results
Five patients were enrolled in this study. All patients received 70.4 Gy (relative biological effectiveness) of irradiation. V95% of the planning target volume before SSP, at the beginning of PT, and at the end of PT was 82.1% ± 11.3%, 98.1% ± 1.1%, and 97.1% ± 0.8%, respectively. The PGA spacers maintained enough thickness (≥1 cm) until the end of PT and disappeared within 8 months after SSP in all patients. No grade ≥3 acute adverse events were observed.
Conclusions
The SMPT is feasible and useful for abdominal or pelvic tumors adjacent to the intestines. This method may be applicable to unresectable tumors located adjacent to normal organs and may expand the indications of PT.
Introduction
Particle therapy (PT) permits delivery of higher radiation doses to the tumor, which may lead to profoundly improved tumor eradication. Recent studies have shown therapeutic superiority of PT for various kinds of malignant tumors.1, 2 However, the application of PT for abdominal or pelvic malignant tumors may be restricted because most of the tumors come in contact with the intestine, which cannot tolerate the radical dose of particle beams. Ishikawa et al3 demonstrated that the dosimetric parameter is an important factor in the occurrence of gastrointestinal bleeding after PT. Therefore, a novel strategy that overcomes this limitation needs to be developed.
Advances in the application of various spacers in radiation therapy or PT have been achieved. Several studies have reported the use of hyaluronic acid or other gels to separate the prostate from the rectum as a spacer for prostate cancer.4, 5, 6, 7, 8, 9, 10 Fukumoto et al11, 12 reported that a nonwoven fabric GORE-TEX sheet could be used for surgical spacer placement (SSP) before PT. However, although the GORE-TEX spacer is useful during PT, it becomes a foreign body after the completion of the therapy.13 Lorenzo et al14 reported their experience using nonabsorbable silicon spacers in the treatment of sacral chordoma using carbon ion therapy. Although these nonabsorbable spacers are useful during PT, they carry a risk for morbidities and their removal may require a second surgery. Therefore, a bioabsorbable spacer seems to be ideal.
We have developed a novel bioabsorbable polyglycolic acid (PGA) spacer and applied it for SSP. We call this method space-making PT (SMPT). This first-in-human phase 1 clinical study of the SMPT aimed to evaluate its safety and efficacy in patients with unresectable malignant tumor located adjacent to normal organs.
Methods and Materials
Trial design
This prospective phase 1, first-in-human, uncontrolled, open-label study was conducted between October 2015 and April 2017. Patient enrollment and SSP were conducted at Kobe University Hospital, and PT was performed at Hyogo Ion beam Medical Center (HIBMC). The study was approved by the Ethics Committee of both Kobe University Hospital and HIBMC and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants. The trial will be externally monitored according to good clinical practice requirements.
Patients
This study enrolled patients with an unresectable malignant tumor located adjacent to normal organs and in whom delivering a curative dose to the PTV via PT appears impossible. The inclusion criteria were as follows: histologically confirmed abdominal or pelvic malignant tumors; no metastasis; eligible for surgical placement of the spacer; age between 20 years and 75 years; Eastern Cooperative Oncology Group performance status 0, 1, and 2; ability to maintain a prone or supine position during PT; and provision of written informed consent. The exclusion criteria were as follows: no prior radiation therapy; no schedules for chemotherapy or immunotherapy before SSP or after completion of PT; another active malignant tumor (ie, other synchronous malignant tumor or metachronous malignant tumor with a disease-free interval of <5 years); intractable infectious disease; peptic ulceration; psychiatric disorder; serious comorbid illness; alcohol abuse; pregnancy; and lack of adequate renal and hepatic function. If the tumor itself is resectable, other malignant tumors are incidentally found, or spacer placement is impractical, the patients are excluded from the study.
PGA spacer and SSP
The PGA spacer was developed by Alfresa Pharma Corporation (Osaka, Japan). It measured 10 × 20 cm and had a thickness of 5, 10, and 15 mm. The PGA spacer has been described in detail previously.15 Briefly, the PGA spacer was originally produced with a surgical suture material made of PGA, a biocompatible synthetic polymeric material. Surgical PGA sutures were manufactured into nonwoven fabric by entangling threads in 3 dimensions. Preclinical evaluations proved that the bioabsorbable PGA spacer had water-equivalent, biocompatible, and thickness-retaining properties and maintained 80% of its thickness for at least 8 weeks.15 The PGA spacer is soft and flexible, and thus its shape is easy to customize to correspond with surrounding tissues to avoid spacer migration.13, 16 Hounsfield units under dry and wet conditions were evaluated using computed tomography (CT) images or cone beam CT. Pressure tests were performed by measuring the thickness of a 100 × 200 × 15 mm spacer (n = 3) after a homogeneous pressure (range, 0.5-2.0 kPa).
