Abstract
Orexins [orexin-A (OXA) and orexin-B (OXB)] are two isoforms of neuropeptides produced by the hypothalamus. The main biological actions of orexins, focused on the central nervous system, are to control the sleep/wake process, appetite and feeding, energy homeostasis, drug addiction, and cognitive processes. These effects are mediated by two G protein-coupled receptor (GPCR) subtypes named OX1R and OX2R. In accordance with the synergic and dynamic relationship between the nervous and immune systems, orexins also have neuroprotective and immuno-regulatory (i.e., anti-inflammatory) properties. The present review gathers recent data demonstrating that orexins may have a therapeutic potential in several pathologies with an immune component including multiple sclerosis, Alzheimer's disease, narcolepsy, obesity, intestinal bowel diseases, septic shock, and cancers.
Keywords: orexins, neuropeptides, GPCR, inflammation, neuroprotection, gastroenterology, autoimmune diseases, cancer
Introduction
The G protein-coupled receptors (GPCR) constitute the largest family of membrane receptors with more than 800 sequences encoded by about 4% of the human genome (1). GPCRs, which act as molecule sensors on the cell surface, lead to signal transduction by activation and/or inhibition of various intracellular signaling pathways leading to final cellular responses (2). Historically, the first structure determination of a GPCR was that of bovine rhodopsin, solved by Palczewski (3). Nearly 10 years later, the first structure of a human GPCR, the β2-adrenergic receptor (βAR), was determined by the group of Rasmussen et al. (4). In 2012, Lefkowitz and Kobilka were awarded with the Nobel Prize in Chemistry “for studies of G-protein-coupled receptors” (5). All GPCRs, also named seven-transmembrane receptor or 7-TM receptors, consist of seven integral α-helices transmembrane domains (H1 to H7) delineating extracellular domains (N-terminal domain and extracellular loops) classically involved in the ligand recognition and intracellular domains (C-terminal domain and intracellular loops) involved in the receptor regulation and signal transduction (6). An eighth α-helix (H8), which would be involved in Gβ/γ binding, has been identified through structural studies of GPCRs (3).
The nature of ligands interacting with GPCRs is characterized by a great diversity, including light, ions, amines, lipids, peptides, proteases, small, and large proteins having multiple properties as neurotransmitters, hormones, pheromones, and odors among others (7). The binding of these various ligands to GPCRs induces a structural conformational change and leads to the activation of G proteins (transducin, Gs, Gi/o, Gq/11, and G12/13). Two major signal transduction pathways that have been associated to GPCRs are the cAMP signal pathway through the adenylyl cyclase effector and the phosphatidylinositol signal pathway through the phospholipase C effector (8). In parallel to its role as a negative regulator of the α subunit, the dissociated Gβ/γ has the ability to modulate signaling pathway cascades including, among others, the regulation of ion channels, the inhibition or activation of adenylyl cyclase, the inhibition of the phosphinositide-3 kinase (PI3K) or the activation of GPCR kinases (βARK) (9).
GPCRs are classified into 6 groups according to IUPHAR nomenclature: rhodopsin-like (class A), secretin-like (class B), metabotropic glutamate (class C), fungal mating pheromone (class D), cyclic AMP receptors (class E), and frizzled/smoothened (class F). This large family of receptors is widely expressed in eukaryotes from yeast to human, and has an essential role in physiological processes, including homeostasis, hormone secretion, neurotransmission, cell differentiation, immunity regulation, vision, metabolism, muscle contraction, olfaction, pain, and many more (10). Related to the large involvement of GPCRs in human physiopathological conditions, these receptors play a major role in inflammatory diseases either by exacerbating and/or inhibiting inflammation (11). Naturally, our intent is not to describe all actions of the multitude of GPCRs in inflammatory contexts, but to outline some of their implications.
GPCRs are able to act directly on immune cells but also on non-immune cells present in specific tissues and organs (12). Among their major actions, they mediate cell migration, phagocyte activation, degranulation, the production of ROS (reactive oxygen species), vascular endothelial permeability and inflammatory nociception (11). Besides these actions, GPCRs are able to regulate inflammatory gene expression (13). GPCR-ligand binding leads to the modulation of transcription factors involved in inflammatory signaling cascades, such as CREB, ERKs, NFAT, c-Jun, STAT3, and NFκB among others (11). GPCRs have been involved in inflammatory diseases such as rheumatoid arthritis (14), sepsis (15), inflammatory bowel diseases (IBD) (16), pancreatitis (17), multiple sclerosis (18), chronic obstructive pulmonary disease (19), renal inflammation (20), and metabolic syndrome involved in obesity and diabetes (21). In that respect, the crosstalk between the actors of inflammation and GPCRs has led to consider these receptors as very promising targets with potential therapeutic applications in inflammatory pathologies. Among the 800 members of the GPCR family, orexin receptors represent an archetype of a putative target for the treatment of chronic inflammatory diseases (22).
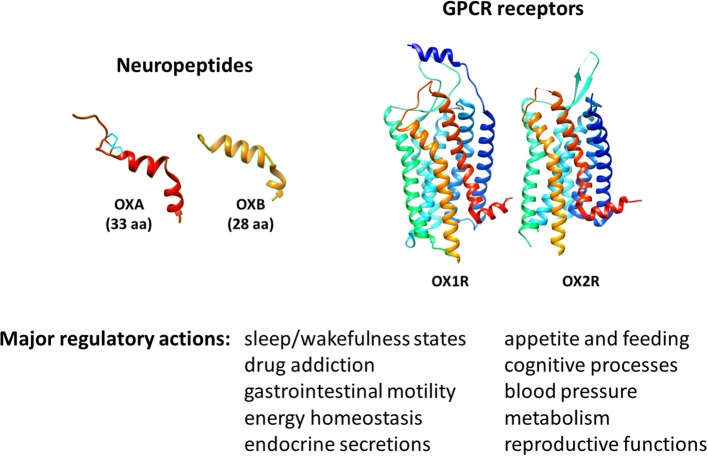
Orexins, also known as hypocretins, comprise two neuropeptides isoforms of 33 and 28 aminoacids, orexin A (OXA/hypocretin-1) and orexin B (OXB/hypocretin-2), respectively (Figure 1). They are encoded by a common precursor polypeptide named prepro-orexin (23). Originally discovered in the hypothalamus in the late nineties, lateral hypothalamic orexin neurons project, and release those peptides widely throughout the central nervous system (CNS) (24, 25). They were initially identified by reverse pharmacology as the endogenous ligands for two orphan GPCR subtypes belonging to the class A family, orexin receptor 1 and 2 (OX1R (Hcrt-1) and OX2R (Hcrt-2), respectively) (23, 25) (Figure 1). Signaling pathways that have been associated to orexin receptors are phospholipase A2, C and D, diacylglycerol lipase, Ca2+, and adenylyl cyclase cascades (26).
