Abstract
Purpose
NRG Oncology's RTOG 0933 demonstrated benefits to memory preservation after hippocampal avoidant whole-brain radiation therapy (HA-WBRT), the avoidance of radiation dose to the hippocampus (using intensity modulated radiation planning and delivery techniques) during WBRT, supporting the hypothesis of hippocampal radiosensitivity and associated memory specificity. However, some patients demonstrated cognitive decline, suggesting mechanisms outside hippocampal radiosensitivity play a role. White matter injury (WMI) has been implicated in radiation therapy–induced neurocognitive decline. This secondary analysis explored the relationship between pretreatment WMI and memory after HA-WBRT.
Methods and Materials
Volumetric analysis of metastatic disease burden and disease-unrelated WMI was conducted on the pretreatment magnetic resonance image. Correlational analyses were performed examining the relationship between pretreatment WMI and Hopkins Verbal Learning Test-Revised (HVLT-R) outcomes at baseline and 4 months after HA-WBRT.
Results
In the study, 113 patients received HA-WBRT. Of 113 patients, 33 underwent pretreatment and 4-month posttreatment HVLT testing and pretreatment postcontrast volumetric T1 and axial T2/fluid-attenuated inversion recovery magnetic resonance imaging. Correlation was found between larger volumes of pretreatment WMI and decline in HVLT-R recognition (r = 0.54, P < .05), and a correlational trend was observed between larger volume of pretreatment WMI and decline in HVLT-R delayed recall (r = 0.31, P = .08). Patients with higher pretreatment disease burden experienced a greater magnitude of stability or positive shift in HVLT-R recall and delayed recall after HA-WBRT (r = –0.36 and r = –0.36, P < .05), compared to the magnitude of stability or positive shift in those with lesser disease burden.
Conclusions
In patients receiving HA-WBRT for brain metastases, extent of pretreatment WMI predicts posttreatment memory decline, suggesting a mechanism for radiation therapy–induced neurocognitive toxicity independent of hippocampal stem cell radiosensitivity. Stability or improvement in HVLT after HA-WBRT for patients with higher pretreatment intracranial metastatic burden supports the importance of WBRT-induced intracranial control on neurocognition.
Summary:
Cognitive decline following whole brain radiotherapy (WBRT) is a concern for clinicians and patients. In patients receiving hippocampal avoidant (HA)-WBRT for brain metastases, extent of MRI determined, pre-treatment white matter injury (WMI) predicts post-treatment memory decline. This suggests a mechanism for radiotherapy induced neurocognitive toxicity independent of hippocampal stem cell radiosensitivity.
Introduction
Metastatic disease to the brain is the most commonly diagnosed malignant brain tumor with an annual incidence as high as 11 per 100,000 per year in the United States alone.1 As systemic chemotherapy and targeted therapies continue to enhance survival in cancer patients, it is anticipated that more patients will present with brain metastases. Therefore, there is considerable interest in trying to mitigate the adverse effects of central nervous system directed therapies, specifically the effect of whole brain radiation therapy (WBRT). WBRT can lead to neurocognitive decline in patients with low-volume metastatic disease to the brain and may be associated with a significant adverse impact on patient quality of life.2, 3
However, the mechanisms and predictive factors of this cognitive decline have yet to be fully explored. It is also unclear why some patients demonstrate significant impairment after WBRT and others are spared. As such, there is considerable clinical interest in predicting which patients will develop cognitive decline after WBRT. This would provide oncologists with a powerful tool to better advise patients about their true risk and degree of neurocognitive impairment, especially in patients that would clearly benefit from WBRT.