Standard preoperative workup before SSP included chest and abdominal radiography, electrocardiogram, respiratory function test, occult blood test, and urinalysis. Regarding operative technique, the abdomen was explored through a midline incision. A distance of at least 10 mm from the tumor and gastrointestinal tract in all directions was maintained to ensure safety of subsequent PT. The PGA spacer was placed between the tumor and adjacent organs and fixed tightly to the peritoneum. The amount and thickness of the PGA spacer were first designed via preoperative CT evaluations and then adjusted during the surgery.
SMPT treatment planning
Treatment planning for PT was performed at HIBMC as described previously.17 Briefly, PT was planned using a CT-based 3-dimensional treatment planning system (Xio-M system; CMS, St Louis, MO; Mitsubishi Electric Corporation, Tokyo, Japan). The target volumes and organs at risk (OARs) were delineated on the CT-magnetic resonance imaging (MRI) fusion images. The clinical target volume (CTV) was defined as the gross tumor volume plus a 5-mm basic margin. The planning target volume (PTV) was defined as the CTV plus a setup margin (5 mm) and an internal margin (1 mm) under the respiratory gating system.18
The relative biological effectiveness (RBE) values for protons and carbon ions at HIBMC are 1.1 and 2 to 3.7, respectively, depending on the depth in the spread-out Bragg peak.19 The dose constraints in the 32-fraction protocol for the small bowel, large bowel, and rectum were a maximum dose (Dmax) of ≤53 Gy (RBE), Dmax of ≤59 Gy (RBE), and volume receiving ≥65 Gy (RBE) (V65) of ≤17% and V40 of ≤35%, respectively. The dose constraint in the 16-fraction protocol for the intestines including the small bowel, large bowel, and rectum was the dose for the most exposed 2-mL volume (D2cc) of <44 Gy (RBE).
The patients were treated using 150-MeV or 210-MeV proton beams or 320-MeV carbon ion beams. The policy for selecting proton therapy or carbon ion therapy was based on the dose distribution, as described previously.17 Although both proton therapy and carbon ion therapy are charged-particle therapies, they have slight differences in their physical characteristics. Regarding monoenergetic beams, carbon ions show superior penumbra but a shorter range compared with protons. Treatment planning details should be agreed following the established conditions. The D95 of the PTV in each patient must be larger than 95% of the prescribed dose. However, if the coverage was difficult to achieve, the doses of OARs were lower than the set criteria.
Evaluation of SMPT
The standard protocol included a physical examination, diagnostic imaging (CT or MRI), and blood testing. The distance between the tumor and adjacent organs was measured using CT images. Toxicities were evaluated using the Common Terminology Criteria for Adverse Events, version 4.0. CT or MRI evaluations were performed before and after SSP, before initiation of PT, during PT (4, 6, and 8 weeks after SSP), and at the end of PT. Treatment planning was performed for each CT image set, and parameters for PTV and OARs were analyzed. The plans created using the CT image set before the initiation of PT were applied for the actual patient treatment (actual plan). Actual plans then were superimposed on newly obtained CT image sets, and each parameter was verified. When the dose constraints for the OARs were violated, a replan was performed if necessary after thorough discussion by several radiation oncologists. After completion of PT, the evaluations were performed every 4 weeks during the follow-up visit.
Estimation of PGA spacer
The thickness and volume of the PGA spacer were evaluated as follows. Data of every CT scan were acquired by 3-dimensional volume scan, and axial sagittal images were reconstructed with 5 mm thickness. The slice position of axial CT images in which target tumor and OAR are closest in the pretreatmental CT scan was chosen, and then the same position of the following CT scan was evaluated. The PGA spacer starts hydrolysis after it contacts water component and is degraded into water and carbon dioxide.
Results
Patients
Five patients (3 men and 2 women) were enrolled in this study. The patient characteristics and treatments are shown in Table 1. Patient age ranged from 26 to 66 years. The Eastern Cooperative Oncology Group performance status of all patients was 0. There were 3 patients (60%) with chordoma, 1 patient (20%) with leiomyosarcoma, and 1 patient (20%) with malignant peripheral nerve sheath tumor. One patient underwent a previous surgery before the spacer placement, and the other 4 patients did not undergo any prior surgery. All patients received a total dose of 70.4 Gy (RBE).
Table 1.