Figure 1.
Molecular 3D representation and biological roles of orexins/OXRs system.
The major biological action of orexins is the regulation of sleep/wakefulness state (24, 27) (Figure 1). Related to this action, one major pathology associated to a deficit of orexin production is narcolepsy with cataplexy, referred to as type 1 narcolepsy (T1N). T1N is characterized by a severe dysregulation of the sleep/wakefulness cycles (28, 29). Accordingly, many academic and pharmaceutical laboratories have developed orexin receptor-targeting molecules, in particular antagonists, to treat insomnia (30, 31). These antagonists have been classified into two types depending on their ability to act on one or both orexin receptors: single orexin-receptor antagonists (SORAs) and dual orexin-receptor antagonists (DORAs). Furthermore, SORAs have been subdivided into two subclasses according to their receptor specificity, SORA1 (such as compound 56) and SORA2 (such as JNJ-42847922), targeting OX1R or OX2R, respectively (32, 33). Recently, the DORA molecule suvorexant (MK-4305) has been approved by the U.S. Food & Drug Administration (FDA) for the treatment of insomnia (34). In addition to their ability to modulate sleep and arousal states, these neuropeptides regulate appetite and feeding, gastrointestinal mobility, energy balance and metabolism, but also play a role in cognitive processes (35–40). Thus, multiple studies have highlighted the therapeutic potential of targeting the orexin system, not only in sleep, cognitive [i.e., Alzheimer's disease (AD)] and metabolic (i.e., obesity) disorders (41–45), but also in ischemic and oxidative stress events (46, 47) and in cancer (48, 49).
In addition to their actions in the CNS, these neuropeptides also play a role in various peripheral organs where they regulate appetite, feeding, gastrointestinal mobility, energy balance, metabolism, blood pressure, neuroendocrine and reproductive functions (36–41, 50) (Figure 1). In parallel, the expression of orexins in peripheral tissues has been investigated using immunochemistry and RT-PCR strategies which detected mainly the prepro-orexin precursor. Despite a large variability in terms of expression levels, orexins have been detected in adrenal glands (51), adipose tissues (51), kidney (52), colon (53), pancreas (52), and reproductive organs including testis (54, 55) and prostate (56). In parallel, orexin receptors are also expressed in peripheral tissues including the gastrointestinal tract, adrenal gland, endocrine pancreas, reproductive system, and adipose tissues (50, 57). In these tissues, a paracrine action of orexins is possible. In fact, the circulating level of orexins in blood in healthy individuals is very low [Range of 2 to 45 pM, representing 1,000 times less than the IC50 of receptors (58–60)]. Although the precise source of orexins in disease conditions remains to be elucidated, the abnormal expression of orexin receptors in certain human pathologies has been demonstrated and may lead to new therapeutic targets. In this sense, the ectopic expression of OX1R in human IBD and digestive cancers has been shown, and the administration of exogenous OXA led to a protective effect of orexins in animal models of these pathologies (22).
The existence of a bidirectional crosstalk between the nervous and immune systems has been revealed in the last decades. In this context, the present review will attempt to highlight the impact of the administration of exogenous orexins in the central nervous (i.e., neuroprotective properties) and immune (i.e., anti-inflammatory properties) systems in physiological and pathophysiological conditions including neuroinflammation, intestinal bowel diseases and systemic inflammation.
Orexins and Neuroinflammation
In the CNS, the established relationship between neurons, microglia, and glial cells is highly dynamic and responsive to the diversity of environmental stimuli. For example, in response to injury, infection or disease, the cellular microenvironment of the CNS produces inflammatory mediators including cytokines, chemokines, adhesion molecules, prostaglandins, and free radicals. Those mediators stimulate the recruitment of additional immune cells as well as the activity of astrocytes and microglia. Particularly, in the healthy brain, microglia, resident macrophage-type immune cells of the CNS that share many characteristics with macrophages, are vital to preserve neuronal health (i.e., to promote formation and elimination of synapses) by maintaining a friendly CNS microenvironment. Indeed, microglial cells are capable of adopting appropriate phenotypic responses (i.e., inflammatory and activated vs. anti-inflammatory and resting) according to the type of stimuli. This immune reactivity of the CNS is beneficial and has to be under tight control to efficiently recover physiological homeostasis; however, long-term and dysregulated neuroinflammation, which is generally accompanied by a chronic inflammatory phenotype of microglia, can trigger deleterious effects on the CNS (i.e., subsequent and progressive neuronal loss). Thus, neuroinflammation is a key mechanism contributing to the progression and exacerbation of neurodegenerative and/or inflammatory diseases of the nervous system.
This concept gained even more credence with the discovery of neuropeptides exerting both neuroprotective and immunomodulatory actions, and becoming an emerging group of biological agents with a great potential for the treatment of immune-mediated CNS disorders such as narcolepsy, metabolic disorders, Alzheimer's disease, and multiple sclerosis. One potential candidate is the orexin system. Indeed, an increasing amount of evidence suggest a novel involvement of the orexin/receptor system in the immune and nervous systems. Particularly, as it will be discussed below, one of the two orexins, OXA, exhibits via activation of orexin receptors, neuroprotective, and immuno-regulatory actions, and thus its administration may be beneficial in the aforementioned diseases.
Orexins Neuroprotective Actions
Recent studies have reported that the hypothalamic neuropeptide OXA (OXA, hypocretin 1) may exert an important role in neuroprotection, in part by reducing apoptosis and inflammation (47, 61, 62). Hence, using orexin/ataxin-3 (O/A3) mice, a transgenic mouse model of neurodegeneration, orexin loss has been linked to neurodegeneration, memory and cognitive deficits, and neuroinflammation (63). Supporting a role for endogenous orexin in neurodegenerative/inflammatory brain pathology, orexin expression was found to be elevated in lesioned CNS areas in murine controlled cortical impact (CCI) and transient common carotid artery occlusion (tCCAO), models of traumatic brain injury and cerebral ischemia, respectively (64, 65). In these studies, the cellular localization of orexin receptors was further investigated by immunofluorescence. Although orexin receptors expression is known to be neuronal in healthy brain tissue, expression by glial cells was also reported in these models. For example, OX1R receptor was found to be upregulated in microglia after CCI (64). Moreover, astrocytes and oligodendrocytes were found to express OX1R after tCCAO (65). Although evidence in human pathology is missing, these studies may suggest a potential action of orexin not only on neurons, but also on glial cells.
