Abstract
Purpose
To demonstrate feasibility and toxicity of linear accelerator–based stereotactic radiation therapy boost (SBRT) for prostate cancer, mimicking a high-dose-rate brachytherapy boost.
Methods and Materials
A phase 1 sequential dose escalation study of SBRT compared 20 Gy, 22 Gy, and 24 Gy to the prostate and 25 Gy, 27.5 Gy, and 30 Gy to the gross tumor volume in 2 fractions, combined with 46 Gy in 23 fractions of external beam radiation. Feasibility of dose escalation (volume receiving 125% and 150% of the dose) while meeting organ-at-risk dose constraints, grade 2 acute and late gastrointestinal and genitourinary toxicity, and freedom from biochemical failure were secondary endpoints.
Results
Thirty-six men with intermediate- and high-risk prostate cancer were enrolled with a median follow-up of 24 months. Sixty-four percent of patients had high-risk features. Nine men were enrolled to dose level 1, 6 to level 2, and 6 to level 3. Another 15 patients were treated at dose level 3 on the continuation study. Dose level 3 achieved superior 125% (23.75 Gy) and 150% (28.5 Gy) dose compared to dose levels 1 and 2, with minimal differences in organ-at-risk doses. Kaplan-Meier estimate of freedom from biochemical failure at 3 years was 93.3%. There were no late grade 2 or 3 gastrointestinal events. The late grade 2 genitourinary toxicity at 2 years was 19.3%. Prostate-specific membrane antigen positron emission tomography was performed at 2 years with no local recurrences.
Conclusions
We have shown that a linear accelerator–based SBRT boost for prostate cancer is feasible and can achieve doses comparable to high-dose-rate boost up to the 150% isodose volumes. Rectal, bladder, and urethral doses remained low, and long-term toxicity was the same as or better than previous reports from high-dose-rate or low-dose-rate boost protocols.
Introduction
Despite 2 randomized trials reporting significant improvements in biochemical control for localized prostate cancer using either a high-dose-rate (HDR)1 or a low-dose-rate (LDR) brachytherapy boost,2 there has been minimal uptake of this paradigm. In fact, registry data suggest that brachytherapy use in prostate cancer is decreasing.3
The reasons why level I evidence from well-conducted trials has not translated into practice include a lack of brachytherapy expertise among radiation oncologists (ROs), the resource-intensive nature of brachytherapy programs, and logistical difficulties in the coordination of several specialties (RO, anesthetist, urologist, medical physicist, and appropriately trained radiation therapists and nursing staff). Access to operating rooms is limited, and there is the cost of disposables, including interstitial seeds. Finally, in the case of HDR brachytherapy, there may be patient discomfort and inconvenience.4 There is also concern regarding increase in urinary toxicity, particularly urethral strictures, which may present late and be problematic.2, 5, 6
External beam radiation therapy (EBRT) alternatives to brachytherapy in both prostate and cervical cancer have been proposed, but historically doses achieved have not been equivalent to those with brachytherapy. With the introduction of magnetic resonance imaging (MRI) to guide contouring, the ability to separate the rectum from the prostate with a spacer,7 real-time image guidance,8, 9 and highly conformal planning with volumetric modulated arc therapy, we proposed that a virtual brachytherapy boost is now achievable with similar nonhomogenous doses compared with interstitial brachytherapy but with reduced urethral and bladder neck toxicity. We present the feasibility, acute and subacute toxicity, and early outcomes of our BOOST for prostate cancER (BOOSTER) phase 1 dose escalation study.
Methods and Materials
After providing informed consent, patients were enrolled into an ethics-approved phase 1 dose escalation study. All patients had localized prostate cancer suitable for curative treatment and were able to undergo either fiducial marker or Calypso beacon placement. Patients with an international prostate symptom score >15 or with bilateral prosthetic hips were excluded. The study was designed as 3 sequential cohorts with increasing doses. A minimum of 6 men was required in each dose level with a minimum 3-month follow-up before proceeding to the next dose level. Patients were eligible for enrollment in concurrent image guided protocols at our institution, which included real-time multileaf collimator tracking with Calypso beacons,8 gating with fiducial marker localization,9 or triggered imaging with TrueBeam software (Varian Medical Systems, Inc., Palo Alto, CA).
All patients had an MRI and from late 2014 also had a prostate-specific membrane antigen (PMSA) positron emission tomography (PET) scan. MRI and PET were fused to the planning computed tomography (CT) scan to aid contouring (including gross tumor volume [GTV] boost). If patients had Calypso transponders in situ, the MRI was obtained before their insertion. Patients were offered the option of hydrogel insertion7; however, this was not mandated.