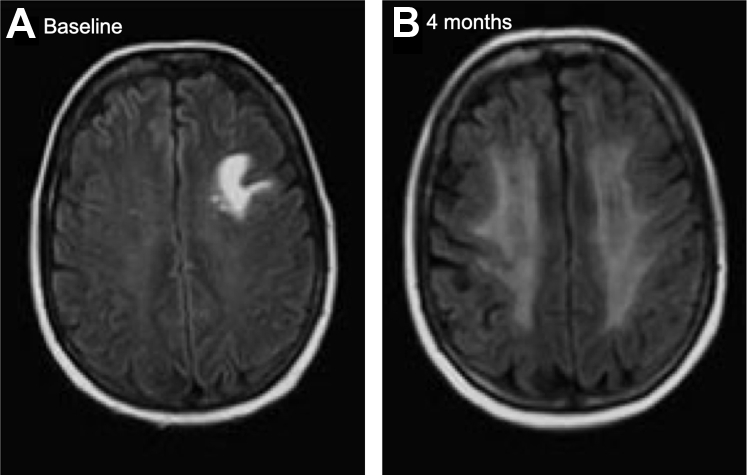
In our previously published work, we have shown that white matter is particularly vulnerable to the effects of WBRT. Symmetric, confluent, hyperintense signal on T2-weighted magnetic resonance imaging (MRI) images is the most common radiographic finding in patients who have been treated with WBRT, particularly in the periventricular deep white matter (Fig 1).4 Fluid-attenuated inversion recovery (FLAIR) is a MRI sequence that produce both a strongly T2-weighted image and suppressed cerebrospinal fluid (CSF) signal. To accomplish this goal, a conventional spin echo (SE) sequence is prefaced by a 180° inversion pulse. A relatively long inversion time is used to allow the longitudinal magnetization of CSF to return to the null point before SE imaging. Thus, the CSF signal is completely suppressed for cortical or periventricular areas, and lesions with typical T2 prolongation in the brain that are adjacent to spinal fluid become much more conspicuous compared with conventional T2 imaging.5 Significant steady increases in MRI FLAIR white matter changes after WBRT at multiple time points up to 1 year posttreatment have been seen. This MRI FLAIR volume is thought to be a surrogate, or biomarker, for presumed white matter injury (WMI) in patients having received WBRT, and WMI volume correlated with the age when treated and with the volume of WMI that was present before the administration of WBRT. This was the first volumetric study to demonstrate a predictive effect of pretreatment WMI on the subsequent development of increasing post treatment WMI.4
Figure 1.
Example of worsening periventricular white matter injury from (A) baseline to (B) 4-month post–whole-brain radiation therapy.
Based on these results, we hypothesized that patients with pretreatment WMI were susceptible for additional injury after WBRT given the vulnerability of white matter to radiation. However, the relationship between WMI and neurocognitive changes after WBRT remained unclear. The presence and extent of WMI have been associated with cognitive impairment in other clinical populations, such as in healthy adult stroke patients and multiple sclerosis, and it is reasonable to assume that WMI underlies the neurocognitive sequela seen in patients treated with WBRT.6, 7, 8, 9, 10 No studies to date have examined this and it is unknown how much WMI a patient can sustain before manifesting neurocognitive symptoms. As such, we sought to explore whether the presence of pretreatment WMI, as represented by MRI FLAIR volume, predicted subsequent neurocognitive decline in patients that underwent WBRT for metastatic brain cancer.
Methods and Materials
As a way of trying to mitigate the neurocognitive effects of WBRT, NRG Oncology embarked on RTOG 0933: A Phase II Trial of Hippocampal Avoidance (HA) During Whole Brain Radiation therapy for Brain Metastases. The hypothesis of this trial was that through the avoidance of radiation dose to the hippocampus (using intensity modulated radiation planning and delivery techniques) during WBRT, the risk of posttreatment neurocognitive decline would be reduced. The primary endpoint of RTOG 0933, a reduction in neurocognitive decline at 4 months posttreatment, was met and reported, and as such, a phase III trial is currently underway (NRG Oncology CC001) to assess HA in a randomized approach.11 Although HA in RTOG 0933 yielded a significant reduction in neurocognitive decline at 4 months posttreatment, there were still patients with some degree of neurocognitive decline. We sought to determine whether baseline WMI as represented by FLAIR abnormality was a predictor for cognitive decline.
Inclusion criteria for RTOG 0933 were previously published and, in brief, included brain metastases outside a 5-mm margin around either hippocampus, a pathologic diagnosis of nonhematopoetic malignancy other than small-cell lung cancer or germ cell malignancy, RTOG recursive partitioning analysis class I or II, and English proficiency.12 Patients <18 years old and those with leptomeningeal metastases, radiographic evidence of hydrocephalus, prior radiation to the brain, planned upfront radiosurgery or surgical resection, contraindication to MRI, serum creatinine 1.4 mg/dL 30 days before study entry, or non-small cell lung cancer–associated brain metastases with 2 organ sites of extracranial metastases were excluded.11 All patients provided study-specific consent and were enrolled by institutions with institutional review board approval.