Data indicate that orexin-induced neuroprotection could rely upon microglial modulation (62, 66). Microglia behave like a sentry capable of efficiently react to endogenous signaling in order to initiate proper neuroinflammatory responses through dynamic transitioning between neurotoxic pro-inflammatory (M1) and neuroprotective (M2) phenotypes. For example, following cerebral ischemic events, microglia can adopt two phenotypes: first an activated neuroprotective M2 phenotype together with the reduction of oxygen levels, and then switch to a pro-inflammatory M1 phenotype, provoking cell death (67). While inflammation is a required normal immune response, chronic M1 pro-inflammatory activation can be detrimental and contributes to subsequent neuronal dysfunction and damage (68). In this context, numerous evidences shed the light on the orexins/receptors system involvement. Indeed, in vivo, OXA exhibited potent neuroprotective actions in several models of rodent focal cerebral ischemia, reducing infarct size (46, 62, 69). This set of data implies a mechanism driven by microglia (46, 62, 69).
Several in vitro studies have demonstrated that OXA promotes both neuronal survival and neuronal protection from death caused by oxidative and hypoxic stress. For example, orexins A and B were capable of efficiently protecting primary rat cortical neurons against cobalt-induced oxidative stress (70). Using SH-SY5Y human neuroblastoma cell line, an in vitro cellular model of dopaminergic neurons in Parkinson's disease, other investigators have shown that OXA elicited neuroprotective actions (i.e., anti-apoptotic and antioxidant effects which are mediated by the PKC and PI3K signaling pathways) against MPP(+) and 6-OHDA-induced neurotoxicity (71–73). These in vitro results might be relevant in light of MS pathogenesis. Indeed, accumulating evidence suggests that oxidative stress, at least in part, contributes to MS pathophysiological processes such as demyelination, axonal damage and neuronal death. In another study, a microarray analysis of neuronal differentiated SH-SY5Y cells treated with OXA revealed the upregulation of somatostatin receptors, vasoactive intestinal peptide (VIP), endothelin-1 (EDN1), and members of the NF-κB pathway, all of which contribute to neuroprotection (74).
Orexins Immuno-Regulatory Properties
In addition to its effects in the nervous system, several studies have shown that OXA can act in vivo as an anti-inflammatory neuropeptide, further supporting its therapeutic potential in neurodegenerative and/or inflammatory disorders. In a rat model of ischemia reperfusion-induced gastric damage, the infusion of OXA: (1) dramatically reduced gastric damage by diminishing the production of reactive oxygen species (ROS) and (2) reduced myeloperoxidase activity in the gastric tissue, suggesting a decrease in polymorphonuclear infiltration and/or activity (75). Later on, using a murine focal cerebral ischemia model, another group demonstrated that the extent of brain lesions were attenuated by the endogenous orexin system, an effect associated with reduced inflammation (i.e., decrease of IL-6 and TNFα levels) (76). More recently, peripheral administration of orexin reduced the levels of proinflammatory mediators (i.e., cytokines and chemokines) and improved the survival of mice in the model of lipopolysaccharide (LPS)-induced endotoxin shock (77). In addition, exposure to LPS down-regulated orexin signaling, supporting the contribution of orexins during an inflammatory event (78). Interestingly, this study demonstrated that peripherally administered OXA was able to cross the blood brain barrier (BBB) under endotoxin shock conditions and acted directly to reduce inflammation in the CNS. This evidence strongly suggests that the orexinergic system can exert its beneficial immuno-regulatory functions not only in inflammatory, but also in immune-driven neurodegenerative diseases.
Despite the scarcity of data regarding the expression of orexin receptors in immune cells, we found that OX1R and OX2R receptors are expressed in murine central and peripheral immune cell tissues, and particularly in sorted T (CD4+ and CD8+) and myeloid (CD11b+) cells (79). We have also described the expression of OX1R in murine colonic lamina propria immune cells (80).
The cellular and molecular mechanisms by which OXA exerts its anti-inflammatory actions in those models have been poorly investigated, with mostly in vitro studies performed. Indeed a direct effect of orexin signaling on microglial cell lines has been shown (62, 66). In normal circumstances, the potent pro-inflammatory agonist lipopolysaccharide (LPS) increases TNF-α production in microglial cell line BV-2 as well as OX1R expression. Interestingly, Xiong et al. reported that a pre-treatment with OXA of the BV-2 cells prior to LPS exposure led to a reduction of TNF-α (62). Although this might suggest an action on innate immune cell mechanisms, the limitation of this work is its in vitro nature. Further studies would be required to demonstrate the relevance of this data as a mechanism for orexin immunoregulatory properties in vivo.
Overall, these recent findings suggest a therapeutic potential of OXA in inflammatory diseases of the CNS.
Orexins in Disease
Narcolepsy
Type 1 narcolepsy (T1N) is a rare but severe chronic neurological sleep disorder (81). Its main symptoms are an excessive daytime sleepiness, cataplexy (sudden loss of muscle tone), fragmented night time sleep with episodes of sleep paralysis and hallucinations (81). T1N is triggered by a selective and almost complete destruction of orexinergic neurons in the lateral hypothalamus (82, 83). Numerous evidence obtained from risk factor studies (i.e., genetic and environmental) and serologic data, suggest that T1N pathogenesis is an autoimmune-based process. A high association of the disease incidence has been found with certain human leukocyte antigen (HLA) class I alleles (i.e., HLA-DQB1*06:02 allele) (84–86), with polymorphisms in the α chain locus of the T-cell receptor (TCR) (87, 88), with the presence of autoantibodies against different CNS antigenic targets identified in the serum and cerebrospinal fluid (CSF) (89–91) and with the vaccination campaigns (i.e., Pandemrix vaccine) against pandemic H1N1 influenza virus (92–94). Even if it has to be confirmed, molecular mimicry has been proposed as a pathophysiological mechanism of the disease (91, 95).