Patients were simulated with and without an indwelling urethral catheter and with a comfortably full bladder and empty rectum. The clinical target volume (CTV) for the virtual HDR component included the prostate and proximal seminal vesicles (SVs) and the GTV as defined by multiparametric MRI or PSMA-PET. The CTV was expanded by 3 mm posteriorly and 5 mm in all other directions to form the planning target volume (PTV). There was no expansion of the GTV. Target volumes for the conventional external beam component (46 Gy in 23 fractions) were the prostate and proximal SVs in intermediate-risk patients or the entire SV and pelvic lymphatics (as per standard guidelines) for high-risk patients. The prostate CTV was expanded by 7 mm in all directions except 5 mm posterior to make the prostate PTV. Androgen deprivation therapy (ADT) was offered to all patients, but patients were allowed to decline ADT use.
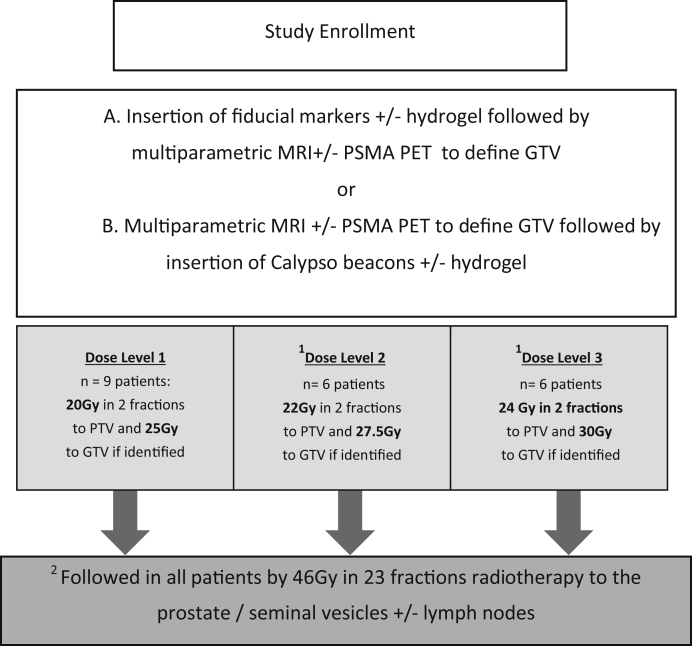
The dose levels for the study are displayed in the CONSORT diagram (Fig 1). A minimum of 6 patients with at least 3-month follow-up was required at each dose level before proceeding to the subsequent dose level. To proceed to the next dose level, there had to be no more than 1 grade 3 toxicity event. If further patients were enrolled while the sixth patient at the current dose level had not reached 3-month follow-up, they were required to be treated on the current (lower) dose level.
Figure 1.
Study schema.
The protocol was designed to increase the dose to the GTV at a proportionally higher rate than the CTV, with the aim to emulate an HDR isodose distribution, but keeping the urethra, bladder, and rectum at stable dose constraints. The final dose level (30 Gy in 2 fractions to the GTV) was modelled to reflect the 150% isodose distribution of an HDR plan, but preferentially directed to the GTV.
After completion of the dose escalation component of the study, another enrollment of 15 patients at the maximum tolerated dose level was undertaken. Secondary endpoints included acute and late radiation toxicity (Common Toxicity Criteria version 4.0) and freedom from biochemical failure (prostate-specific antigen [PSA] nadir + 2).
Results
Twenty-one patients were included in the initial dose finding study and an additional 15 patients in the continuation study at the maximum dose level. Patient characteristics are presented in Table 1. The median age was 69 years (range, 55-85 years) and the median initial PSA was 9.0 (range, 4.3-130). Baseline international prostate symptom score ranged from 0 to 15 (median, 5). Histologic grading as per the International Society of Urological Pathology 2017 classification was grade group 2 (GS 3 + 4), 3 (GS 4 + 3), 4 (GS 4 + 4), and 5 (GS 5 + 4) in 25%, 33%, 14%, and 28% of patients, respectively, with 64% in the high-risk group and 36% intermediate-risk group. ADT was used in 61% of the patients; 16 of 23 were high risk and 6 of 13 were intermediate risk. No GS 5 + 5 patients were included.
Table 1.
Patient demographics (n = 36)
| Characteristics | Median (range) or no. of patients |
|---|---|
| Age, y | 69 (55-85) |
| iPSA | 9.0 (4.3-130) |
| AJCC T stage | |
| T1c to T2a | 12 |
| T2b to T2c | 12 |
| T3a | 7 |
| T3b | 5 |
| ISUP 2017 grade | |
| 2 | 9 |
| 3 | 12 |
| 4 | 5 |
| 5 | 10 |
| Risk group | |
| Intermediate | 13 |
| High | 23 |
| ADT use | 22 |
| Image guidance | |
| Calypso | 11 |
| Kim gating | 23 |
| TrueBeam gating | 3 |
| Dose levels | |
| 1 | 9 |
| 2 | 6 |
| 3 | 21 |
Abbreviations: ADT = Androgen deprivation therapy; AJCC = American Joint Committee on Cancer; iPSA = initial PSA; ISUP = International Society of Urological Pathology.
