Abstract
Germline heterozygous PTEN mutations cause subsets of Cowden syndrome (CS) and Bannayan-Riley-Ruvalcaba syndrome (BRRS); these subsets are characterized by high risks of breast, thyroid, and other cancers and, in one subset, autism spectrum disorder (ASD). Up to 10% of individuals with PTENMUT CS, CS-like syndrome, or BRRS have germline SDHx (succinate dehydrogenase, mitochondrial complex II) variants, which modify cancer risk. PTEN contributes to metabolic reprogramming; this is a well-established role in a cancer context. Relatedly, SDH sits at the crossroad of the electron transport chain and tricarboxylic acid (TCA) cycle, two central bioenergetic pathways. Intriguingly, PTENMUT and SDHMUT individuals have reduced SDH catalytic activity, resulting in succinate accumulation; this indicates a common genotype-independent biochemical alteration. Here, we conducted a TCA targeted metabolomics study on 511 individuals with CS, CS-like syndrome, or BRRS with various genotypes (PTEN or SDHx, mutant or wild type [WT]) and phenotypes (cancer or ASD) and a series of 187 population controls. We found consistent TCA cycle metabolite alterations in cases with various genotypes and phenotypes compared to controls, and we found unique correlations of individual metabolites with particular genotype-phenotype combinations. Notably, increased isocitrate (p = 1.2 × 10−3), but reduced citrate (p = 5.0 × 10−4), were found to be associated with breast cancer in individuals with PTENMUT/SDHxWT. Conversely, increased lactate was associated with neurodevelopmental disorders regardless of genotype (p = 9.7 × 10−3); this finding was replicated in an independent validation series (n = 171) enriched for idiopathic ASD (PTENWT, p = 5.6 × 10−4). Importantly, we identified fumarate (p = 1.9 × 10−2) as a pertinent metabolite, distinguishing individuals who develop ASD from those who develop cancer. Our observations suggest that TCA cycle metabolite alterations are germane to the pathobiology of PTEN-related CS and BRRS, as well as genotype-independent ASD, with implications for potential biomarker and/or therapeutic value.
Keywords: PTEN hamartoma tumor syndrome, autism spectrum disorder, neurodevelopmental disorders, hereditary cancer, targeted metabolomics, Krebs cycle
Main Text
Cowden syndrome (CS [MIM: 158350]) and Bannayan-Riley-Ruvalcaba syndrome (BRRS [MIM: 153480]) represent two well-defined PTEN-related overgrowth disorders. CS is a multisystem autosomal dominant disorder characterized by elevated lifetime risks for both benign and malignant tumors of the breast and thyroid, as well as neurodevelopmental disorders such as autism spectrum disorder (ASD).1, 2, 3 CS is difficult to clinically recognize due to the extensive degree of phenotypic variability and the existence of individual features which mimic normal variation or other disorders.4 BRRS is a rare autosomal dominant congenital disorder classically characterized by macrocephaly in combination with intestinal hamartomatous polyposis, vascular malformations, lipomas, hemangiomas, and genital freckling.5 Similar to CS, individuals with BRRS can present with a spectrum of mild to severe phenotypic manifestations, including neurodevelopmental delays.6
The tumor suppressor gene phosphatase and tensin homolog (PTEN [MIM: 601728]), which encodes a dual-specificity phosphatase which classically counteracts the PI3K/AKT/mTOR growth-promoting cascade, is the first CS and BRRS susceptibility gene.7 Germline PTEN mutations occur in ∼25% of CS/CS-like individuals who meet International Cowden Consortium (ICC) operational diagnostic criteria (Table S1)8 and in up to ∼60% of BRRS cases.9 More recent studies have shown that up to 20% of individuals with ASD and macrocephaly have germline PTEN mutations.3, 10, 11, 12 In the PTEN wild type (WT) subset of CS/CS-like individuals (who meet full diagnostic criteria minus one), ∼10% harbor germline heterozygous variants in genes encoding three of the four subunits of succinate dehydrogenase or mitochondrial complex II (SDHB [MIM: 185470], SDHC [MIM: 602413], and SDHD [MIM: 602690]; collectively referred to as SDHx).13 Mitochondrial complex II lies at the crossroads between the electron transport chain and the tricarboxylic acid (TCA or Krebs) cycle, catalyzing the oxidation of succinate to fumarate. Individuals harboring SDHx variants show an increased risk of breast cancer and papillary thyroid cancer that surpasses the risk mediated by mutant PTEN alone.14 Functional studies show that SDHx variants result in ROS-mediated stabilization of HIF-1α, destabilization and decreased protein levels of p53 due to defective interaction with NQO1, and resistance to apoptosis.14 Indeed, mitochondrial dysfunction is not an unusual suspect at the crux of metabolic perturbations which lead to tumorigenesis.15, 16, 17
Biochemical screening of organic acids identified elevated plasma succinate levels in a pilot series of 55 CS/CS-like individuals with PTEN, SDHB, and SDHD mutations compared to controls.16 Mutations in SDHB and SDHD make sense in terms of succinate accumulation due to mitochondrial complex II dysfunction. However, the finding of succinate accumulation in PTEN mutation positive (SDHx WT) individuals intriguingly suggests a common biochemical perturbation in CS/CS-like individuals independent of genotype. A subsequent biochemical analysis of urine and plasma organic acids and amino acids in another pilot series of 69 individuals with ASD, with or without macrocephaly and in the presence or absence of germline PTEN mutations, did not reveal genotype- or phenotype-driven associations with metabolite levels.18 However, the lack of elevated succinate levels in the ASD cases compared to the original series of CS/CS-like individuals without ASD reflects potential differences in metabolite signatures in a phenotype-dependent manner, and that may have been missed due to the limited sample size in each of the two pilot series. Thus, our current study sought to identify common and distinct TCA cycle metabolite alterations in an expanded prospective series of CS, CS-like, and BRRS individuals to ascertain genotype- and/or phenotype-dependent metabolic signatures.