Clinical imaging
In the original trial, 113 patients were accrued from March 2011 through November 2012, and of those, 42 patients were analyzable at 4 months. Baseline MRI FLAIR and postcontrast T1 imaging submission was not required in the RTOG 0933 trial. As a result, the 23 participating institutions were individually contacted to request the baseline MRI FLAIR and postcontrast T1 images acquired before trial enrollment. The baseline images for 39 of the 42 patients treated with HA-WBRT were acquired. Of these, 6 image sets were deemed inevaluable because the image quality was poor as a result of significant artifact. Therefore, 33 baseline image sets were able to be evaluated. The images that were evaluated were MRI FLAIR sequences acquired with 5 mm thick axial slices and the T1 with contrast sequences with 1-mm-thick axial slices.
Volumetric analysis
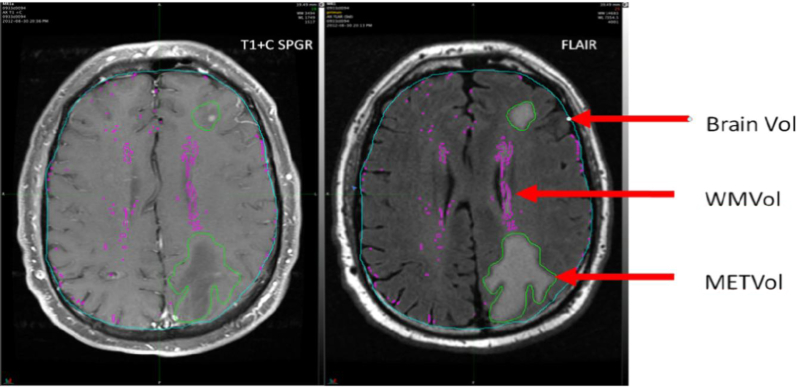
The image sets were standardized to account for inter-scanner variability, which consisted of a 3-step process including bias field correction, anisotropic diffusion noise reduction, and signal intensity normalization. A volume for the metastatic disease (METVol) was contoured manually. The METVol was defined as the enhancing lesion on the T1 with contrast scan plus any contiguous MRI FLAIR signal associated with the contrast enhancing lesion. Pretreatment MRI FLAIR white matter volumes (WMVol) were automatically generated with an autosegmentation technique in MIM (MIM Software, Cleveland, OH, version 6.5.4). WMVol were created by empirically thresholding the MRI FLAIR images at 1.5 standard deviation (SD) of the mean MRI FLAIR intensity value for the entire brain (Fig 2). The volumes were independently assessed by 2 nonpartisan observers blinded to the cognitive data and were edited to remove misclassified areas (eg, chorid plexus, septum pellucidum). Total brain volume was calculated through an autosegmentation tool in MIM. Both the METVol and WMVol were divided by total brain volume in order to create corrected volumes to account for variability in total brain volumes and the volume of available brain to assess for WMI.
Figure 2.
Representative example of brain volume, white matter volumes, and volume for the metastatic disease.
Cognitive assessment
All patients underwent pretreatment and 4-month post-WBRT memory testing using the Hopkins verbal learning test-revised (HVLT-R). Alternate forms were used between the 2 exams to reduce practice effects. The test involves memorizing a list of 12 targets for 3 consecutive trials (total recall), identifying the 12 targets from a list of semantically related or unrelated items (immediate recognition), and recalling the 12 targets after a 20-minute delay (delayed recall). The timing of HVLT-R IR immediately after HVLT-R TR, as opposed to after HVLT-R DR, represents a departure from HVLT-R used in more contemporary studies, but it was in keeping with the method of administration for the control cohort.13 This approach has been used in prior phase III cooperative group studies.14, 15
Statistical analysis
All data analyses were performed using SAS/STAT software (SAS, Cary, NC; version 9.4 of the SAS System for Windows). The data was approximately normally distributed so 2-tailed Pearson correlations were used to examine the relationship between our clinical variables of interest and memory performance before and 4 months posttreatment. Change scores of cognitive assessments were calculated by subtracting the 4-month follow-up raw score from the baseline raw score (baseline–follow-up), and positive change scores reflected greater decline whereas negative change scores reflected improvement. Corrected METVol and WMVol were treated as a continuous variables in all analyses. A significance level of 0.05 was used.