In order to study the autoimmune mechanisms involved in the development of narcolepsy and particularly to discover the effector immune cells responsible for the selective orexin-secreting neuron destruction, a novel mouse model of narcolepsy has been generated (96). Mice were designed to express a “neo-self-antigen” [i.e., hemagglutinin (HA)] specifically in hypothalamic orexin-expressing neurons (named Orex-HA). To induce the disease, they were then adoptively transferred with effector neo-self-antigen-specific T cells either CD4+ Th1 or cytotoxic CD8+ (CTLs). Both HA-specific T cells were able to infiltrate the hypothalamus and cause local inflammation. However, only CTLs were capable of leading to a narcoleptic-like phenotype mimicking human T1N clinical manifestations such as cataplexy and sleep attacks. The latter phenotype was accompanied with a selective and drastic destruction of orexin+ neurons due to a direct and antigen-dependent CTL-mediated cytotoxicity. This work thus emphasizes that narcolepsy pathogenesis is strongly mediated by the immune system (i.e., CTLs play a central effector role) and suggests that novel therapeutic strategies including OXA should trigger the protection of orexin-secreting neurons.
Studies using orexin receptor transgenic mice have suggested a major role for OX2R in narcolepsy. Indeed, narcolepsy in dogs has been associated with a deficiency of the OX2R (29), and narcolepsy-cataplexy symptoms have been observed in OX2R- and not in OX1R- deficient mice (97, 98). Moreover, wakefulness is inhibited only by OX2R and dual orexin receptor antagonists, but not by selective OX1R antagonists (99). Recently, it was shown that peripheral administration of a potent non-peptidic OX2R agonist, YNT-185, significantly ameliorated narcolepsy symptoms in mice (100). This study supports a therapeutic use for orexin receptor agonists (in particular OX2R agonists) as a therapy in narcolepsy.
High Fat Diet (HFD)-Induced Obesity
Given the fact that: (1) the orexin system efficiently controls appetite and feeding as well as the energy balance and metabolism and (2) OXA exhibits potent neuroprotective function, for example by attenuating oxidative stress-induced cell death, another team was interested in deciphering how the dynamic orexin-microglia dialogue might interfere with brain health to induce obesity through high fat diet in saturated fatty acids (SFA) [i.e., palmitic acid (PA, C16:0)] exposure (66). Chronic dietary intake enriched in PA contributes to hypothalamic neurodegeneration (i.e., neuronal cell death and apoptosis) in part through earlier onset of increased oxidative stress, overproduction of ROS, insulin resistance, and hippocampal neuroinflammation (i.e., release of circulating proinflammatory cytokines from microglial cells) (63, 101–105). Additionally, HFD-induced ROS leads to an impairment in hypothalamic gene expression profiles linked to obesity pathogenesis including downregulation of the neuronal anti-apoptotic protein B cell lymphoma 2 (Bcl-2), but upregulation of the pro-apoptotic protein B cell lymphoma 2 associated X protein (Bax) (106, 107).
Using the immortalized murine BV-2 microglial cell line, authors have shown that PA treatment: (1) increases OX1R gene expression but not OX2R and (2) causes the BV-2 cell line to shift toward a pro-inflammatory M1 state (66). In parallel, other teams demonstrated that PA diet activates microglia to an M1 phenotype, resulting in the release of pro-inflammatory cytokines such as TNF-α and IL-6, under either a NFκB- or a toll like receptor 4 (TLR-4)-dependent pathway (66, 103, 108, 109). Further, microglial activation by SFA via TLR-4 contributes to neuronal cell death (108). However, OXA efficiently blocked the harmful effects of PA. Indeed, OXA is capable of promoting a neuroprotective anti-inflammatory M2-like microglial phenotype at the expense of the PA-induced neurotoxic pro-inflammatory microglial M1 phenotype. This was characterized by increased expression of the M2 microglial marker arginase-1, while inhibiting the production of pro-inflammatory TNFα, IL-6 and inducible nitric oxide synthase (iNOS) mediators (66). In addition, using an immortalized murine hypothalamic neuronal cell line (named as mHypoA-1/2), Duffy et al. showed that OXA protects hypothalamic neurons against PA-induced hypothalamic microglial dysregulation (110). This beneficial effect was accompanied with: (1) diminished caspase-3/7 apoptosis, stabilization of Bcl-2 gene expression, and subsequent decrease of Bax/Bcl-2 gene expression ratio, (2) inhibition of ROS production, and (3) a reversion of PA-induced changes in intracellular metabolism, basal/maximum respiration, ATP production and reserve capacity (110). These data support the concept that OXA can efficiently block the actions of PA and may act as a potent immuno-regulator of M1/M2 phenotype microglia, reducing pro-inflammatory cytokines and increasing anti-inflammatory cytokines to promote a beneficial neuronal microenvironment.
Alzheimer's Disease (AD)
Alzheimer's disease is primarily characterized by the loss of pyramidal neurons and synapses in the cerebral cortex as well as in some subcortical regions such as the hippocampus. This event results in general in brain atrophy as well as expanded ventricular volume. Alongside intracellular aggregates of hyper phosphorylated tau proteins and extracellular deposits of amyloid-β (Aβ) aggregates (111), both clinical and preclinical studies have provided recent data clearly determining that AD is a multistep disorder in which chronic and uncontrolled neuroinflammatory processes play an important role for its development. Initially, to preserve healthy brain function, inflammatory responses against Aβ deposits from microglia and astrocytes coordinate efficient phagocytic removal and enzymatic breakdown of amyloid peptides, respectively. However, AD patients present excessive tau protein and Aβ deposition that overcomes physiological clearance, resulting in continued microglial stimulation. The latter significantly leads to an overproduction of pro-inflammatory cytokines which foster dysregulated neurodegeneration (i.e., death of otherwise healthy proximal neurons) in the brain microenvironment (112, 113). In addition, cellular debris and damage-associated molecular patterns from these degenerating neurons can further enhance the stimulation of microglia and the production of inflammatory mediators. It has been shown that diet factors, such as PA, potentiate the risk of not only developing obesity but also cognitive disorders such as AD. In this regard, as mentioned before, several teams have demonstrated the beneficial effects of OXA by antagonizing the proinflammatory actions of PA diet (62, 63, 66, 110). The pathogenic role of excessive inflammation in AD suggests that an antiinflammatory treatment may exert beneficial actions in the disease.