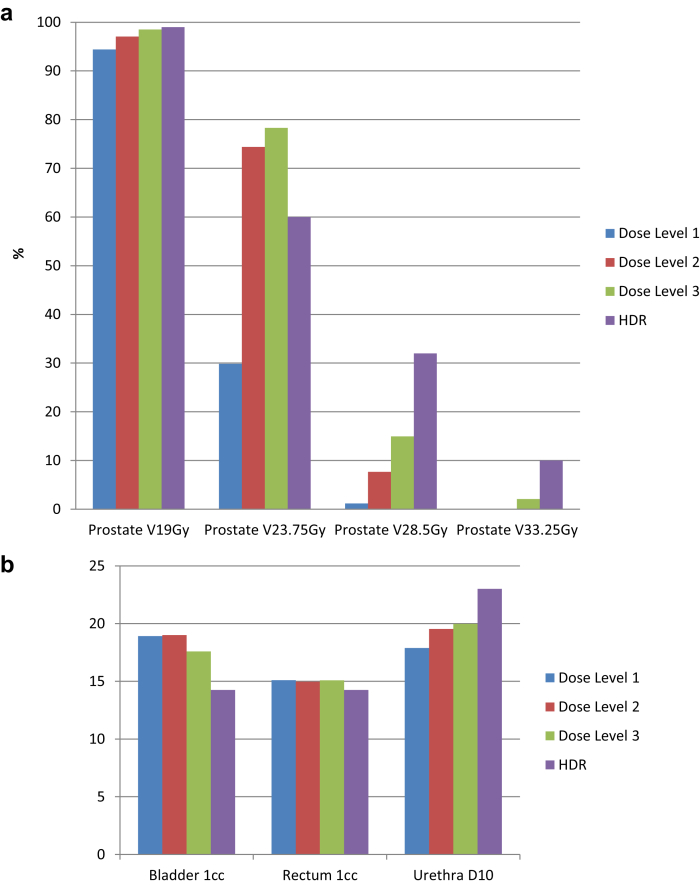
Median follow-up was 24 months (range, 10-54 months). Freedom from biochemical failure (PSA nadir + 2) at 3 years was 93.3%. None of the intermediate-risk patients have had a biochemical failure to date. Dose heterogeneity was successfully increased with each dose level while maintaining the rectal, urethral, and bladder dose constraints (Figs 2 and 3). Figure 2 shows typical isodose distributions for each of the dose levels. If viewed in association with Figure 3, it can be seen that despite increased dose heterogeneity (V125% increasing from 30% at dose level 1 to >70% at dose levels 2 and 3; V150% increasing from 1% to 8% and 15%, respectively) the rectal, bladder, and urethral doses have remained relatively constant. Figure 3 compares the dose heterogeneity and organ-at-risk (OAR) doses from our study with typical HDR patients.5
Figure 2.
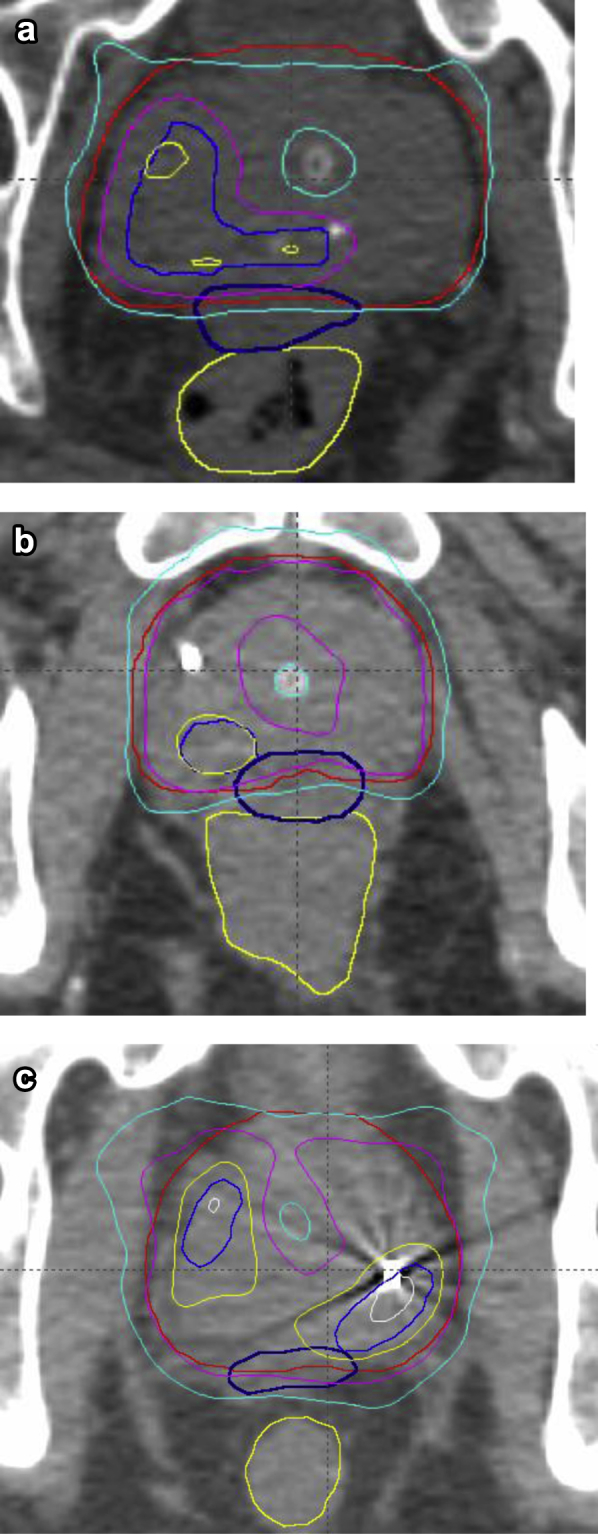
Comparison of the increasing dose heterogeneity among dose levels 1, 2 and 3. Prescription dose was 19 Gy in 2 fractions. White = 33.25 Gy (175%); yellow = 28.5 Gy (150%); pink = 23.75 Gy (125%); cyan = 19 Gy (100%). (a) Dose level 1. (b) Dose level 2. (c) Dose level 3. French blue = GTV; red = PTV; dark blue = hydrogel; yellow = rectum. Compared to dose level 1, dose level 2 has improved coverage of 23.75 Gy and some areas of 28.5 Gy to the GTV. Dose level 3 has larger areas of 28.5 Gy with improved GTV coverage. The urethra and rectum are kept at similar doses in each dose level. Abbreviations: GTV = gross tumor volume; MRI = magnetic resonance imaging; PSMA-PET = prostate specific membrane antigen- positron emission tomography; PTV = planning target volume. (A color version of this figure is available at https://doi.org/10.1016/j.adro.2019.03.015.)
Figure 3.