Patient and tumor characteristics
| Patient | Type of disease | Site of disease | Previous surgery | Source of therapy | Dose (GyE) | Fractions |
|---|---|---|---|---|---|---|
| 1 | Leiomyosarcoma | Retroperitoneal | Yes | Carbon ion | 70.4 | 32 |
| 2 | Chordoma | Sacrum | None | Carbon ion | 70.4 | 32 |
| 3 | Chordoma | Sacrum | None | Proton | 70.4 | 32 |
| 4 | Peripheral nerve sheath tumor | Sacrum | None | Proton | 70.4 | 32 |
| 5 | Chordoma | Sacrum | None | Proton | 70.4 | 16 |
PGA spacer and SSP
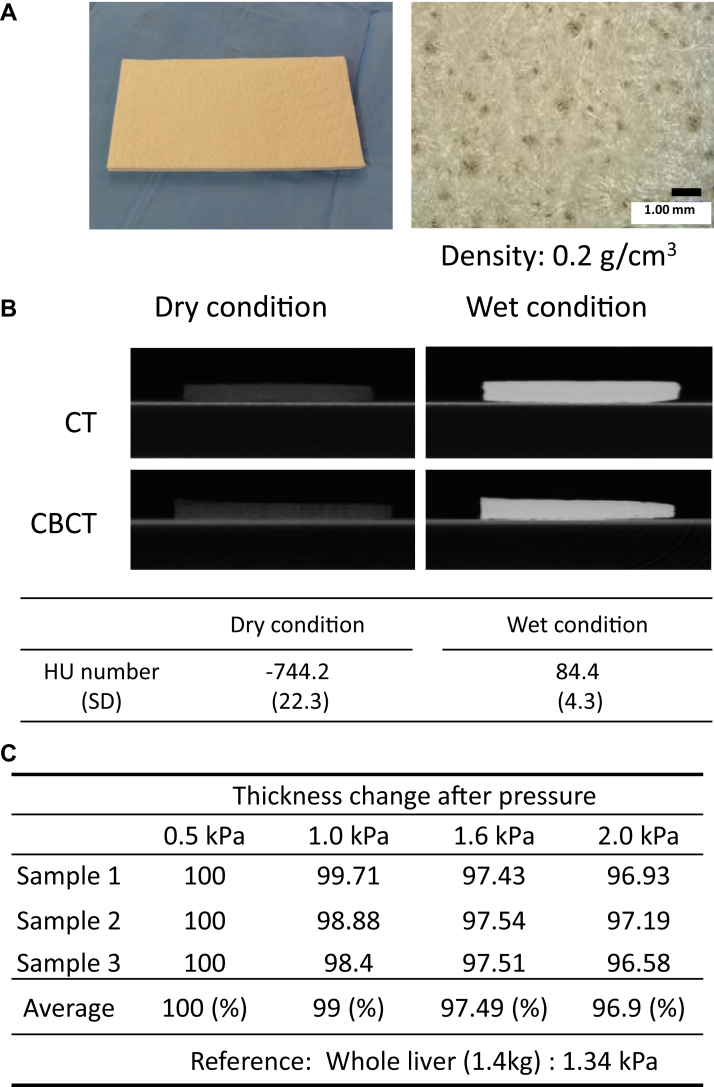
Features and Hounsfield units under dry and wet conditions are shown in Figure 1A and 1B. The density is 0.2 g/cm3. A weight of whole liver (approximately 1.4 kg) was adapted as a reference pressure (approximately 1.34 kPa). The PGA spacer remained 97.5% and 96.9% of its thickness under 1.6 kPa and 2.0 kPa pressures (Fig 1C), respectively. From these results, the retention force seems sufficient for clinical use.
Figure 1.
(A) Macroscopic (left) and microscopic (right) features of the polyglycolic acid (PGA) spacer. (B) Computed tomography (CT) or cone beam computed tomography (CBCT) images and Hounsfield unit number of the PGA spacer under dry and wet conditions. (C) Pressure tests of the PGA spacer. Three independent tests were performed using 0.5 to 3.0 kPa pressures.
The mean operative time was 194 minutes, and the mean amount of blood loss was 116 mL. None of the patients received blood transfusion. No patient developed operation-related complications.