Sleep is critical for physiological brain function allowing the clearance of neurotoxic waste products such as Aβ (114) and stimulates synapse formation and maintenance during the learning process (115, 116). In contrast, sleep deprivation leads to inflammation, reactive glia response, reduced Aβ clearance ability (114, 117) and subsequently strongly increases its levels in the hippocampus and cortex (41) in AD-relevant mouse models. In this sense, increasing evidence in mice and in human suggests that sleep disruption may exacerbate the progression of Alzheimer's neuropathology and cognitive deterioration including memory (118–121). In this context, Duncan et al. tested whether a chronic administration of a dual orexin receptor antagonist (DORA) would favor sleep enhancement and attenuate the development of AD by reducing neuropathology, neuroinflammation, and cognitive deficits. For this purpose, an AD-relevant mouse preclinical model (i.e., 5XFAD mice) which exhibits AD-like features (i.e., sleep disruptions, neuropathology, neuroinflammation, and cognitive deficits including spatial memory) was chosen for the study. In 5XFAD mice, DORA significantly increased light-phase sleep and restored natural sleep patterns (122). Nevertheless, it did not impact neuropathological and neuroinflammatory features of the disease with similar Aβ levels and plaque density in comparison with untreated-DORA mice. 5XFAD mice did not exhibit cognitive deficits in this study. Therefore, the authors could not evaluate whether or not DORA-induced increased sleep improved cognitive functions (122). This set of findings suggests that OXA antagonist analogs (DORA) may be used to improve sleep pattern in AD patients, but its impact on neuroinflammation remains unknown.
Thus, we might speculate that whereas OXA agonists might decrease inflammation (i.e., in the case of high fat diet associated pathology) and potentially AD, antagonist molecules might be beneficial by improving sleep patterns in AD patients with sleep deficits. Further research is needed to determine the best orexin-based therapy in AD.
Multiple Sclerosis (MS)
Multiple sclerosis is a chronic demyelinating disease of the central nervous system. MS is initially characterized by episodes of acute symptoms, followed by partial or complete recovery (relapsing-remitting MS), until remissions no longer occur and disability continuously progresses (progressive MS). Despite its complex pathogenesis, it is established that chronic inflammation in the spinal cord and brain is driven by a Th1/Th17 autoimmune component of the disease. This is characterized by exacerbated neurodegeneration and failure of central nervous system repair mechanisms. Thus, most of MS therapies are immunomodulatory. However, current treatments are only partially effective at the earliest phases of the pathology slowing its progression of disability and also reducing its severity and incidence of exacerbations with somehow important side effects, and have no major impact on its progressive phase (123–125). In addition to inflammation, axonal and neuronal pathologies are central components during MS.
The aforementioned evidences suggest that OXA may present potent therapeutic properties for MS by acting on both inflammatory and neurodegenerative components of the disease. An upregulation of hypothalamic orexin receptor mRNA expression has been described upon experimental autoimmune encephalomyelitis (EAE, a widely used MS mouse model) (126). The same team has shown that intracerebroventricular (ICV) delivery of OXA starting before disease onset, attenuated the clinical score of EAE (127). Nevertheless, this brief study did not address whether or not OXA was able to dampen key components in MS pathogenesis, such as Th1 and Th17 encephalitogenic responses and neurodegeneration. Compared to ICV administration, peripheral administration of OXA might be more interesting from a therapeutic standpoint. Whereas, IP-delivered OXA might easily reach immune organs, a desired local action at the level of the CNS, notably to provide neuroprotection, would require that this neuropeptide crosses the BBB. There is not much information in this sense, but one study demonstrated that intravenously delivered OXA was capable of crossing the BBB from the blood by simple diffusion (128). Furthermore, as mentioned before, peripherally (IP) administered OXA was capable of crossing the BBB and reach the CNS in a study of LPS-induced systemic inflammation (77). This suggests that OXA administered peripherally might act both at CNS and peripheral levels. In order to respond to these issues in MS mouse models, we investigated the curative potential of peripheral OXA administration in the clinical development of ongoing established chronic myelin oligodendrocyte glycoprotein 35-55 (MOG35−55)-induced EAE (a mouse model for progressive MS). Moreover, we studied the impact of this treatment on inflammatory and neurodegeneration processes that underlie the pathogenesis of EAE. We found that an intraperitoneal OXA administration to mice undergoing established chronic MOG35−55-induced EAE had a striking curative effect by alleviating the clinical symptoms and histopathological features of the disease. This was associated to a global reduction of the inflammatory response in the CNS, including a decrease of immune cell infiltration (i.e., CD4+ T cells) and the expression of immune cell mediators (chemokines such as MCP-1/CCL2 and IP-10/CXCL10, and cytokines such as IFN-γ, IL-17, TNF-α, IL-10, and TGFβ) (79). In parallel, OXA diminished demyelination, astrogliosis and microglial activation. The immunomodulatory effects of OXA were not observed in the periphery, since OXA failed to inhibit in vitro draining lymph node cell responses to MOG35−55 (proliferation and Th1/Th17 cytokine production) (79). Overall, this set of results provided the proof-of-concept that peripheral administration with OXA may be beneficial in MS.
IBD
Intestinal bowel diseases (IBD) encompassing Crohn's disease (CD) and ulcerative colitis (UC) are characterized by chronic inflammation of the intestinal mucosa (129). UC is a crippling disease characterized by relapsing-remitting cycles affecting exclusively the mucosa of the colon and rectum following a distal to proximal inflammatory gradient (130). This inflammatory disease was described during the acute phase by the change of mucosal structure resulting of an alteration of mucus-secreting goblet cells, crypt distortion, and crypt abscesses induced by an immune cell infiltration trough the epithelium (130). In this respect, the presence of these lesions might, at least in the longer term, evolve toward dysplasia and colorectal cancer (CRC) (131). The major symptoms are abdominal pain, persistent diarrhea including bloody stools, weight loss, and large fatigue (130). The incidence of this pathology is about 300 per 100,000 in the USA with a general prevalence of IBD of 0.3 % in North America, Oceania and Europe (132). At date, the exact cause of UC remains mainly unknown. UC is a multiple pathogenic disease involving various factors, including genetic susceptibility, environmental impact, dysbiosis, dysregulation of innate and adaptive immune response, inflammasome signaling pathway, regulatory RNAs and endoplasmic reticulum (ER) cellular stress (133).