Comparison of dose levels 1 to 3 (stereotactic radiation therapy boost) and high-dose-rate (HDR) brachytherapy for (a) percentage of target (prostate) volume receiving 19 Gy (100%), 23.75 Gy (125%), 28.5 Gy (150%), and 33.25 Gy (175%) and (b) dose to 1 mL of bladder and rectum and dose to 10% of the intraprostatic urethra. This shows increasing regions of high dose inside the prostate as patients proceed through the dose escalation levels, but this has not been at the expense of bladder, rectum, or urethra doses (b).
In the initial dose finding study 2 patients (1 each in dose level 1 and dose level 3) required catheterization after the first SBRT fraction. In both patients, the second stereotactic boost dose was omitted, and an adjustment to the standard fractionation was made to deliver 60 Gy in 30 fractions instead of 46 Gy 23 fractions. Both patients at last follow-up had a PSA <0.2. In the first patient the catheter was removed at day 6, and with 4 years of follow-up, he has not reported any further urinary symptoms. The second patient's catheter was removed at day 5. He remained well until he developed hematuria, dysuria, and urgency (grade 2) at 12 months postradiation. These symptoms resolved with the aid of an anticholinergic and at last follow-up (24 months) he has had no further symptoms.
Other acute grade 2 genitourinary (GU) toxicities were reported in 3 patients at dose level 2 and 4 patients at dose level 3 (including the continuation study). This toxicity was due to a combination of frequency and dysuria, with onset commonly 1 to 2 weeks into the standard-fractionation treatment. Symptoms responded to α-blockers, anticholinergics, and in 1 patient treatment of a urinary tract infection (subsequent to catheterization at simulation). No acute grade 2 or 3 gastrointestinal (GI) toxicities were recorded. There were no acute or late grade 3 GU or GI toxicities in any patient.