Estimation and efficacy of the PGA spacer
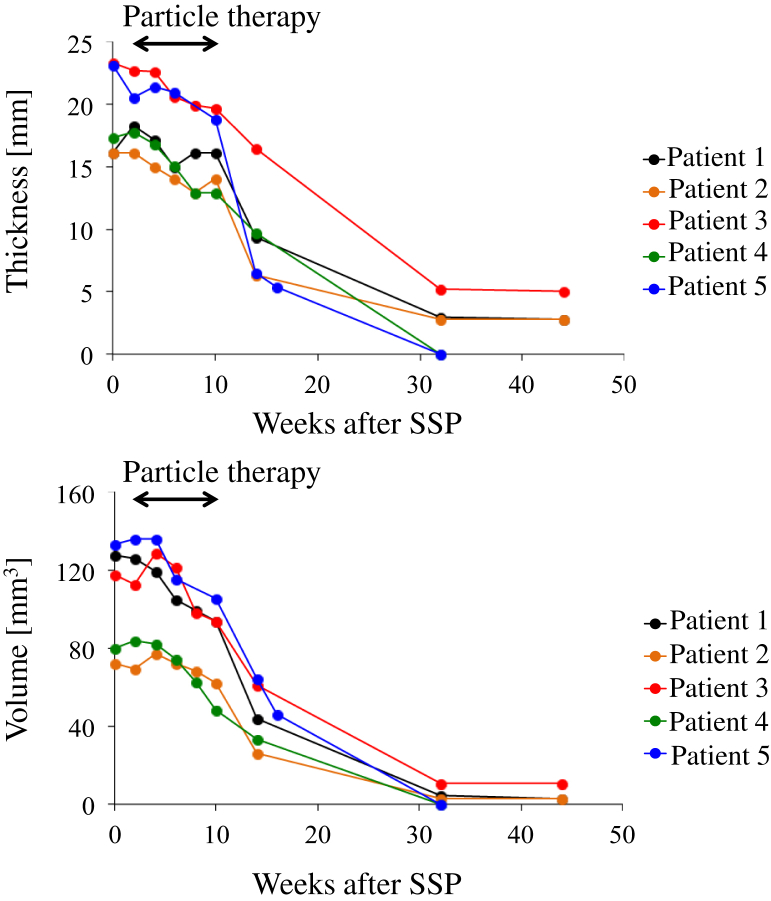
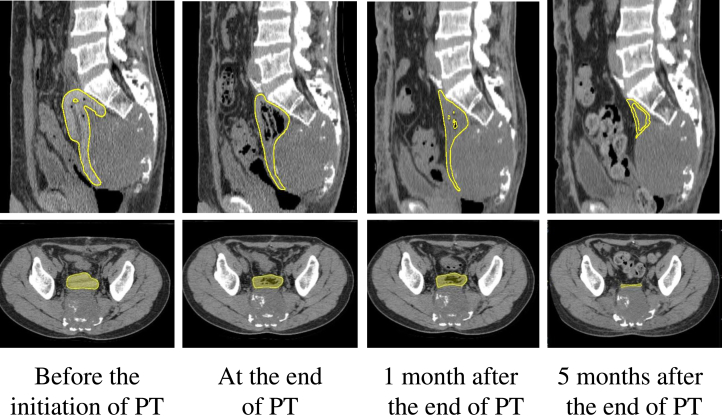
The thickness and volume of the PGA spacer were measured in all patients. As expected, the thickness was preserved by 80% from the initial spacer placement to 8 weeks of PT. Starting from the 10th week after placement, the thickness and volume of the PGA spacer were reduced to less than 10% in 32 weeks (Fig 2). A representative feature for changes in the PGA spacer on CT images is shown in Figure 3. A certain portion of generated carbon dioxide is enclosed within the PGA spacer, leading to estimated thickness and volume that looks enlarged. Enclosed carbon dioxide is also seen in the CT images of revised Figure 3. At 8 weeks, the carbon dioxide was completely absorbed.
Figure 2.
Serial volume and thickness changes of the polyglycolic acid spacer after surgical spacer placement in 5 patients.
Figure 3.
Sagittal and axial computed tomography images of serial volume changes of the polyglycolic acid spacer in a patient with sacral chordoma. Yellow = contours of the placed polyglycolic acid spacer. Abbreviation: PT = particle therapy.