Currently, the treatment of UC is dependent on the severity of disease. The first line of treatment involves anti-inflammatory drugs, including 5-aminosalicylates and corticosteroids (134). The use of immune system suppressors such as azathioprine, methotrexate, cyclosporine, anti-TNFα (Infliximab) and anti-integrin/α4β7 (Vedolizumab) is also prescribed alone or in combination (134). Unfortunately, the failure of medication, a significant degradation in the quality of life and/or severe flare-ups including acute severe colitis, perforation, uncontrollable bleeding and risk of cancer leads to perform surgery consisting of total colectomy (134). In this context, the identification of new targets represents a major goal in the treatment of this pathology. GPCRs may constitute these innovative new targets. Indeed, most of those receptors are potential targets in colitis such as chemokine receptors (135), cannabinoid receptors (136), histamine receptors (137), and neuropeptide receptors (138). Recently, it was shown that OX1R was expressed in inflamed mucosa from patients having UC (80), but not in normal mucosa (139). It should be emphasized that an UC rat model reproducing chronic mucosal inflammation by injection of adjuvant mixture containing proteins from UC patients, revealed an upregulation of OXA in colon (140). Moreover, an epidemiologic analysis from narcoleptic patients indicated a higher prevalence of immunopathological diseases, including among others Crohn's disease and ulcerative colitis (141). In the classical DSS-induced acute colitis mouse model, OX1R was found to be highly expressed in inflamed colon mucosa whereas the receptor was not expressed in normal mucosa (80). Three intraperitoneal injections of OxA by week in this animal model resulted in an anti-inflammatory effect characterized by the restoration of the intestinal barrier and the inhibition of TNFα, IL-1α, IL-1β, IL-6, IFNγ, IL-17 cytokines and the MCP-1 chemokine in the colon mucosa (80). More recently, Tunisi et al. have demonstrated that OxA prevents the disruption of the intestinal barrier induced by LPS in Caco-2 cells and in vivo (142). The use of a genetically modified murine model where the IL-10 and NAPDH Oxydase 1 (NOX1) genes were invalidated, which mimics the chronic phase of human UC (80), has demonstrated the same anti-inflammatory effect of OXA. Moreover, these OXA-induced anti-inflammatory effects were specific because they were reverted by: (1) the SORA molecule SB-408124 which is an OX1R specific antagonist (80) and (2) the use of an OX1R−/− mouse model in which UC was induced by DSS (80). The anti-inflammatory effect of OXA was mainly mediated by the activation of intracellular calcium releasing signaling pathway and by the inhibition of the NFκB activation (Figure 2) (80). In this study, OX1R was expressed by T lymphocytes and its activation by OXA led to an inhibition of pro-inflammatory cytokines (80).
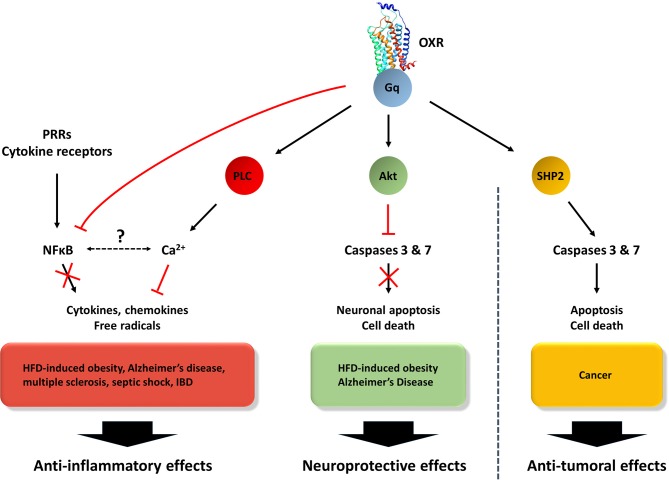
Figure 2.
Orexins/OXR system-modulated signaling patways involved in anti-inflammatory, neuroprotective and anti-tumoral effects. The orexin/receptors system may trigger: (1) anti-inflammatory functions through the inhibition of NFκB and the activation of PLC/Ca2+ pathways, (2) in the CNS, neuroprotective actions via the inhibition of caspases 3/7 by Akt pathway and (3) in the context of cancer, anti-tumoral effects through the activation of caspases 3/7 by SHP2 signaling pathway (60).
Digestive Cancers
Considering that chronic inflammation encompassing IBD, pancreatitis, hepatic fibrosis and metabolic syndrome constitute a high-risk factor to develop cancers (131), the role of orexins in inflammation represents a major question. In 2011, our group had demonstrated that OX1R but not OX2R was highly expressed in colon cancer cell lines and colorectal tumors from patients (139). It should be noted that: (1) no detection of prepro-orexin was observed in normal and tumoral colonic epithelia (139) and (2) OX1R was not detected in normal colon epithelium (22). Orexins treatment of digestive cancer cell lines derived from colon, pancreas and liver cancers (22, 139, 143) induced a strong cell death by apoptosis. The orexin-induced apoptosis was mediated by the phosphorylation of two tyrosine-based motifs (ITIM) present in the receptor sequence. This triggered successive signaling events (Figure 2), such as: the recruitment of the phosphotyrosine phosphatase SHP2, the phosphorylation of the p38 mitogen/stress-activated protein kinase and the translocation of the proapoptotic protein Bax in the mitochondria, leading to apoptosome formation and caspase (3 and 7) activation (144, 145). In a preclinical model, where cancer cells lines derived from colon, pancreas, liver and prostate cancers were sub-cutaneously xenografted, OXA treatment induced a strong reduction of tumor volume (22). This anti-tumoral effect of OXA was also observed in the patient-derived xenograft model (143). The expression of OX1R in digestive cancers had occurred at an early stage since the dysplastic cells present in colon polyps or pancreatic intraepithelial neoplasia (PanIN) lesions highly expressed OX1R (22, 143).
Septic Shock
Sepsis is a systemic infection syndrome representing one of the most important causes of admission in the intensive care unit and potentially life threatening (146). Septic shock, which is associated to organ(s) dysfunction, is the culmination of sepsis through a continuum between infection to severe sepsis (147). It should be noted that the difference between sepsis and the systemic inflammatory syndrome (SIRS) is only related to the presence of one identifiable focus of infection in sepsis (147). Clinical signs associated with septic shock encompass fever, hypotension, tachycardia, oliguria, respiratory distress, skin marbling, confused thinking, and they can evolve to coma. Except for the intensive care associated with organ failure, including heart, kidney, respiratory tract, liver and brain, the treatment of septic shock consists of intravenous injection of empiric antibiotics, vasopressor medications, insulin and corticosteroids (148).
Although the pathological mechanisms involved in organ failure associated to septic shock are not completely understood, some candidate factors involved have been identified. An exacerbated secretion of inflammatory cytokines such as TNFα, IL-6, IL-1β, and MCP-1 has been described in sepsis (147). Associated with this cytokine storm, NFκB, which plays a central role in the induction of transcription pro-inflammatory genes, has been involved in septic shock (147). Indeed, the use of NFκB inhibitors as pyrrolidine dithiocarbamate and parthenolide in lipopolysaccharide (LPS)-induced septic shock murine models improved organ failure and hypotension (149). Cellular apoptosis process also plays a prominent role in septic shock. For example, T and B cell apoptosis has been reported in septic shock patients, leading to immunosuppression (147). Apoptosis of intestinal and lung epithelial cells has been also observed in autopsied patients (150). In addition, LPS, which is one of the major component of gram negative bacteria walls, is involved in septic shock. LPS interacts with the complex toll-like receptor 4 (TLR4)/myeloid differentiation factor 2 (MD-2). TLR4 is expressed in various cells such as macrophages, dendritic cells, adipocytes, enterocytes and mucosal cells, in which LPS induces cytokine and interferon secretion via NFκB activation (151).