Results
Thirty-three patients were assessed with a median age in years of 58 (range 28-81). Descriptive statistics for WMVol and MetVol are provided in Table 1. Correlations between pretreatment memory performance and METVol and WMVol can be found in Table 2. Pretreatment, there were no significant correlations between either METVol or WMVol and HVLT-R total recall, delayed recall, or immediate recognition memory performance; however, there was a trend between WMVol and HVLT total recall (ρ = –0.30, P = .09) with greater white matter abnormality associated with lower pretreatment learning ability. Age at time of treatment was significantly correlated in the positive direction with WMVol (ρ = 0.38, P = .03), suggesting later age at time of treatment is associated with greater pretreatment white matter abnormalities (Table 3).
Table 1.
Magnetic resonance imaging FLAIR volume characteristics
| FLAIR volume (mL) | (n = 33) |
| Mean | 13.53 |
| SD | 10.66 |
| Median | 9.31 |
| Minimum to maximum | 2.63-45.76 |
| Q1-Q3 | 6.96-18.41 |
| Met volume (mL) | (n = 33) |
| Mean | 44.87 |
| SD | 77.03 |
| Median | 7.26 |
| Minimum to maximum | 0.00-319.24 |
| Q1-Q3 | 0.88-38.33 |
| Whole brain volume (mL) | (n = 33) |
| Mean | 1415.88 |
| SD | 231.57 |
| Median | 1395.00 |
| Minimum to maximum | 920.00-2070.00 |
| Q1-Q3 | 1274.00-1546.00 |
| Corrected FLAIR volume | (n = 33) |
| Mean | 0.0096 |
| SD | 0.0077 |
| Median | 0.0068 |
| Minimum to maximum | 0.0020-0.0377 |
| Q1-Q3 | 0.0050-0.0127 |
| Corrected FLAIR volume adjusted for met volume | (n = 33) |
| Mean | 0.0099 |
| SD | 0.0078 |
| Median | 0.0068 |
| Minimum to maximum | 0.0020-0.0379 |
| Q1-Q3 | 0.0053-0.0131 |
| Corrected met volume | (n = 33) |
| Mean | 0.0303 |
| SD | 0.0479 |
| Median | 0.0059 |
| Minimum to maximum | 0-0.1748 |
| Q1-Q3 | 0.0006-0.0309 |
Abbreviations: FLAIR = fluid-attenuated inversion; Q1 = first quartile; Q3 = third quartile.
Table 2.
Correlations with pretreatment HVLT-R recall score (n = 33)
| HVLT-R total recall | HVLT-R immediate recognition | HVLT-R delayed recall | ||
|---|---|---|---|---|
| FLAIR volume | ρ | −0.33 | −0.30 | −0.33 |
| P value | .062 | .091 | .058 | |
| Met volume | ρ | −0.24 | 0.10 | −0.20 |
| P value | .17 | .57 | .25 | |
| Corrected FLAIR volume | ρ | −0.30 | −0.25 | −0.27 |
| P value | .091 | .16 | .12 | |
| Corrected met volume | ρ | −0.19 | 0.11 | −0.16 |
| P value | .30 | .54 | .37 | |
| Corrected FLAIR volume Adjusted for met volume | ρ | −0.301 | −0.25 | −0.29 |
| P value | .08 | .16 | .11 | |
Abbreviations: FLAIR = fluid-attenuated inversion; HVLT-R = Hopkins Verbal Learning Test-Revised.
Table 3.
Correlations of age with posttreatment HVLT-R and pretreatment MRI FLAIR volume (n = 33)
| HVLT-R recall change score | ρ | −0.40 |
| P value | .0093 | |
| HVLT-R delayed recall change score | ρ | −0.29 |
| P value | .064 | |
| HVLT-R recognition change score | ρ | −0.01 |
| P value | .97 | |
| FLAIR volume | ρ | 0.36 |
| P value | .39 | |
| Corrected FLAIR volume | ρ | 0.38 |
| P value | .031 | |
| Corrected FLAIR volume adjusted for met volume | ρ | 0.37 |
| P value | .036 |
Abbreviations: FLAIR = fluid-attenuated inversion; HVLT-R = Hopkins Verbal Learning Test-Revised; MRI = magnetic resonance imaging.