Improvement of distance between CTV and adjacent organs via SSP
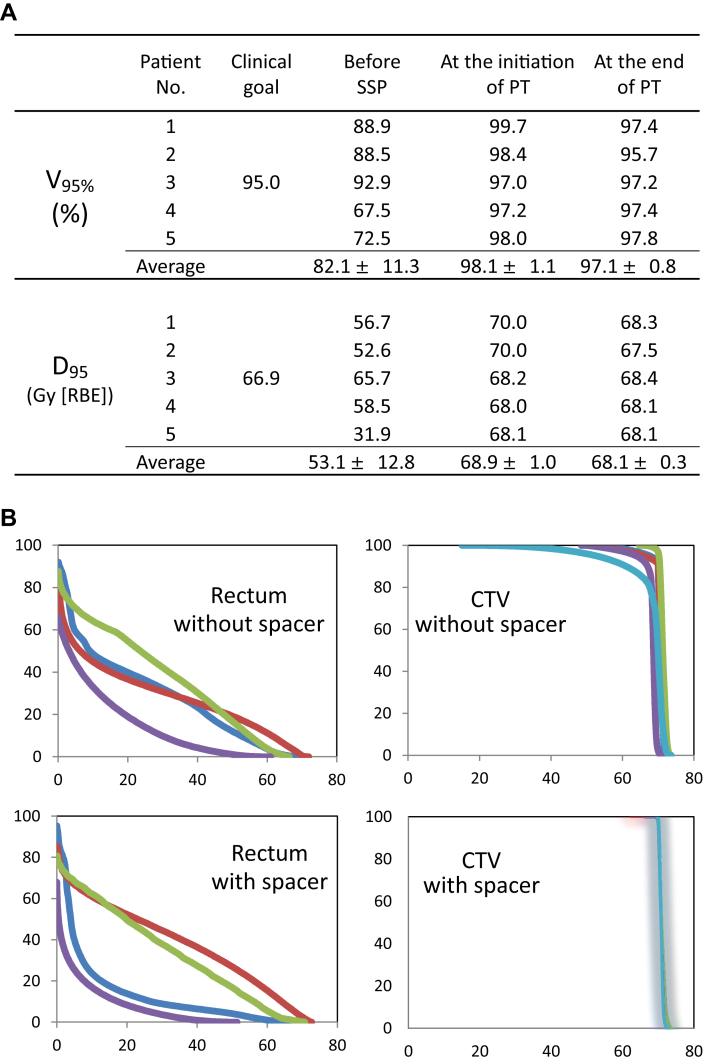
Figure 4A shows the changes in V95% (volume receiving ≥95% of the prescribed dose) and D95 (dose irradiated to ≥95% of the target volume) of the PTV in all patients. The PTV V95% before SSP, at the initiation of PT, and at the end of PT was 82.1% ± 11.3%, 98.1% ± 1.1%, and 97.1% ± 0.8%, respectively. The PTV D95 before SSP, at the initiation of PT, and at the end of PT was 53.1 ± 12.8 Gy (RBE), 68.9 ± 1.0 GyE (RBE), and 68.1 ± 0.3 GyE (RBE), respectively. The V95% and D95 should be ≥ 95% and ≥66.9 Gy (RBE; 95% of the total dose), respectively, for curative intent (clinical goals). None of the patients met the clinical goals before SSP; however, all met the clinical goals throughout the PT shown in the dose-volume histogram (Fig 4B).
Figure 4.
(A) Changes of planning target volume parameters. (B) Comparison of clinical target volume coverage and organ at risk (rectum) with or without the polyglycolic acid spacer in the dose-volume histogram in 4 patients whose tumors were located in the pelvis. Abbreviations: D95 = dose irradiated to ≥95% of the target volume; PT = particle therapy; SSP = surgical spacer placement; V95% = volume receiving ≥95% of the prescribed dose.
The changes in OAR parameters in all patients are demonstrated in Table 2. Violations of dose constraints were observed in 2 patients, but replan was only needed in 1 patient. In this patient (no. 4 in Table 2), violations of the dose constraint for the large bowel were observed at 6 and 8 weeks after SSP. However, the exceeding volume was minute, and thus a replan was not made. In the other patient (no. 5 in Table 2), a violation of the dose constraint for the rectum was observed at 6 weeks after SSP. The exceeding dose necessitated a replan that resulted in the D2cc being decreased to 42.1 Gy (RBE). In both patients, violations of the dose constraints for the large bowel and rectum were observed at the end of PT.
Table 2.