Five hundred eleven eligible CS, CS-like and BRRS individuals were identified based on genotypic and phenotypic characteristics (Table 1) and in accordance with our research protocol 8458-PTEN, approved by the Cleveland Clinic Institutional Review Board. Both females (68.5%) and males (31.5%) were represented, and the median age at consent was 45 years (range 1–89). The Cleveland Clinic (CC) score, roughly an estimate of age-related disease burden,8 ranged from 0 to 69 (median 13), corroborating the broad spectrum of phenotypic burden and PTEN germline mutation status. Of 511 research participants, 309 (60.5%) harbored germline pathogenic PTEN mutations, 79 (15.5%) harbored germline SDHx mutations or variants, and 112 (21.9%) met clinical diagnostic criteria but were WT for both PTEN and SDHx. Neurodevelopmental disorders and solid tumors were the two predominant clinical phenotypic features. Among all cases, 111 (21.7%) had neurodevelopmental features, chief of which were ASD and developmental delays (DD). Of these 111, the median age at consent was 7 years (range 1–62). A total of 285 (55.8%) individuals had a cancer diagnosis, with 145 (50.9%) of those having second malignant primary neoplasms (SMNs). In the remaining 124 cases without cancer and without ASD or DD, the median age at consent was 39 years (range 1–74). We also identified 98 population controls (median age 37; range 2–76) who were WT for PTEN and SDHx (Table S2).
Table 1.
Demographic and Clinical Characteristics of 511 Cowden/Cowden-Like Syndrome and Bannayan-Riley-Ruvalcaba Syndrome Cases
| median age at consent (range) | 45 (1–89) |
| median CC score (range) | 13 (0–69) |
| Gender | |
| female | 350 (68.5%) |
| male | 161 (31.5%) |
| Genotypes | |
| PTEN | 309 |
| SDHx | 79 |
| PTEN/SDHx | 11 |
| PTEN/SDHx wildtype | 112 |
| Macrocephaly | |
| yes | 337 (65.9%) |
| no | 116 (22.7%) |
| unknown | 58 (11.4%) |
| Neurodevelopmental Features | 111 (21.7%) |
| macrocephaly | 108 |
| autism spectrum disorder | 45 |
| global developmental delay | 64 |
| variable delay | 27 |
| mental retardation | 12 |
| learning disabilities | 10 |
| Cowden Component Malignancies | |
| breast cancer | 142 (27.8%) |
| thyroid cancer | 99 (19.4%) |
| renal cell cancer | 50 (9.8%) |
| endometrial cancer | 48 (9.4%) |
| colon cancer | 16 (3.1%) |
| melanoma | 13 (2.5%) |
| Other Malignancies | |
| ovarian cancer | 24 (4.7%) |
| testicular cancer | 3 (0.6%) |
| bladder cancer | 1 (0.2%) |
| other gastrointestinal cancer | 2 (0.4%) |
We first sought to investigate whether TCA metabolite levels (Figure S1) are altered in CS/CS-like and BRRS cases compared to population controls, while taking PTEN/SDHx mutation status, age, and gender into consideration. Immortalized lymphoblastoid cell lines (LBLs) from case and control peripheral blood samples (n = 780) were generated following standard procedures and then cultured in vitro for metabolic profiling (see Supplemental Material and Methods in Supplemental Data). Extracted metabolites were measured through the use of liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis. Sample measurements were obtained in five batches and normalization was applied before we performed global comparisons. Quality control measures resulted in seven out of nine TCA metabolites (citrate, isocitrate, succinate, fumarate, malate, lactate, and 2-hydroxyglutarate) with reliable signal-to-noise estimates (Figure S1). To look for correlations between genotypic and/or phenotypic features and metabolite levels, we applied linear regression models controlling for age and gender (see Supplemental Material and Methods in Supplemental Data).
First, we looked for metabolite associations with phenotype, irrespective of genotype status. As such, we found that increased citrate (p = 2.9 × 10−3) and lactate (p = 6.1 × 10−4) but decreased isocitrate (p = 3.0 × 10−5) and succinate (p = 2.8x10−3) levels were associated with CS/CS-like or BRRS compared to controls (Figure 1A–1E). Moreover, increased citrate (p = 6.8x10−4) but decreased isocitrate (p = 1.9x10−3) levels were correlated with having a cancer diagnosis (any malignancy) relative to controls. These observations, compared to controls, remained consistent for individuals diagnosed with thyroid cancer (increased citrate, p = 1.9 × 10−3; decreased isocitrate, p = 7.2 × 10−3), but not for those diagnosed with breast cancer. We focus our analyses on breast and thyroid cancers because they are the most prevalent component malignancies reported in PTEN mutation positive CS.19 Considering the subset of individuals with ASD/DD (Table 1), we observed similar correlation patterns, i.e., increased citrate (p = 4.6 × 10−2) and lactate (p = 9.7 × 10−3), along with decreased isocitrate (p = 3.2 × 10−2) and succinate (p = 1.3 × 10−2); but we additionally noted the distinct association of those with ASD/DD with decreased fumarate (p = 7.2 × 10−3). These data indicate that TCA cycle metabolite alterations show a clear and consistent pattern in CS/CS-like and BRRS cases with various phenotypic features compared to controls.