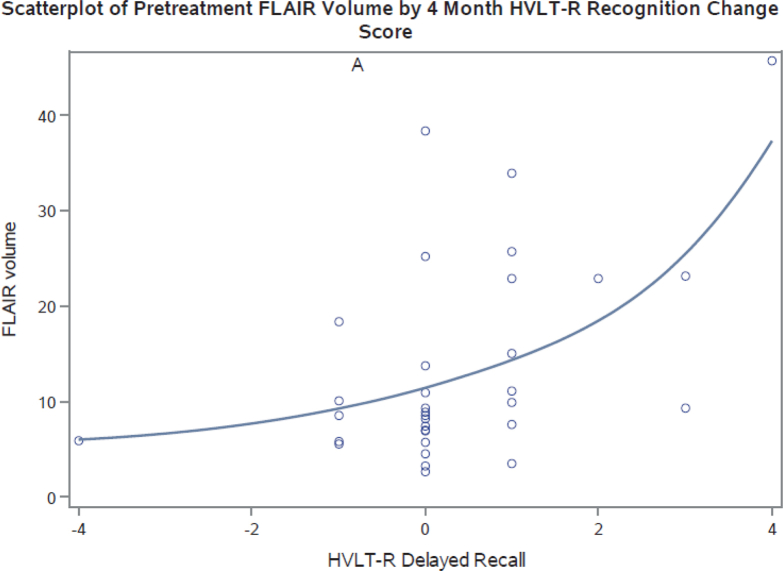
Posttreatment, a significant positive correlation was observed between WMVol and change in HVLT-R immediate recognition memory performance at 4 months (ρ = 0.54, P = .001), suggesting greater pretreatment white matter abnormality was associated with greater declines in immediate recognition memory performance (Table 4; Fig 3). A trend was seen between WMVol and change in HVLT-R delayed recall performance at 4 months posttreatment (ρ = 0.31, P = .08), indicating that greater amounts of white matter abnormality before WBRT was associated with greater declines in delayed recall performance. No significant relationship was observed between WMVol and HVLT-R total recall. MetVol was significantly negatively correlated with HVLT-R total recall (ρ = –0.36, P = .04) and delayed recall (ρ = –0.36, P = .04), but no correlation was noted with immediate recognition performance (ρ = –0.19, P = .28), suggesting that greater disease volume before treatment was associated with a greater magnitude of stability or positive shift in HVLT-R total recall and delayed recall after HA-WBRT compared with the magnitude of stability or positive shift in those with lesser disease burden. Age is also significantly associated with posttreatment HVLT-R total recall (ρ = –0.40, P = .009; Table 3).
Table 4.
Correlations with posttreatment HVLT-R change score at 4 months (n = 33)
| HVLT-R total recall | HVLT-R immediate recognition | HVLT-R delayed recall | ||
|---|---|---|---|---|
| FLAIR volume | ρ | 0.08 | 0.49 | 0.26 |
| P value | .67 | .0038 | .15 | |
| Met volume | ρ | −0.34 | −0.17 | −0.37 |
| P value | .053 | .34 | .032 | |
| Corrected FLAIR volume | ρ | 0.08 | 0.54 | 0.31 |
| P value | .65 | .0012 | .078 | |
| Corrected met volume | ρ | −0.36 | −0.19 | −0.36 |
| P value | .042 | .28 | .039 | |
| Corrected FLAIR volume adjusted for met volume | ρ | 0.06 | 0.53 | 0.29 |
| P value | .73 | .0016 | .10 | |
Abbreviations: FLAIR = fluid-attenuated inversion; HVLT-R = Hopkins Verbal Learning Test-Revised.
Figure 3.
Scatter plot of baseline magnetic resonance imaging fluid–attenuated inversion recovery volume.