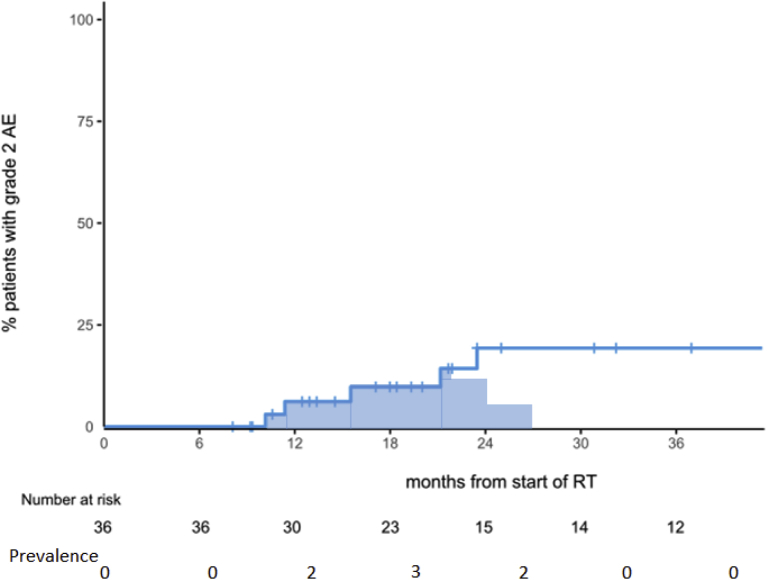
No late grade 2 GI events were recorded. Late grade 2 GU toxicity was reported in 5 patients (actuarial rate at 2 years of 19.3%). Late grade 2 GU toxicity is shown in Figure 4 with both Kaplan-Meier estimates and prevalence. Prevalence peaked between 12 and 24 months with 3 of 23 patients (13%). Late toxicity was frequency in 3 patients, urgency in 1 patient, and a urethral stricture in 1 patient. This patient had concurrent penile/urethral psoriasis, and a small stricture was dilated 24 months postradiation. At 36 months, he has had no further urinary issues. In the 14 patients with >30 months follow-up, no patients reported grade 2 toxicity (ie, prevalence = 0%). There were no late grade 3 events.
Figure 4.
Cumulative incidence and prevalence of grade 2 urinary toxicity. Number at risk refers to patients on study still in follow-up at that time point. Prevalence refers to absolute number of patients in follow-up with a grade 2 urinary toxicity at that time point. Abbreviation: RT = radiation therapy.
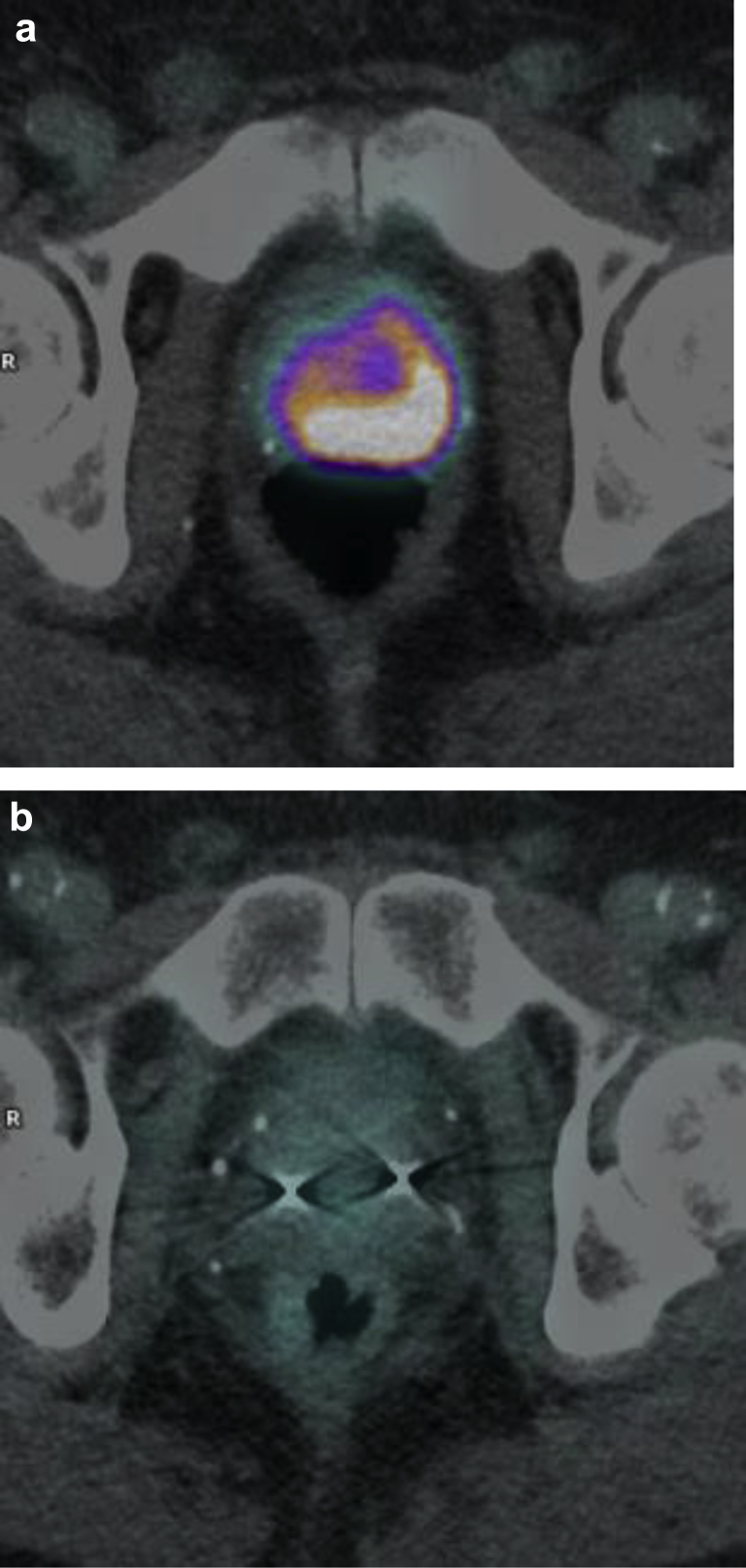
There have been 3 biochemical failures. In 2 of the 3 patients PSMA at the time of failure showed distant disease with no PSMA uptake identified locally in the prostate. In the third patient, at 3.5 years of follow-up his PSA was 2.5 (nadir 0.2). Because of progressive airway disease, he declined further investigations. His 2-year PSMA PET 18 months prior showed no evidence of disease. In the remaining 33 patients, there have been no biochemical failures. Fifteen patients have been followed up for >2 years, all of whom have undergone a 24-month PSMA PET scan. There has been a complete metabolic response (Fig 5) in all patients (100% local metabolic control). There has been 1 death, without evidence of recurrent prostate cancer.
Figure 5.
Patient treated on protocol with androgen deprivation for 18 months combined with stereotactic radiation therapy boost, then 46 Gy in 23 fractions. (a) Pretreatment positron emission tomography showing large tumor extending bilaterally and (b) 24-month positron emission tomography showing complete response (prostate-specific antigen 0.07 and testosterone 13.9 nmol/L.)
Discussion