Figure 1.
Alteration of TCA Cycle Metabolites in CS, CS-like syndrome, or BRRS Cases Compared to Population Controls
A general logistic regression model was applied to evaluate the association between metabolite levels and genotype and/or phenotype, with age and gender as covariates. Heatmaps (A and F) depict TCA cycle metabolites (top label) and significance of associations relative to various phenotypic or genotypic group comparisons (left label). Blue color corresponds to an association with decreased metabolite levels, whereas orange color corresponds to an association with increased metabolite levels. Color intensity corresponds to different thresholds of p value significance (scale on the right).
(A) Heatmap depicting the association between TCA cycle metabolite levels and phenotypes, irrespective of genotype status, compared to population controls.
(B–E) Stratified gender depiction of increased citrate and lactate, but decreased isocitrate and succinate in all CS, CS-like syndrome, or BRRS cases (n = 511) compared to population controls (n = 98). Metabolite levels were log-transformed and center-scaled to have a mean of 0 and a standard deviation of 1. The line in the middle of each scatter plot is plotted at the mean. Error bars represent the 95% confidence intervals.
(F) Heatmap depicting the association between TCA cycle metabolite levels and genotypes, irrespective of phenotype status. The first three rows represent comparisons between individuals of different genotypes and population controls. The last two rows represent comparisons between individuals of various genotypes.
Second, we sought to determine whether TCA cycle metabolite levels may be influenced by underlying genotype status. We first compared metabolites from individuals with germline PTEN mutations (WT for SDHx, PTENMUT/SDHWT) to metabolites from controls (WT for both PTEN and SDHx). Our analysis showed germline PTEN mutation as a genetic feature associated with increased 2-hydroxyglutarate (p = 2.4x10−2), citrate (p = 1.8x10−3), and lactate (p = 7.2x10−3), but decreased isocitrate (p = 5.0 × 10−4) and succinate (p = 4.3 × 10−3) (Figure 1F). Relatedly, increased citrate (p = 1.9 × 10−2) but reduced fumarate (p = 4.4 × 10−2) and isocitrate (p = 1.9 × 10−2) were associated with germline SDHx variants (WT PTEN, PTENWT/SDHMUT) compared to controls. Interestingly, CS/CS-like and BRRS individuals who are WT for both PTEN and SDHx (PTENWT/SDHWT) only showed weak association with increased lactate levels (p = 1.3 × 10−2). To elucidate whether these differences are indeed genotype-dependent (versus linked to the presence of a disease state in cases relative to controls), we compared TCA metabolite levels between mutant and WT individuals. Relative to WT CS, CS-like syndrome, or BRRS cases (PTENWT/SDHWT), increased 2-hydroxyglutarate (p = 1.4 × 10−2), citrate (p = 2.5 × 10−4), and lactate (p = 1.1 × 10−2) but reduced malate (p = 6.0 × 10−4) and succinate (p = 4.3 × 10−3) were associated with the PTENMUT/SDHWT genotypic feature. However, only increased malate (p = 3.8 × 10−2) was associated with the PTENWT/SDHMUT genotype compared to PTENWT/SDHWT cases. Interestingly, increased 2-hydroxyglutarate and decreased malate were distinctive metabolites in PTENMUT/SDHWT compared to WT individuals. So far, these genotype-level analyses suggest that PTEN and SDHx are strong predictive genetic features for TCA metabolite levels in cases versus controls (Figure 1F).
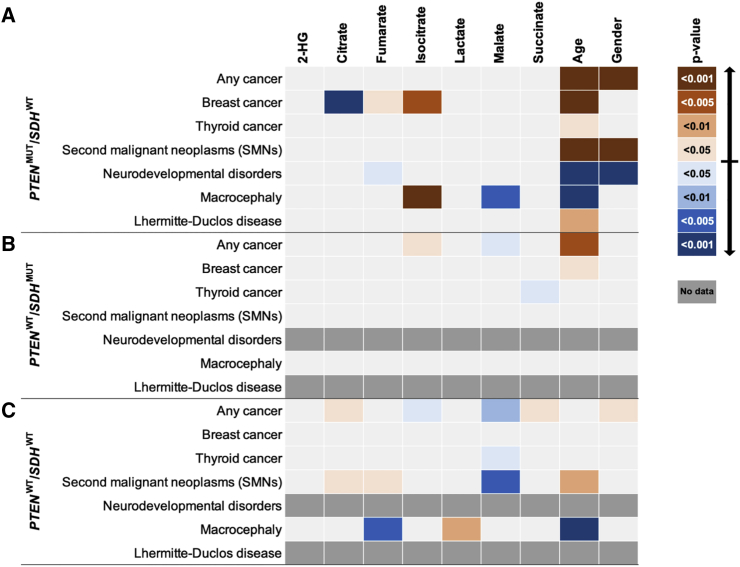
Because genotype is the dominant risk factor contributing to the metabolite differences of cases versus controls, we performed stratified analyses to tease out whether specific metabolites are associated with specific phenotypic features within defined genotypic subgroups. Within the PTENMUT/SDHWT subset of individuals, we found increased levels of fumarate (p = 3.0 × 10−2) and isocitrate (p = 1.2 × 10−3) but reduced citrate (p = 5.0 × 10−4) to be associated with breast cancer compared to PTENMUT/SDHWT individuals without breast cancer (Figure 2A). Additionally, decreased fumarate (p = 1.8 × 10−2) was correlated with the presence of ASD/DD. Finally, increased isocitrate (p = 1.0 × 10−3) but decreased malate (p = 1.4 × 10−3) were both correlated with the presence of macrocephaly. Within the subset of individuals with SDHx variants (PTENWT/SDHMUT), we observed increased isocitrate (p = 1.9 × 10−2) but decreased malate (p = 1.6 × 10−2) to be associated with a cancer diagnosis, compared to individuals with the same genotype but without cancer. Additionally, reduced levels of succinate (p = 3.2 × 10−2) were specifically associated with the presence of a thyroid cancer diagnosis compared to PTENWT/SDHMUT cases without thyroid cancer (Figure 2B).