Change in organ at risk parameters
| Patient no. | OAR | Dose constraint | Before SSP | Before the initiation of PT | At the initiation of PT | 4 wk after SSP | 6 wk after SSP | 8 wk after SSP | At the end of PT |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SB | Dmax ≤53 Gy (RBE) | 53.0 | 19.8 | 18.5 | 18.3 | 19.9 | 20.3 | 27.3 |
| 2 | LB | Dmax ≤59 Gy (RBE) | 57.1 | 3.3 | 3.9 | 4.6 | 6.0 | 2.9 | 8.9 |
| Rectum | V65 ≤ 17% | 1.7 | 0.2 | 0.6 | 2.0 | 6.0 | 6.2 | 6.0 | |
| V40 ≤ 35% | 29.0 | 7.8 | 8.4 | 9.0 | 13.2 | 13.9 | 12.3 | ||
| 3 | Rectum | V65 ≤ 17% | 9.4 | 8.3 | 2.8 | 8.1 | 3.1 | 13.1 | 12.9 |
| V40 ≤ 35% | 34.1 | 29.8 | 20.4 | 26.6 | 23.5 | 33.0 | 33.6 | ||
| 4 | SB | Dmax ≤53 Gy (RBE) | 51.4 | 23.0 | 22.8 | 29.2 | 39.7 | 19.9 | 48.5 |
| LB | Dmax ≤59 Gy (RBE) | 58.5 | 58.9 | 58.1 | 47.7 | 63.9∗ | 65.6∗ | 66.0∗ | |
| Rectum | V65 ≤ 17% | 2.6 | 2.0 | 1.1 | 0.8 | 4.2 | 12.6 | 11.5 | |
| V40 ≤ 35% | 33.8 | 27.9 | 22.6 | 25.1 | 29.1 | 36.0 | 31.9 | ||
| 5 | Rectum | D2cc < 44 Gy (RBE) | 43.5 | 30.7 | 40.8 | 27.2 | 58.2∗ | − | 60.6∗ |
Abbreviations: Dmax = maximum dose; D2cc = dose for the most exposed 2-mL volume; LB = large bowel; OAR = organ at risk; PT = particle therapy; RBE = relative biological effectiveness; SB = small bowel; SSP = surgical spacer placement; Vx = volume receiving ≥ x Gy (RBE).
Violation of the dose constraints.
Adverse events
For SSP, 1 patient who previously underwent surgery for the primary tumor experienced ileus (grade 2) at 12 days after SSP. This improved with conservative treatment and fasting, and the planned schedule of PT was conducted without delay.
For PT, acute dermatitis was observed in all patients (grade 1 in 3 and grade 2 in 2). One patient experienced grade 2 bone fracture in the sacrum; however, it developed too early to be an adverse effect of PT. We considered that the fracture was probably due to both the tumor-induced fragility of the sacrum and mechanical stress from playing tennis as the patient played tennis every week.
Discussion
This study evaluated the safety and efficacy of the SMPT in the first-in-human clinical study. SMPT is a promising method designed to allow for increased tumor dose while limiting exposure to adjacent organs without retention of a foreign body after treatment. Presumably, the most appropriate indication for this method is very large tumors occurring in the pelvis, such as sacral chordoma. Recently, several institutions have reported the efficacy of PT in various kinds of bone and soft-tissue sarcoma, including chordoma.17, 20, 21, 22 However, in cases in which the tumor is located adjacent to normal organs, delivering curative doses to the tumor will be difficult even if PT is adapted. Moreover, the outcomes of unresectable chordoma are poor, and effective systemic treatment regimens using single or multiple chemotherapeutic agents have not been reported until recently.23, 24 Because SMPT is a curative strategy, it may be the most effective treatment option for unresectable sacral chordoma.
Bioabsorbable nonwoven fabric spacer is a novel tool in PT or radiation therapy. Therefore, its possible benefits and risks need to be carefully discussed. The PGA spacer is designed to maintain approximately 80% of its thickness within 8 weeks and to spontaneously decrease in volume thereafter.15 In our institution, PT protocols for bone and soft-tissue sarcoma are the longest compared with those for other malignant tumors. A 32-fraction protocol spans 7 weeks, and a 16-fraction protocol spans less than 4 weeks. PT is scheduled to start within 10 days after SSP. In general, SMPT for sarcoma is completed within 8 weeks after SSP. In the current trial, the PGA spacer maintained >80% of its thickness for 10 weeks. These results show that the PGA spacer is feasible in most PT protocols. Another advantage of the PGA spacer is that its thickness can be customized according to tumor shape or anatomic difficulty. In this study, validations of the site of placement and thickness were carefully discussed by radiation oncologists and surgeons before and during surgery. As shown in Figure 3, a spacer >15 mm thick can be implanted at a shallow site, whereas the maximum feasible thickness at deeper sites is only 5 mm. Therefore, PGA spacers with various thicknesses are necessary.