With the use of a conventional linear accelerator (linac) coupled with real-time imaging, we have emulated nonhomogeneous HDR-like doses with an SBRT boost. Our technique was feasible, with orderly progression through the 3 dose levels. Most importantly, we have approached HDR-like isodoses with acceptable GI and GU toxicity. We know from the HDR experience that urethral toxicity can manifest many years after the procedure and longer follow-up is needed.5 One potential advantage of an SBRT boost is the lack of trauma to the urethra from needle insertion. In addition, caudal displacement of needles with HDR has been well documented, resulting in migration of the dose cloud away from the target to the membranous urethra or GU diaphragm.10 Hence, the OAR doses shown in Figure 3b for HDR may not have been delivered. This criticism may also be levelled at SBRT owing to intrafraction motion, but with real-time imaging, dose reconstruction has shown this not to be the case.8
As with all prostate cancer series, much longer follow-up is needed to determine local control and recurrence free survival, but the 2-year PSMA outcomes are promising, with all 15 patients who have undergone their scheduled 2-year posttreatment PSMA scan exhibiting a complete metabolic response. Although PSMA PET is unproven as a surrogate endpoint, we have previously reported our experience in delineating failure in those with rising PSA (Phoenix definition) and found PSMA to be both reliable and specific.11 With PSMA PET not currently approved in the United States, this endpoint is of limited utility in that population. However, as access becomes easier, PSMA surrogates will be an area of interest.
The lower use of ADT in our cohort (only 61% of patients) may reflect a bias in patient selection. All patients were offered ADT, but many declined because they perceived the toxicity as greater than the potential gain. It is possible some patients enrolled in our study with the goal of avoiding ADT, offsetting the gains with higher biological doses. This was not an aim of the study.
Ultra-high-dose escalation with a brachytherapy boost has been found to be superior to standard-fractionation EBRT in 2 randomized studies for biochemical control.1, 2 Although the control arm of the initial randomized control trial was considered low, the more recent publication from Morris et al2 used a contemporary dose of 78 Gy in 39 fractions and demonstrated a difference of 86% versus 75% biochemical failure at 7 years in a moderate- to high-risk cohort. It should be noted that there has been no comparison of HDR boost with dose escalated radiation or HDR boost to LDR boost, and extrapolation of these techniques needs to be done with caution. An increase in toxicity and barriers to accessing a brachytherapy program may explain the poor uptake of this treatment paradigm. In comparison, our linac-based program has the potential for lower toxicity and wider adoption.