Figure 2.
Altered TCA Cycle Metabolites Correlate with Distinct Genotype-Phenotype Combinations
Heatmaps depicting stratified analyses to determine associations among distinct metabolites and specific phenotypic features within the PTENMUT/SDHWT (A), PTENWT/SDHMUT (B), and PTENWT/SDHWT (C) groups, compared to cases who have identical genotypes, respectively, but who do not have the phenotype of interest. A general logistic regression model was applied with age and gender as covariates. Heatmaps depict TCA cycle metabolites (top label) and significance of associations relative to various phenotypic or genotypic group comparisons (left label). Blue color corresponds to an association with decreased metabolite levels, whereas orange color corresponds to an association with increased metabolite levels. Color intensity corresponds to different thresholds of p value significance (scale on the right). Dark gray (no data) fields indicate the absence of cases fulfilling the corresponding genotype/phenotype combinations.
The third subset of analyses focuses only on PTEN and SDHx WT CS/CS-like and BRRS cases (PTENWT/SDHWT). Intriguingly, although we did not observe notable associations, except for increased lactate, in this WT subset of individuals compared to controls (Figure 1F), multiple associations were observed when specific phenotypic features were taken into consideration (Figure 2C). These metabolite differences appear to be purely associated with phenotypic features in PTENWT/SDHWT CS, CS-like syndrome, or BRRS individuals. Increased citrate (p = 1.9 × 10−2) and succinate (p = 3.6 × 10−2) but decreased isocitrate (p = 3.9 × 10−2) and malate (p = 9.4 × 10−3) were associated with the presence of cancer (any malignancy) in WT CS, CS-like syndrome, or BRRS cases compared to WT cases without cancer. Second, decreased malate (p = 1.2 × 10−2) was specifically associated with the presence of a thyroid cancer diagnosis in PTENWT/SDHWT CS, CS-like syndrome, or BRRS individuals. Interestingly, increased citrate (p = 3.9 × 10−2) and fumarate (p = 3.5 × 10−2) but reduced malate (p = 3.6 × 10−3) correlated with the presence of SMNs in these PTENWT/SDHWT CS, CS-like syndrome, or BRRS cases compared to CS, CS-like syndrome, or BRRS cases with the same genotype but without SMNs. Finally, increased lactate (p = 1.1 × 10−2) but decreased fumarate (p = 4.5 × 10−3) were associated with the presence of macrocephaly in PTENWT/SDHWT CS, CS-like syndrome, or BRRS individuals. Overall, these stratified analyses corroborate the impact of genotype-phenotype interactions on the identity and levels of altered TCA metabolites in CS/CS-like and BRRS individuals.
We next sought to explore whether metabolic differences exist among individuals belonging to the two predominant phenotypic features, neurodevelopmental disorders and cancer (Table 1). Indeed, decreased fumarate (p = 1.9 × 10−2) was associated with the presence of ASD/DD compared to those with any cancer (Figure 3). This observation remains consistent (decreased fumarate, p = 3.2 × 10−2) if we consider the subset of individuals with germline PTEN mutations (PTENMUT/SDHWT) for both phenotypes. Interestingly, in addition to an association with decreased fumarate (p = 4.3 × 10−2), we also observed decreased succinate (p = 3.9 × 10−2) but increased isocitrate (p = 4.0 × 10−2) to be associated with the presence of ASD/DD compared to the presence of a thyroid cancer diagnosis. These observations indicate that particular TCA metabolites, consistently fumarate, may be pertinent to distinguish individuals with neurodevelopmental disorders compared to those with cancer.
Figure 3.
TCA Metabolite Signatures in Individuals with Neurodevelopmental Disorders Compared to Those with Cancer
A general logistic regression model was applied to evaluate the association of metabolite levels in neurodevelopmental disorders (ASD/DD) versus cancer, with age, gender, and genotype as covariates. Heatmap depicts TCA cycle metabolites (top label) and significance of associations relative to various phenotypic and/or genotypic group comparisons (left label). Blue color corresponds to an association with decreased metabolite levels, whereas orange color corresponds to an association with increased metabolite levels. Color intensity corresponds to different thresholds of p value significance (scale on the right).