One possible risk of the PGA spacer is unexpected migration or dislocation from the placement site. To avoid this complication, the PGA spacer was placed using nonabsorbable strings and tightly sewed to the peritoneum or surrounding tissues. As a result, no migration was observed in our series of patients. Another possible complication is adhesion. In this study, 1 patient who previously underwent surgery at the same site experienced ileus, possibly caused by adhesion. Fortunately, the ileus was managed via conservative treatments with fasting, and the planned PT schedule was not delayed. Notably, the other 4 patients who did not undergo any prior surgery before SSP did not experience similar symptoms. In the preclinical study of the PGA spacer, adhesion was minimal.15 These findings show that adhesion should be considered and carefully monitored during the treatment period, particularly in patients who underwent prior surgery. Furthermore, methods to avoid or reduce adhesion, such as the use of an antiadhesion agent in SSP, may be recommended.
The SMPT is expected to be applied for the treatment of pediatric malignancies. Weber et al25 demonstrated the effectiveness of proton therapy for various pediatric malignancies, such as chondrosarcoma, rhabdomyosarcoma, Ewing sarcoma, and osteosarcoma. Proton therapy should be considered particularly in young patients and anatomically challenging cases in which irradiation to tissues within high-dose areas can lead to impaired growth and development, particularly in young children. Proton therapy or carbon ion therapy may substantially decrease long-term complications in tumors that are typically located adjacent to dose-limiting structures, such as the small bowel and bladder, and the use of the PGA spacer seems to be more effective to decrease the risks of complications in such cases. To the best of our knowledge, no study has reported on the use or efficacy of the SSP. We are now planning a phase 2 study of the SMPT for pediatric malignancies.
Pinkawa et al9 reported that spacing between the rectum and prostate by using a hydrogel spacer is a useful method for decreasing the rectal doses. In a single-blind randomized phase 3 trial conducted by Hamstra et al26 the hydrogel spacer was safe to apply, well tolerated, and resulted in a significant rectal dose reduction. Furthermore, the hydrogel spacer remained beneficial at 15 months for bowel toxicity, and quality of life was maintained or improved in the 3-year median follow-up period. However, the method was direct injection of gels as guided by transrectal ultrasound, and the applicability of this method seems to be limited in prostate cancer.27 Although the concept of the gel spacer is similar, its indication differs from that of the nonwoven fabric PGA spacer, as shown in Table E1 (available online at https://doi.org/10.1016/j.adro.2019.05.002).
Conclusions
The SMPT is feasible and useful for abdominal or pelvic tumors adjacent to the intestines. This method may be applicable to unresectable tumors located adjacent to normal organs and may expand the indications of PT. Further large-scale investigation to validate the applicability and safety of SPMT are warranted in the near future.
Acknowledgments
We thank Junko Suzuki and Sachiko Inubushi for their scientific assistance. We thank Editage (available at: www.editage.jp) for English language editing.
Footnotes
Sources of support: This work was supported by Grants-in-Aid for Exploratory Research (grant number, 16H05391 to RS) from the Ministry of Education and Culture, Sports, Science, and Technology of Japan and from the Japan Agency for Medical Research and Development (grant numbers, 16ck0106034h0003 and 18ck0106301h0002 to R.S.).
Disclosures: Costs of the clinical trial were partially supported by the Alfresa Pharma Corporation.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2019.05.002.
Supplementary data
References
- 1.Brada M., Pijls-Johannesma M., De Ruysscher D. Proton therapy in clinical practice: Current clinical evidence. J Clin Oncol. 2007;25:965–970. doi: 10.1200/JCO.2006.10.0131. [DOI] [PubMed] [Google Scholar]
- 2.Schulz-Ertner D., Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25:953–964. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa H., Tsuji H., Kamada T. Risk factors of late rectal bleeding after carbon ion therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:1084–1091. doi: 10.1016/j.ijrobp.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 4.Prada P.J., Fernandez J., Martinez A.A. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2007;69:95–102. doi: 10.1016/j.ijrobp.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 5.Prada P.J., Gonzalez H., Menéndez C. Transperineal injection of hyaluronic acid in the anterior perirectal fat to decrease rectal toxicity from radiation delivered with low-dose-rate brachytherapy for prostate cancer patients. Brachytherapy. 2009;8:210–217. doi: 10.1016/j.brachy.