There have been 5 published studies investigating stereotactic boost in addition to standard fractionation EBRT: 4 reports with the CyberKnife platform11, 12, 13, 14 and 1 with intensity modulated radiation therapy.15 The largest experience is from Katz et al,13 who reported 73 patients with both intermediate- (n = 41) and high-risk (n = 32) disease. Patients were treated with CyberKnife to deliver between 18 and 21 Gy in 3 fractions in addition to 45 Gy in 25 fractions of EBRT. With a median follow-up of 33 months, BFFS was 89.5% and 77.7% for the intermediate- and high-risk patients, respectively. A 5-mm expansion from the prostate to the PTV was used except posteriorly, where the margin was 3 mm. There was a 7% rate of grade 2 acute GU and GI toxicity, with late grade 2 estimates at 3 years of 5.5% (GU) and 8.2% (GI). Despite larger boost doses, we have shown lower grade 2 GI toxicity and similar GU toxicity in our series. The former may be due to our use of a hydrogel spacer to spare the rectum. Three other series also using CyberKnife were reported between 2008 and 2012.11, 12, 14 These studies used smaller margins of 0 to 2 mm posteriorly and 3 to 5 mm in other directions and reported low rates of grade 2 and grade 3 toxicity. Only 2 of these reports specifically attempted to reproduce the dose heterogeneity of HDR, with large areas of the PTV receiving >125% (40%-45%) and >150% (5%-10%) of the prescribed dose. These CyberKnife virtual HDR plans are similar to the plans seen in dose level 3 of our study (Fig 3a). We consider that achieving a nonhomogenous dose akin to brachytherapy is important with respect to efficacy and reducing toxicity. Furthermore, if we simply delivered a uniformly homogenous SBRT dose, then the SBRT boost is simply a hybrid fractionation schedule combining elements of extreme hypofractionation (for 2 fractions) with conventional fractionation at 2 Gy per fraction per day, rather than true virtual brachytherapy. It should be noted that we only achieved doses similar to HDR up to, but not exceeding, the 150% dose (eg, >28 Gy in 2 fractions). It is unknown if small volumes of exceptionally high dose will have a biological impact. The initial results of our study, which show excellent local control, are some evidence that the dose response may not extend to this dose range.
Miralbell et al15 reported a linac-based intensity modulated radiation therapy boost in 50 patients in 2010. This series used an endorectal balloon but no image guidance, which may be the cause of the unacceptably high 5-year estimates of 26% late GI grade 2 toxicity. The endorectal balloon spares the posterior rectal wall but in some cases may push a larger proportion of the anterior rectal circumference into the high-dose region. This is not an issue with hydrogel, where the entire rectal circumference is displaced posteriorly without the day-to-day variation of a balloon.
The use of our linac-based approach has implications for the generalizability of this study. All departments have access to modern linacs, but few can access the CyberKnife. The only caveat is access to real-time motion management. We used 3 types of real-time image guidance in the form of Calypso, kilovoltage intrafraction monitoring, and Varian triggered imaging, demonstrating that multiple solutions for motion management exist. There are both patient convenience and departmental workflow benefits of hypofractionation, whether with brachytherapy or external beam radiation alone, compared to a conventionally fractionated approach. The optimal technique and dose fractionation schedule, however, has yet to be defined. Quality of life data from Toronto compared 5-fraction SBRT with HDR and found significant differences in urinary function and bother, bowel function, and sexual function and bother domains in favor of the SBRT group.16 It is unknown how an SBRT linac-based boost compares to a 20-fraction, or even 5-fraction, approach, especially if these moderately or extremely hypofractionated approaches are escalated with a simultaneous integrated boost to MRI- or PSMA-defined dominant intraprostatic lesions, as in the Fluoxetine for Motor Recovery after Acute Ischaemic Stroke (FLAME) trial.17 With a recent publication18 reporting SBRT as monotherapy in 2142 patients from 10 studies (median follow-up, 6.9 years), with excellent biochemical and toxicity outcomes, the role of HDR or SBRT boost may also become less relevant. The caveat to this is the low number of high-risk patients included in SBRT monotherapy series to date. It is reasonable to hypothesize that EBRT boost will be indicated only in bulky, high-grade cancers, with SBRT monotherapy suitable for all other patients. Future studies are needed to answer this.
Conclusions
A linac-based SBRT boost for prostate cancer is feasible and can achieve doses comparable to HDR boost, ≤150% isodose volumes. Low rectal, bladder, and urethral doses can be achieved. To date we have shown very acceptable toxicity, with no grade 3 events.
Acknowledgments
The authors thank the Luan and Yoong Foundation for the generous support of Radiation Oncology Research in our department.