Discussion
This secondary analysis is the first to examine MRI FLAIR as a biomarker for white matter integrity and demonstrates that volumetric extent of pretreatment MRI FLAIR abnormality predicts for memory decline after HA-WBRT. The hypothesis is that MRI FLAIR volume acts as a surrogate for white matter tract injury. If a patient presents with a large volume of pretreatment MRI FLAIR abnormality, this could be an indicator that their brains are susceptible to further microvascular injury after WBRT. Age was also a factor that correlated with pretreatment MRI FLAIR volumes: increased age was associated with a greater volume of pretreatment MRI FLAIR volume. This is consistent with the extant literature showing that age increases risk for harboring microvacular disease.16, 17 Age in this analysis also is moderately correlated, although statistically significant, with post-WBRT neurocognitive declines in HVLT-R total recall (Table 3). Clinically, the effects of radiation therapy on an aging patient's brain are important correlations. As patients age, their brains are more susceptible to the WMI effects of radiation therapy, which subsequently has a negative impact upon neurocognition. Owing to the limited sample size in this analysis, the effect of WMI cannot be determined to be independent of age or other confounders but will be explored in subsequent, larger trials.
It was previously demonstrated that MRI FLAIR volume significantly increases after WBRT and that the presence of pretreatment MRI FLAIR abnormality is a predictor of subsequent changes or injury to the white matter after treatment.4 These data add to this literature by showing that the pretreatment MRI FLAIR abnormality also correlates with subsequent memory decline. One limitation in the RTOG 0933 data used in this analysis is the lack of imaging available at 4 months. However, from previous work, it is reasonable to assume that at 4 months, treatment-related changes to the white matter are already developing and that the degree of these changes may relate to the degree of memory decline. We will be able to further assess this in the currently open NRG Oncology-CC001: A Randomized Phase III trial of Memantine and Whole-Brain Radiation therapy With or Without Hippocampal Avoidance in Patients with Brain Metastases, where a collection of pretreatment MRI imaging in addition to 6 month posttreatment imaging are enrollment requirements for the trial. In addition, the sample size in this study was small, which limited the statistical power in these analyses. This current data will potentially be validated in a much larger imaging and neurocognitive dataset with the NRG Oncology-CC001 results. Pretreatment imaging submission was an enrollement requirement in NRG Oncology-CC001. The NRG Oncology-CC001 secondary analysis will therefore benefit from improved statistical power compared with the present study.
It is important to note that all of these patients in this analysis received HA-WBRT with intensity modulated radioation therapy (IMRT). It will be interesting to determine whether the heterogeneous dose distribution inherent to IMRT will have a subsequent impact on the increased development of MRI FLAIR volume changes. This will be further assessed in the NRG Oncology-CC001 patient population, where patients are randomized between standard WBRT (non-IMRT) and HA-WBRT (IMRT). It will also be of interest to learn if the hippocampus is truly the target to be spared with WBRT to prevent neurocognitive decline. Perhaps some patients have a predilection for developing neurocognitive decline just because their white matter is significantly more susceptible to any radiation therapy injury and therefore will benefit least from hippocampal avoidance.
In addition, some patients with brain metastases have cognitive disability before initiating WBRT because of the burden of their intracranial disease. Patients with higher pretreatment disease burden experienced a greater magnitude of stability or positive shift in HVLT-R total recall and delayed recall after HA-WBRT compared with the magnitude of stability or positive shift in those with lesser disease burden. It is underreported that patients can actually stabilize or have a positive shift in cognitive function after WBRT, as opposed to decline, because their disease responds to therapy. These data help to support this hypothesis and underscore the need for a balanced approach to selecting patients for or withholding WBRT.
The present analysis is also limited by the cognitive testing performed to evaluate patients in RTOG 0933. In this trial, patients were administered the HVLT-R to assess memory functioning. WBRT is known to affect white matter and other neurocognitive domains. Therefore, processing speed and executive functions are likely at high risk for decline after radiation as these functions have been shown to depend on white matter integrity.18, 19 It is suspected that including these neurocognitive domains will increase the sensitivity in detecting and perhaps predicting neurocognitive change posttreatment. This data is included in the collection for the NRG Oncology CC001 trial.
The ability to predict neurocognitive decline after WBRT in the metastatic disease population would be a powerful tool for oncologists. Our work has shown that pretreatment MRI FLAIR volume maybe one means to better assess this risk. This hypothesis is being evaluated in the accruing NRG Oncology CC001 phase III study.