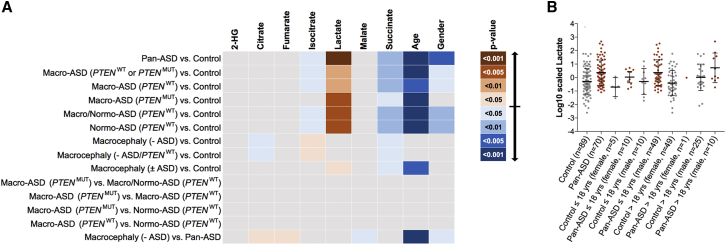
Since the majority of our accrued cases with syndromic ASD/DD (96.4%) harbored germline PTEN mutations, it became prudent to evaluate whether TCA metabolite levels are also altered in individuals with idiopathic neurodevelopmental disease. We hence evaluated TCA metabolite levels in an independent series of cases enriched for idiopathic ASD, with or without macrocephaly (Table 2). This series also included 89 healthy individuals consisting of unaffected members of the probands’ families. To investigate whether metabolic changes are PTEN-genotype-dependent, we also included an independent series of individuals with ASD who harbor germline PTEN mutations. If TCA cycle metabolite alterations are dependent on the PTEN genotype, then we would expect distinct metabolite profiles between individuals with ASD who have germline PTEN mutations and those with ASD who are WT for PTEN. Regardless of genotype or head circumference, increased lactate (p = 5.6 × 10−4) but decreased isocitrate (p = 3.6 × 10−2) and succinate (p = 6.6 × 10−3) were associated with the presence of ASD compared to non-ASD controls (Figure 4A). These observations remained consistent among various subsets of individuals (with or without macrocephaly and with or without germline PTEN mutations); these results suggest that the metabolic alterations within the TCA cycle are ASD state dependent but PTEN status independent. Interestingly, increased isocitrate (p = 4.2 × 10−2) but decreased citrate (p = 4.9 × 10−2) and succinate (p = 2.1 × 10−2) were associated with the presence of macrocephaly (without ASD), and isocitrate showed a correlation in the opposite direction to what we had observed for individuals with ASD (decreased levels), including those with macrocephaly (macro-ASD). Notably, elevated lactate levels were consistently associated with ASD (p = 2.0 × 10−5) compared to non-ASD controls (Figure 4B). Additionally, family-based analyses corroborated the correlation of elevated lactate with ASD relative to non-ASD members within the same family (Figure S2). Overall, this association was consistent in 23 out of 39 (59%) families with ASD (8/18 [44.4%] with idiopathic normo-ASD, 8/11 [72.7%] with idiopathic macro-ASD, and 9/13 [69.2%] with PTENMUT macro-ASD).
Table 2.
Demographic and Clinical Characteristics of the ASD Validation Series (n = 171)
| Control (non-ASD) | 89 (52%) |
| female | 54 (60.7%) |
| male | 35 (39.3%) |
| median age at consent (range) | 41 (2–67) |
| Pan-ASD | 70 (41%) |
| Idiopathic Normo-ASD | 41 (59%) |
| Female | 6 (14.6%) |
| male | 35 (85.4%) |
| median age at consent (range) | 8 (2–45) |
| Idiopathic Macro-ASD | 15 (21%) |
| female | 0 (0.0%) |
| male | 15 (100%) |
| median age at consent (range) | 12 (5-38) |
| PTEN Macro-ASD | 14 (20%) |
| female | 5 (35.7%) |
| male | 9 (64.3%) |
| median age at consent (range) | 7 (2–24) |
| Macrocephaly or PTEN (no-ASD) | 12 (7%) |
| macrocephaly | 7 (58%) |
| female | 4 (57.1%) |
| male | 3 (42.9%) |
| median age at consent (range) | 42 (35–61) |
| Macro-PTEN (no ASD) | 5 (42%) |
| female | 4 (80.0%) |
| male | 1 (20.0%) |
| median age at consent (range) | 34 (3–47) |
Figure 4.
ASD Validation Series and Relevance of TCA Cycle Metabolites to Idiopathic Disease
(A) Evaluation of TCA metabolite levels in an independent series of cases (n = 82) enriched for idiopathic ASD, with or without macrocephaly, as well as n = 89 healthy individuals consisting of unaffected (non-ASD, control) members of the probands’ families. Heatmap depicts associations among distinct metabolites and specific genotype/phenotype combinations compared to non-ASD healthy controls or among ASD cases (within-cases comparisons). A general logistic regression model was applied, and age and gender were covariates. Heatmap depicts TCA cycle metabolites (top label) and significance of associations relative to various phenotypic or genotypic group comparisons (left label). Blue color corresponds to an association with decreased metabolite levels, whereas orange color corresponds to an association with increased metabolite levels. Color intensity corresponds to different thresholds of p value significance (scale on the right).
(B) Stratified age and gender depiction of the association of increased lactate in ASD, irrespective of genotype or macrocephaly, compared to non-ASD controls. Metabolite levels were log-transformed and center-scaled to have a mean of 0 and a standard deviation of 1. The line in the middle of each scatter plot is plotted at the mean. Error bars represent the standard deviations.
Overall, we found multiple associations between TCA metabolite profiles and genotypic and phenotypic features within CS/CS-like and BRRS individuals. TCA metabolite measurements were obtained from blood-derived lymphoblastoid cell lines (LBLs). While LBL interrogation to represent the germline is well accepted in the heredity field, it is tempting to speculate whether such metabolite alterations are also consistent in an affected tissue-specific manner. However, presuming identical effects within affected tissues and/or global effects on the whole organism may be a simplistic view for a complex process that warrants further investigation. Indeed, systematic studies investigating concordance of metabolite levels between affected tissues (e.g., breast cancer) and a surrogate source (e.g., blood, urine) are lacking. Additionally, we note that the opposite risk direction of age being younger age for neurodevelopmental features (p = 9.4 × 10−13) versus older age for cancer (p = 1.4 × 10−11) is due to the inherent nature of our cohort, which includes individuals presenting with ASD/DD or BRRS at a younger age (median age at consent 7 years, range 1–62) than that of individuals presenting with a cancer phenotype (median age at consent 52 years, range 3–89). Interestingly, we have shown particular individual TCA metabolites to be implicated in tumorigenic phenotypes. For example, within the PTENMUT/SDHWT subset of individuals, we found increased levels of isocitrate (p = 1.2 × 10−3) but reduced citrate (p = 5.0 × 10−4) to be associated with breast cancer, compared to PTENMUT/SDHWT individuals without breast cancer. In the TCA cycle, citrate is converted to isocitrate via cis-aconitate through the Fe-S cluster enzyme aconitase or aconitate hydratase.20 While aconitase activity is inhibited, leading to citrate accumulation in normal prostate epithelium,21 catalytic activity is restored in prostate cancer.22, 23 Importantly, this metabolic shift toward decreased citrate is a useful marker for distinguishing normal from transformed prostate epithelia. Additionally, aconitase expression was found to be an independent prognostic factor in gastric cancer.24 Interestingly, aconitase inhibition has been observed in fumarate hydratase (FH) deficient cancer cells due to elevated fumarate,25 where FH is another TCA enzyme catalyzing fumarate to malate conversion. Whether perturbations in individual TCA metabolites will have a global impact on the TCA cycle and ATP production is a hypothesis warranting further investigation.