Footnotes
Sources of support: Varian Medical Systems have partially funded the tracking study that enabled this analysis.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Hoskin P.J., Rojas A.M., Bownes P.J. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103:217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Morris W.J., Tyldesley S., Rodda S. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98:275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Wang L.L., Begashaw K., Evans M. Patterns of care and outcomes for men diagnosed with prostate cancer in Victoria: An update. ANZ J Surg. 2018;88:1037–1042. doi: 10.1111/ans.14722. [DOI] [PubMed] [Google Scholar]
- 4.Chen J.Y., Hruby G., Stockler M.R. Patient-reported outcomes of prostate high-dose-rate brachytherapy boost comparing an outpatient and inpatient protocol: A two-center chronologic cohort study. Brachytherapy. 2011;10:454–460. doi: 10.1016/j.brachy.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Bece A., Patanjali N., Jackson M. High-dose-rate brachytherapy boost for prostate cancer: Outcomes and genitourinary toxicity. Brachytherapy. 2015;14:670–676. doi: 10.1016/j.brachy.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan L., Williams S.G., Tai K.H. Urethral stricture following high-dose rate brachytherapy for prostate cancer. Radiother Oncol. 2009;91:232–236. doi: 10.1016/j.radonc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Whalley D., Hruby G., Alfieri F. SpaceOAR hydrogel in dose-escalated prostate cancer radiotherapy: Rectal dosimetry and late toxicity. Clin Oncol (R Coll Radiol) 2016;28:e148–e154. doi: 10.1016/j.clon.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Keall P.J., Colvill E., O'Brien R. Electromagnetic-guided MLC tracking radiation therapy for prostate cancer patients: Prospective clinical trial results. Int J Radiat Oncol Biol Phys. 2018;101:387–395. doi: 10.1016/j.ijrobp.2018.01.098. [DOI] [PubMed] [Google Scholar]
- 9.Keall P.J., Ng J.A., Juneja P. Real-time 3D image guidance using a standard linac: Measured motion, accuracy, and precision of the first prospective clinical trial of kilovoltage intrafraction monitoring-guided gating for prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2016;94:1015–1021. doi: 10.1016/j.ijrobp.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Simnor T., Li S., Lowe G. Justification for inter-fraction correction of catheter movement in fractionated high-dose-rate brachytherapy treatment of prostate cancer. Radiother Oncol. 2009;93:253–258. doi: 10.1016/j.radonc.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Fuller D.B., Naitoh J., Lee C. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: Dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys. 2008;70:1588–1597. doi: 10.1016/j.ijrobp.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Jabbari S., Weinberg V.K., Kaprealian T. Stereotactic body radiotherapy as monotherapy or post-external beam radiotherapy boost for prostate cancer: Technique, early toxicity, and PSA response. Int J Radiat Oncol Biol Phys. 2012;82:228–234. doi: 10.1016/j.ijrobp.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Katz A.J., Santoro M., Ashley R. Stereotactic body radiotherapy as boost for organ-confined prostate cancer. Technol Cancer Res Treat. 2010;9:575–582. doi: 10.1177/153303461000900605. [DOI] [PubMed] [Google Scholar]
- 14.Oermann E.K., Slack R.S., Hanscom H.N. A pilot study of intensity modulated radiation therapy with hypofractionated stereotactic body radiation therapy (SBRT) boost in the treatment of intermediate- to high-risk prostate cancer. Technol Cancer Res Treat. 2010;9:453–462. doi: 10.1177/153303461000900503. [DOI] [PubMed] [Google Scholar]
- 15.Miralbell R., Molla M., Rouzaud M. Hypofractionated boost to the dominant tumor region with intensity modulated stereotactic radiotherapy for prostate cancer: A sequential dose escalation pilot study. Int J Radiat Oncol Biol Phys. 2010;78:50–57. doi: 10.1016/j.ijrobp.2009.07.1689. [DOI] [PubMed] [Google Scholar]
- 16.Helou J., Morton G., Zhang L. A comparative study of quality of life in patients with localized prostate cancer treated at a single institution: Stereotactic ablative radiotherapy or external beam + high-dose rate brachytherapy boost. Radiother Oncol. 2014;113:404–409. doi: 10.1016/j.radonc.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Monninkhof E.M., van Loon J.W.L., van Vulpen M. Standard whole prostate gland radiotherapy with and without lesion boost in prostate cancer: Toxicity in the FLAME randomized controlled trial. Radiother Oncol. 2018;127:74–80. doi: 10.1016/j.radonc.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Kishan A.U., Dang A., Katz A.J. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open. 2019;2:e188006. doi: 10.1001/jamanetworkopen.2018.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]