Footnotes
Sources of support: This project was supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), U24CA180803 (Imaging and Radiation Oncology Core), UG1CA189867 (National Cancer Institute (NCI) Community Oncology Research Program) from the NCI, and clinicaltrials.govNCT01227954.
Disclosures: Drs Bovi, DeNittis, Dominello, Greenspoon, Huang, Kachnic, Kundapur, Laack, Machtay, Paulson, and Sabsevitz have nothing to disclose.
Dr Chao reports personal fees from Varian and personal fees from Novocure. Dr Gondi reports personal fees from UpToDate, personal fees from AbbVie, personal fees from INSYX Therapeutics, and nonfinancial support from Novocure. Dr Mehta reports personal fees from INSYS, personal fees from Remedy, personal fees from IBA, personal fees from Varian, personal fees from Celgene, personal fees from Abbvie, personal fees from Astra-Zeneca, personal fees from Tocagen, personal fees from Blue Earth, and other (Board Of Directors) from Oncoceutics outside the submitted work. Dr Moore reports other from Clovis, other from Astra Zeneca, other from Tesaro, other from Immunogen, other from Genentech/Roche, other from VBL Therapeutics, and other from Merck. Dr Robinson reports grants and personal fees from Varian, grants from Elekta, and other from Radialogica. Dr Pugh reports other from Millenniu. Dr Shahin reports grants and personal fees from Tesaro, personal fees from Clovis Oncology, grants and personal fees from Astra Zeneca, and personal fees from Pacira Pharmaceuticals.
References
- 1.Eichler A.F., Loeffler J.S. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 2.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laack N.N., Brown P.D. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31:702–713. doi: 10.1053/j.seminoncol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Sabsevitz D.S., Bovi J.A., Leo P.D. The role of pre-treatment white matter abnormalities in developing white matter changes following whole brain radiation: a volumetric study. J Neurooncol. 2013;114:291–297. doi: 10.1007/s11060-013-1181-8. [DOI] [PubMed] [Google Scholar]
- 5.Brant-Zawadzki M., Atkinson D., Detrick M. Fluid-attenuated inversion recovery (FLAIR) for assessment of cerebral infarction. Initial clinical experience in 50 patients. Stroke. 1996;27:1187–1191. doi: 10.1161/01.str.27.7.1187. [DOI] [PubMed] [Google Scholar]
- 6.Burton E.J., Kenny R.A., O'Brien J. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke. 2004;35:1270–1275. doi: 10.1161/01.STR.0000126041.99024.86. [DOI] [PubMed] [Google Scholar]
- 7.DeCarli C., Murphy D.G., Tranh M. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 8.Mungas D., Jagust W.J., Reed B.R. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien J.F., Wiseman R., Burton E.J. Cognitive associations of subcortical white matter lesions in older people. Ann N Y Acad Sci. 2002;977:436–444. doi: 10.1111/j.1749-6632.2002.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 10.Randolph J.J., Wishart H.A., Saykin A.J. FLAIR lesion volume in multiple sclerosis: Relation to processing speed and verbal memory. J Int Neuropsychol Soc. 2005;11:205–209. doi: 10.1017/s1355617705050253. [DOI] [PubMed] [Google Scholar]
- 11.Gondi V., Pugh S.L., Tome W.A. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaspar L., Scott C., Rotman M. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 13.Mehta M.P., Rodrigus P., Terhaard C.H. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 14.Sun A., Bae K., Movsas B. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: Neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfson A.H., Bae K., Komaki R. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:77–84. doi: 10.1016/j.ijrobp.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas E., De Vos R., Jansen Steur E.N. Are Alzheimer’s disease, hypertension, and cerebrocapillary damage related? Neurobiol Aging. 2000;21:235–243. doi: 10.1016/s0197-4580(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 17.Farkas E., Luiten P.G. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs H.I., Leritz E.C., Williams J.V. Association between white matter microstructure, executive functions, and processing speed in older adults: The impact of vascular health. Hum Brain Mapp. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magistro D., Takeuchin H., Nejad K.K. The relationship between processing speed and regional white matter volume in healthy young people. PLoS One. 2015;10:e0136386. doi: 10.1371/journal.pone.0136386. [DOI] [PMC free article] [PubMed] [Google Scholar]