Several GPCRs and their ligands have been involved in septic shock and/or in its treatment, including chemokine receptors (i.e., CCR2, CX3CR1, and CXCR1), neuropeptides (i.e., VIP, neuropeptide Y, ocytocin, vasopressin, neurotensin, orexins, substance P, and apelin), proteases [i.e., thrombin (PAR1 and PAR2)], lipid derivatives [i.e., N-arachidonylglycine (GPR18)] and amines (i.e., catecholamines, dopamine histamine, melatonin) (152–156).
The standard animal model used to study the role of GPCRs, particularly orexin receptors, in systemic inflammatory responses in the absence of infection, has been the endotoxemia model induced by LPS injection (157). In parallel, other models using either live bacteria administration or cecal ligation and puncture (CLP) which exposes the cecal content rich in bacteria into the peritoneal cavity have been used (157). In the early 2010s, Deutschman et al. using the CPL mouse model had demonstrated that the orexinergic activity was strongly reduced (158). This inhibition was associated with a reduction of respiratory, heart, temperature and arousal rates (158). Conversely, the intravenous injection of OXA reverted these clinical signs. Other reports indicate that LPS or TNFα (a major cytokine involved in septic shock) were also able to suppress orexin neuronal activity (159). More recently, the use of orexin-neuron ablated mouse model (OX/ataxin-3 transgenic mouse model) injected with LPS revealed a high mortality rate as compared to wild type mice (160). Moreover, the injection of LPS in wild type mice reduced OXA tissue content compared to untreated mice (160). Yanagisawa's group had clearly shown that the subcutaneous diffusion of OXA using an osmotic pump in LPS-induced endotoxin shock mice improve the survival of these mice (77). OXA ameliorated hypothermia and bradycardia associated to LPS-induced endotoxin shock, and reduced the secretion of TNFα, CCL3, IFNγ, IL-17, and IL-6 (77).
Conclusions
Recent literature suggest that the orexin/receptor system can be added to the list of nervous system mediators exhibiting immunoregulatory properties. Overall, the in vivo and in vitro studies gathered here strongly indicate that, in addition to their conventional actions (Figure 1), orexins are neuropeptides with important neuroprotective and anti-inflammatory properties. This may expand their current interest as therapeutic agents from sleep disorders to neurodegenerative disorders with/without a neuroinflammatory component (i.e., HFD-induced obesity, Alzheimer's disease, narcolepsy and multiple sclerosis), acute inflammatory diseases (i.e., septic shock) and chronic inflammatory diseases (i.e., inflammatory bowel diseases and associated cancers) (Figure 3). Based on the data gathered in this review, Figure 2 summarizes potential molecular mechanisms leading to these effects.
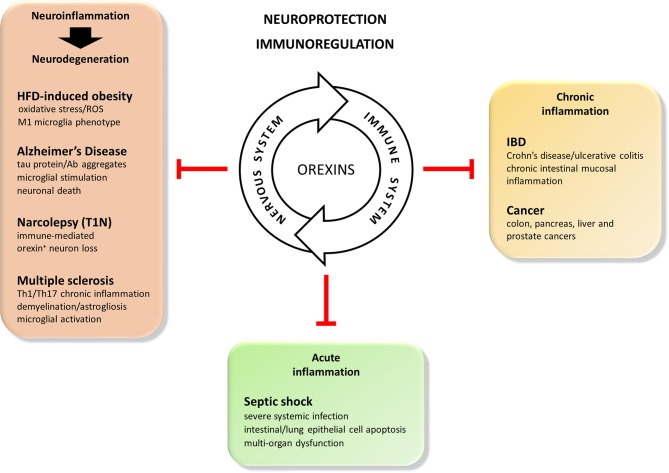
Figure 3.
Beneficial actions of the orexins in immune mediated disorders. Orexin neuropeptides are known to efficiently regulate sleep and arousal states, appetite and feeding, gastrointestinal motility, metabolism, cognitive processes. More recently, considering the synergic and dynamic cross-talk between the nervous and immune systems, they also display potent neuroprotective and immunoregulatory features. Therefore, the orexin/receptors system may be consider as a potential therapeutical tool for the treatment of: (1) acute inflammatory-induced diseases such as septic shock, (2) chronic inflammatory inflammatory-induced diseases such as inflammatory bowl diseases (IBD) or cancer, and (3) immune-mediated neurodegenerative diseases of the central nervous system such as high fat diet (HFD)-induced obesity, Alzheimer's disease, narcolepsy (T1N), or multiple sclerosis.
Author Contributions
AC, TV, PN, VG, CA, and YVT have actively participated in the writing of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Our work on orexins was supported in part by Inserm Transfert, and co-supported by European Union and Région Normandie. Europe gets involved in Normandie with European Regional Development Fund (ERDF). This work was also supported by INSERM U1149/The Inflammation Research Center (CRI), the Institut National du Cancer (INCA) [grant number N°2013-213] and the Ligue Contre le Cancer [grant numbers R16020HH, GB/MA/CD/EP-12062].