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Wilder R.B., Barme G.A., Gilbert R.F. Cross-linked hyaluronan gel reduces the acute rectal toxicity of radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:824–830. doi: 10.1016/j.ijrobp.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 7.Daar E., King L., Nisbet A. Viscosity changes in hyaluronic acid: Irradiation and rheological studies. Appl Radiat Isotopes. 2010;68:746–750. doi: 10.1016/j.apradiso.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Susil R.C., Mcnutt T.R., Deweese T.L. Effects of prostate-rectum separation on rectal dose from external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1251–1258. doi: 10.1016/j.ijrobp.2009.07.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinkawa M., Corral N.E., Caffaro M. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2011;100:436–441. doi: 10.1016/j.radonc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Noyes W.R., Hosford C.C., Schultz S.E. Human collagen injections to reduce rectal dose during radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1918–1922. doi: 10.1016/j.ijrobp.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu S., Hori Y., Fukumoto T. Surgical spacer placement and proton radiotherapy for unrespectable hepatocellular carcinoma. World J Gastroenterol. 2010;16:1800–1803. doi: 10.3748/wjg.v16.i14.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumoto T., Komatsu S., Hori Y. Particle beam radiotherapy with a surgical spacer placement for advanced abdominal leiomyosarcoma results in a significant clinical benefit. J Surg Oncol. 2010;101:97–99. doi: 10.1002/jso.21417. [DOI] [PubMed] [Google Scholar]
- 13.Ogino T., Sekimoto M., Nishimura J. Intraluminal migration of a spacer with small bowel obstruction: A case report of rare complication. World J Surg Oncol. 2012;10:30. doi: 10.1186/1477-7819-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenzo C., Andrea P., Barbara V. Surgical spacer placement prior carbon ion radiotherapy (CIRT): An effective feasible strategy to improve the treatment for sacral chordoma. World J Surg Oncol. 2016;14:211. doi: 10.1186/s12957-016-0966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akasaka H., Sasaki R., Miyawaki D. Preclinical evaluation of bioabsorbable polyglycolic acid spacer for particle therapy. Int J Radiat Oncol Biol Phys. 2014;90:1177–1185. doi: 10.1016/j.ijrobp.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 16.Riaz A.A., Ismail M., Barsam A. Mesh erosion into the bladder: A late complication of incisional hernia repair. A case report and review of the literature. Hernia. 2004;8:158–159. doi: 10.1007/s10029-003-0187-0. [DOI] [PubMed] [Google Scholar]
- 17.Demizu Y., Jin D., Sulaiman N.S. Particle therapy using protons or carbon ions for unresectable or incompletely resected bone and soft tissue sarcomas of the pelvis. Int J Radiat Oncol Biol Phys. 2017;98:367–374. doi: 10.1016/j.ijrobp.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Minohara S., Kanai T., Endo M. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1097–1103. doi: 10.1016/s0360-3016(00)00524-1. [DOI] [PubMed] [Google Scholar]
- 19.Kagawa K., Murakami M., Hishikawa Y. Preclinical biological assessment of proton and carbon ion beams at Hyogo Ion Beam Medical Center. Int J Radiat Oncol Biol Phys. 2002;54:928–938. doi: 10.1016/s0360-3016(02)02949-8. [DOI] [PubMed] [Google Scholar]
- 20.Imai R., Kamada T., Araki N. Carbon ion radiotherapy for unresectable localized axial soft tissue sarcoma. Cancer Med. 2018;7:4308–4314. doi: 10.1002/cam4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamada T., Tsujii H., Blakely E.A. Carbon ion radiotherapy in Japan: An assessment of 20 years of clinical experience. Lancet Oncol. 2015;16:e93–e100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K., Imai R., Kamada T. Impact of carbon ion radiotherapy for primary spinal sarcoma. Cancer. 2013;119:3496–3503. doi: 10.1002/cncr.28177. [DOI] [PubMed] [Google Scholar]
- 23.Stacchiotti S., Casali P.G. Systemic therapy options for unresectable and metastatic chordomas. Curr Oncol Rep. 2011;13:323–330. doi: 10.1007/s11912-011-0176-x. [DOI] [PubMed] [Google Scholar]
- 24.Stacchiotti S., Gronchi A., Fossati P. Best practices for the management of local-regional recurrent chordoma: A position paper by the chordoma Global consensus Group. Ann Oncol. 2017;28:1230–1242. doi: 10.1093/annonc/mdx054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber D.C., Habrand J.L., Hoppe B.S. Proton therapy for pediatric malignancies: Fact, figures and costs. A joint consensus statement from the pediatric subcommittee of PTCOG, PROS and EPTN. Radiother Oncol. 2018;128:44–55. doi: 10.1016/j.radonc.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Hamstra D.A., Mariados N., Sylvester J. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 27.Hedrick S.G., Fagundes M., Case S. Validation of rectal sparing throughout the course of proton therapy treatment in prostate cancer patients treated with SpaceOAR®. J Appl Clin Med Phys. 2017;18:82–89. doi: 10.1002/acm2.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.