Intriguingly, TCA metabolite alterations were also observed in individuals with idiopathic ASD, suggesting that the TCA cycle may be a common pathway impacted in syndromic and sporadic neurodevelopmental disorders. We found elevated lactate to be consistently associated with ASD/DD compared to population controls and/or related family members who do not have ASD. Indeed, dysregulation of mitochondrial metabolism has been previously reported in ASD. Several studies have shown elevated levels of lactate or increased lactate/pyruvate ratio in children with autism.26, 27, 28, 29, 30 Additionally, in vivo magnetic resonance spectroscopic imaging demonstrated elevated lactate in the brains of individuals with ASD,31 as was observed in lymphoblastoid cell lines derived from individuals with ASD/DD in this study. Some studies speculate that ASD with mitochondrial dysfunction represents a distinct subgroup of individuals with frank mitochondrial disease.32 Certainly, longitudinal studies will be important to evaluate dynamic levels of lactate in individuals with ASD and correlations with the natural history of the disease. Importantly, the contribution of PTEN dysfunction to metabolic reprogramming and increased lactate production (i.e., Warburg effect) have been extensively studied in the context of carcinogenesis.33 Although we observed elevated lactate levels to be associated with ASD regardless of the PTEN genotype, we speculate that individuals with germline PTEN mutations may have elevated levels of lactate at baseline, potentially resulting in intrinsic “adaptation” to these levels. However, another possibility is variable tissue-specific levels of lactate that could influence organ-specific manifestations in individuals with germline PTEN mutations.
Finally, an outstanding clinical challenge is the ability to predict among germline PTEN mutation carriers, who will develop neurodevelopmental disorders chief of which is ASD versus cancer.34 While studies have shown distinct PTEN protein structural and functional characteristics of germline PTEN mutations in those with ASD versus those with cancer,35, 36, 37, 38, 39, 40 absolute PTEN genotype-phenotype correlations are lacking and downstream clinical phenotypes difficult to predict in humans.34 Here, we show that decreased fumarate is associated with ASD/DD versus cancer. In the TCA cycle, fumarate is formed from succinate oxidation by succinate dehydrogenase. Fumarate is then converted to malate by FH. Intriguingly, germline heterozygous mutations in FH (MIM: 136850) cause the neoplasia predisposition syndrome hereditary leiomyomatosis and renal cell cancer (HLRCC [MIM: 150800]), resulting in pro-tumorigenic fumarate accumulation.41 Indeed, TCA cycle intermediates, traditionally linked to oxidative phosphorylation, are now clearly shown to act in signal transduction as well. Excessive accumulation of succinate or fumarate have been shown to suppress the homologous recombination (HR) DNA-repair pathway required for the resolution of DNA double-strand breaks and for maintenance of genomic integrity.17 The role of fumarate in neurodevelopmental disorders is less understood. However, fumarate is involved in the metabolism of glutamate, an excitatory neurotransmitter. Abnormalities in excitatory and inhibitory synaptic signaling have been implicated in the pathobiology of ASD.42 Recently, alterations in gut glutamate metabolism have been shown to be associated with gut microbiome composition in children with ASD.43 In addition to reduced fumarate, the latter study found notably decreased 2-keto-glutaramic acid and L-aspartic acid in the guts of children with ASD compared to children with typical development. It is therefore tantalizing to speculate whether fumarate may be a pertinent predictive biomarker to distinguish individuals who will develop ASD/DD versus those who will develop cancer. Overall, our data suggest that TCA cycle metabolite alterations are germane to the pathobiology of PTEN-related CS, CS-like syndrome, or BRRS and to that of genotype-independent ASD, with potential implications for risk prediction for the individual and for therapeutic metabolic correction.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We are grateful to our research participants and their families who contributed to this study. We thank the Genomic Medicine Biorepository of the Cleveland Clinic Genomic Medicine Institute and our database and clinical research teams. We also thank Hannah Chen for technical assistance in genotyping DNA samples. This work was supported in part by the National Cancer Institute (P01CA124570, R01CA118989), the Ambrose Monell Foundation, the American Cancer Society (RPG-02-151-01-CCE), the Breast Cancer Research Foundation, and the Doris Duke Distinguished Clinical Scientist Award (all to C.E.), as well as the National Institutes of Health (U54NS092090 to T.W.F. and C.E.). L.Y. is an Ambrose Monell Foundation Cancer Genomic Medicine Fellow at the Cleveland Clinic Genomic Medicine Institute and was an International Fulbright Science and Technology Doctoral Fellow at the Cleveland Clinic Genomic Medicine Institute and was a recipient of the Dr. Michael H. Fakih Pre-Doctoral Scholarship. C.E. is the Sondra J. and Stephen R. Hardis Chair of Cancer Genomic Medicine at the Cleveland Clinic and an American Cancer Society (ACS) Clinical Research Professor.
Published: September 26, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.09.004.
Web Resources
OMIM, https://www.omim.org/
RStudio version 1.1.463, https://www.rstudio.com
Supplemental Data
References
- 1.Marsh D.J., Coulon V., Lunetta K.L., Rocca-Serra P., Dahia P.L., Zheng Z., Liaw D., Caron S., Duboué B., Lin A.Y. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum. Mol. Genet. 1998;7:507–515. doi: 10.1093/hmg/7.3.507. [DOI] [PubMed] [Google Scholar]
- 2.Orloff M.S., Eng C. Genetic and phenotypic heterogeneity in the PTEN hamartoma tumour syndrome. Oncogene. 2008;27:5387–5397. doi: 10.1038/onc.2008.237. [DOI] [PubMed] [Google Scholar]
- 3.Butler M.G., Dasouki M.J., Zhou X.P., Talebizadeh Z., Brown M., Takahashi T.N., Miles J.H., Wang C.H., Stratton R., Pilarski R., Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yehia L., Ni Y., Sesock K., Niazi F., Fletcher B., Chen H.J.L., LaFramboise T., Eng C. Unexpected cancer-predisposition gene variants in Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome patients without underlying germline PTEN mutations. PLoS Genet. 2018;14:e1007352. doi: 10.1371/journal.pgen.1007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorlin R.J., Cohen M.M., Jr., Condon L.M., Burke B.A. Bannayan-Riley-Ruvalcaba syndrome. Am. J. Med. Genet. 1992;44:307–314. doi: 10.1002/ajmg.1320440309. [DOI] [PubMed] [Google Scholar]
- 6.Peiretti V., Mussa A., Feyles F., Tuli G., Santanera A., Molinatto C., Ferrero G.B., Corrias A. Thyroid involvement in two patients with Bannayan-Riley-Ruvalcaba syndrome. J. Clin. Res. Pediatr. Endocrinol. 2013;5:261–265. doi: 10.4274/Jcrpe.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liaw D., Marsh D.J., Li J., Dahia P.L., Wang S.I., Zheng Z., Bose S., Call K.M., Tsou H.C., Peacocke M. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 8.Tan M.H., Mester J., Peterson C., Yang Y., Chen J.L., Rybicki L.A., Milas K., Pederson H., Remzi B., Orloff M.S., Eng C. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am. J. Hum. Genet. 2011;88:42–56. doi: 10.1016/j.ajhg.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh D.J., Dahia P.L., Zheng Z., Liaw D., Parsons R., Gorlin R.J., Eng C. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat. Genet. 1997;16:333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- 10.Herman G.E., Butter E., Enrile B., Pastore M., Prior T.W., Sommer A. Increasing knowledge of PTEN germline mutations: Two additional patients with autism and macrocephaly. Am. J. Med. Genet. A. 2007;143A:589–593. doi: 10.1002/ajmg.a.31619. [DOI] [PubMed] [Google Scholar]
- 11.Orrico A., Galli L., Buoni S., Orsi A., Vonella G., Sorrentino V. Novel PTEN mutations in neurodevelopmental disorders and macrocephaly. Clin. Genet. 2009;75:195–198. doi: 10.1111/j.1399-0004.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 12.Varga E.A., Pastore M., Prior T., Herman G.E., McBride K.L. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet. Med. 2009;11:111–117. doi: 10.1097/GIM.0b013e31818fd762. [DOI] [PubMed] [Google Scholar]
- 13.Ni Y., Zbuk K.M., Sadler T., Patocs A., Lobo G., Edelman E., Platzer P., Orloff M.S., Waite K.A., Eng C. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am. J. Hum. Genet. 2008;83:261–268. doi: 10.1016/j.ajhg.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni Y., He X., Chen J., Moline J., Mester J., Orloff M.S., Ringel M.D., Eng C. Germline SDHx variants modify breast and thyroid cancer risks in Cowden and Cowden-like syndrome via FAD/NAD-dependant destabilization of p53. Hum. Mol. Genet. 2012;21:300–310. doi: 10.1093/hmg/ddr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Hobert J.A., Mester J.L., Moline J., Eng C. Elevated plasma succinate in PTEN, SDHB, and SDHD mutation-positive individuals. Genet. Med. 2012;14:616–619. doi: 10.1038/gim.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulkowski P.L., Sundaram R.K., Oeck S., Corso C.D., Liu Y., Noorbakhsh S., Niger M., Boeke M., Ueno D., Kalathil A.N. Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat. Genet. 2018;50:1086–1092. doi: 10.1038/s41588-018-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobert J.A., Embacher R., Mester J.L., Frazier T.W., 2nd, Eng C. Biochemical screening and PTEN mutation analysis in individuals with autism spectrum disorders and macrocephaly. Eur. J. Hum. Genet. 2014;22:273–276. doi: 10.1038/ejhg.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan M.H., Mester J.L., Ngeow J., Rybicki L.A., Orloff M.S., Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res. 2012;18:400–407. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd S.J., Lauble H., Prasad G.S., Stout C.D. The mechanism of aconitase: 1.8 A resolution crystal structure of the S642a:citrate complex. Protein Sci. 1999;8:2655–2662. doi: 10.1110/ps.8.12.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavanagh J.P. Sodium, potassium, calcium, magnesium, zinc, citrate and chloride content of human prostatic and seminal fluid. J. Reprod. Fertil. 1985;75:35–41. doi: 10.1530/jrf.0.0750035. [DOI] [PubMed] [Google Scholar]
- 22.Singh K.K., Desouki M.M., Franklin R.B., Costello L.C. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol. Cancer. 2006;5:14. doi: 10.1186/1476-4598-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsui K.H., Chung L.C., Wang S.W., Feng T.H., Chang P.L., Juang H.H. Hypoxia upregulates the gene expression of mitochondrial aconitase in prostate carcinoma cells. J. Mol. Endocrinol. 2013;51:131–141. doi: 10.1530/JME-13-0090. [DOI] [PubMed] [Google Scholar]
- 24.Wang P., Mai C., Wei Y.L., Zhao J.J., Hu Y.M., Zeng Z.L., Yang J., Lu W.H., Xu R.H., Huang P. Decreased expression of the mitochondrial metabolic enzyme aconitase (ACO2) is associated with poor prognosis in gastric cancer. Med. Oncol. 2013;30:552. doi: 10.1007/s12032-013-0552-5. [DOI] [PubMed] [Google Scholar]
- 25.Kerangueven F., Eisinger F., Noguchi T., Allione F., Wargniez V., Eng C., Padberg G., Theillet C., Jacquemier J., Longy M. Loss of heterozygosity in human breast carcinomas in the ataxia telangiectasia, Cowden disease and BRCA1 gene regions. Oncogene. 1997;14:339–347. doi: 10.1038/sj.onc.1200818. [DOI] [PubMed] [Google Scholar]
- 26.Coleman M., Blass J.P. Autism and lactic acidosis. J. Autism Dev. Disord. 1985;15:1–8. doi: 10.1007/BF01837894. [DOI] [PubMed] [Google Scholar]
- 27.László A., Horváth E., Eck E., Fekete M. Serum serotonin, lactate and pyruvate levels in infantile autistic children. Clin. Chim. Acta. 1994;229:205–207. doi: 10.1016/0009-8981(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 28.Lombard J. Autism: A mitochondrial disorder? Med. Hypotheses. 1998;50:497–500. doi: 10.1016/s0306-9877(98)90270-5. [DOI] [PubMed] [Google Scholar]
- 29.Correia C., Coutinho A.M., Diogo L., Grazina M., Marques C., Miguel T., Ataíde A., Almeida J., Borges L., Oliveira C. Brief report: High frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J. Autism Dev. Disord. 2006;36:1137–1140. doi: 10.1007/s10803-006-0138-6. [DOI] [PubMed] [Google Scholar]
- 30.Weissman J.R., Kelley R.I., Bauman M.L., Cohen B.H., Murray K.F., Mitchell R.L., Kern R.L., Natowicz M.R. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS ONE. 2008;3:e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goh S., Dong Z., Zhang Y., DiMauro S., Peterson B.S. Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: evidence from brain imaging. JAMA Psychiatry. 2014;71:665–671. doi: 10.1001/jamapsychiatry.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossignol D.A., Frye R.E. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol. Psychiatry. 2012;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y.R., Chen M., Pandolfi P.P. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat. Rev. Mol. Cell Biol. 2018;19:547–562. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 34.Yehia L., Ngeow J., Eng C. PTEN-opathies: from biological insights to evidence-based precision medicine. J. Clin. Invest. 2019;129:452–464. doi: 10.1172/JCI121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez-Escudero I., Oliver M.D., Andrés-Pons A., Molina M., Cid V.J., Pulido R. A comprehensive functional analysis of PTEN mutations: implications in tumor- and autism-related syndromes. Hum. Mol. Genet. 2011;20:4132–4142. doi: 10.1093/hmg/ddr337. [DOI] [PubMed] [Google Scholar]
- 36.Spinelli L., Black F.M., Berg J.N., Eickholt B.J., Leslie N.R. Functionally distinct groups of inherited PTEN mutations in autism and tumour syndromes. J. Med. Genet. 2015;52:128–134. doi: 10.1136/jmedgenet-2014-102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leslie N.R., Longy M. Inherited PTEN mutations and the prediction of phenotype. Semin. Cell Dev. Biol. 2016;52:30–38. doi: 10.1016/j.semcdb.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 38.Mighell T.L., Evans-Dutson S., O’Roak B.J. A saturation mutagenesis approach to understanding PTEN lipid phosphatase activity and genotype-phenotype relationships. Am. J. Hum. Genet. 2018;102:943–955. doi: 10.1016/j.ajhg.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith I.N., Thacker S., Jaini R., Eng C. Dynamics and structural stability effects of germline PTEN mutations associated with cancer versus autism phenotypes. J. Biomol. Struct. Dyn. 2019;37:1766–1782. doi: 10.1080/07391102.2018.1465854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith I.N., Thacker S., Seyfi M., Cheng F., Eng C. conformational dynamics and allosteric regulation landscapes of germline pten mutations associated with autism compared to those associated with cancer. Am. J. Hum. Genet. 2019;104:861–878. doi: 10.1016/j.ajhg.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomlinson I.P., Alam N.A., Rowan A.J., Barclay E., Jaeger E.E., Kelsell D., Leigh I., Gorman P., Lamlum H., Rahman S., Multiple Leiomyoma Consortium Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 42.Nelson S.B., Valakh V. excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87:684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M., Wan J., Rong H., He F., Wang H., Zhou J., Cai C., Wang Y., Xu R., Yin Z., Zhou W. Alterations in gut glutamate metabolism associated with changes in gut microbiota composition in children with autism spectrum disorder. mSystems. 2019;4 doi: 10.1128/mSystems.00321-18. e00321–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.