References
- 1.Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schioth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. (2006) 88:263–73. 10.1016/j.ygeno.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 2.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. (2010) 9:373–86. 10.1038/nrd3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. (2000) 289:739–45. 10.1126/science.289.5480.739 [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. (2011) 477:549–55. 10.1038/nature10361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenakin T. Making receptors a reality: the 2012 Nobel Prize in Chemistry. Trends Pharmacol Sci. (2013) 34:2–5. 10.1016/j.tips.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Couvineau A, Ceraudo E, Tan YV, Nicole P, Laburthe M. The VPAC1 receptor: structure and function of a class B GPCR prototype. Front Endocrinol. (2012) 3:139. 10.3389/fendo.2012.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edward Zhou X, Melcher K, Eric Xu H. Structural biology of G protein-coupled receptor signaling complexes. Protein Sci. (2019) 28:487–501. 10.1002/pro.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weis WI, Kobilka BK. The molecular basis of G protein-coupled receptor activation. Annu Rev Biochem. (2018) 87:897–919. 10.1146/annurev-biochem-060614-033910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boularan C, Kehrl JH. Implications of non-canonical G-protein signaling for the immune system. Cell Signal. (2014) 26:1269–82. 10.1016/j.cellsig.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couvineau A, Laburthe M. VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins. Br J Pharmacol. (2012) 166:42–50. 10.1111/j.1476-5381.2011.01676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin. (2012) 33:342–50. 10.1038/aps.2011.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammermann T, Kastenmuller W. Concepts of GPCR-controlled navigation in the immune system. Immunol Rev. (2019) 289:205–231. 10.1111/imr.12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veldhuis NA, Poole DP, Grace M, McIntyre P, Bunnett NW. The G protein-coupled receptor-transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacol Rev. (2015) 67:36–73. 10.1124/pr.114.009555 [DOI] [PubMed] [Google Scholar]
- 14.Neumann E, Khawaja K, Muller-Ladner U. G protein-coupled receptors in rheumatology. Nat Rev Rheumatol. (2014) 10:429–36. 10.1038/nrrheum.2014.62 [DOI] [PubMed] [Google Scholar]
- 15.Polat G, Ugan RA, Cadirci E, Halici Z. Sepsis and septic shock: current treatment strategies and new approaches. Eur J Med. (2017) 49:53–8. 10.5152/eurasianjmed.2017.17062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Salhy M, Solomon T, Hausken T, Gilja OH, Hatlebakk JG. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J Gastroenterol. (2017) 23:5068–85. 10.3748/wjg.v23.i28.5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pabreja K, Mohd MA, Koole C, Wootten D, Furness SG. Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. Br J Pharmacol. (2014) 171:1114–28. 10.1111/bph.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du C, Xie X. G protein-coupled receptors as therapeutic targets for multiple sclerosis. Cell Res. (2012) 22:1108–28. 10.1038/cr.2012.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nayak AP, Deshpande DA, Penn RB. New targets for resolution of airway remodeling in obstructive lung diseases. F1000Res. (2018) 7:F1000. 10.12688/f1000research.14581.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoes e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1–7) and Mas receptor axis in inflammation and fibrosis. Br J Pharmacol. (2013) 169:477–92. 10.1111/bph.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riddy DM, Delerive P, Summers RJ, Sexton PM, Langmead CJ. G protein-coupled receptors targeting insulin resistance, obesity, and type 2 diabetes mellitus. Pharmacol Rev. (2018) 70:39–67. 10.1124/pr.117.014373 [DOI] [PubMed] [Google Scholar]
- 22.Couvineau A, Dayot S, Nicole P, Gratio V, Rebours V, Couvelard A, Voisin T. The anti-tumoral properties of Orexin/Hypocretin hypothalamic neuropeptides: an unexpected therapeutic role. Front Endocrinol. (2018) 9:573. 10.3389/fendo.2018.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. (2009) 61:162–76. 10.1124/pr.109.001321 [DOI] [PubMed] [Google Scholar]
- 24.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. (1998) 95:322–7. 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. (1998) 92:696 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- 26.Kukkonen JP. Orexin/hypocretin signaling. Curr Top Behav Neurosci. (2017) 33:17–50. 10.1007/7854_2016_49 [DOI] [PubMed] [Google Scholar]
- 27.Mieda M, Yanagisawa M. Sleep, feeding, and neuropeptides: roles of orexins and orexin receptors. Curr Opin Neurobiol. (2002) 12:339–45. 10.1016/S0959-4388(02)00331-8 [DOI] [PubMed] [Google Scholar]
- 28.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. (1999) 98:437–51. 10.1016/S0092-8674(00)81973-X [DOI] [PubMed] [Google Scholar]
- 29.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. (1999) 98:365–76. 10.1016/S0092-8674(00)81965-0 [DOI] [PubMed] [Google Scholar]
- 30.Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. (2014) 171:283–93. 10.1111/bph.12261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roecker AJ, Cox CD, Coleman PJ. Orexin receptor antagonists: new therapeutic agents for the treatment of insomnia. J Med Chem. (2016) 59:504–30. 10.1021/acs.jmedchem.5b00832 [DOI] [PubMed] [Google Scholar]
- 32.Bonaventure P, Shelton J, Yun S, Nepomuceno D, Sutton S, Aluisio L, et al. Characterization of JNJ-42847922, a Selective Orexin-2 receptor antagonist, as a clinical candidate for the treatment of insomnia. J Pharmacol Exp Ther. (2015) 354:471–82. 10.1124/jpet.115.225466 [DOI] [PubMed] [Google Scholar]
- 33.Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman BT, et al. A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther. (2015) 352:590–601. 10.1124/jpet.114.220392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H−1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. (2010) 53:5320–32. 10.1021/jm100541c [DOI] [PubMed] [Google Scholar]
- 35.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. (1998) 18:9996–10015. 10.1523/JNEUROSCI.18-23-09996.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology. (2005) 182:75–83. 10.1007/s00213-005-0040-5 [DOI] [PubMed] [Google Scholar]
- 37.Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. (2007) 27:14239–47. 10.1523/JNEUROSCI.3878-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. (2010) 67:753–60. 10.1016/j.biopsych.2009.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, de Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci USA. (2011) 108:13305–10. 10.1073/pnas.1015633108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sears RM, Fink AE, Wigestrand MB, Farb CR, de Lecea L, Ledoux JE. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc Natl Acad Sci USA. (2013) 110:20260–5. 10.1073/pnas.1320325110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. (2009) 326:1005–7. 10.1126/science.1180962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Zou B, Xiong X, Pascual C, Xie J, Malik A, et al. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. J Neurosci. (2013) 33:5275–84. 10.1523/JNEUROSCI.3200-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butterick TA, Billington CJ, Kotz CM, Nixon JP. Orexin: pathways to obesity resistance? Rev Endocr Metab Disord. (2013) 14:357–64. 10.1007/s11154-013-9259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roh JH, Jiang H, Finn MB, Stewart FR, Mahan TE, Cirrito JR, et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer's disease. J Exp Med. (2014) 211:2487–96. 10.1084/jem.20141788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mavanji V, Butterick TA, Duffy CM, Nixon JP, Billington CJ, Kotz CM. Orexin/hypocretin treatment restores hippocampal-dependent memory in orexin-deficient mice. Neurobiol Learn Mem. (2017) 146:21–30. 10.1016/j.nlm.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan LB, Dong HL, Zhang HP, Zhao RN, Gong G, Chen XM, et al. Neuroprotective effect of orexin-A is mediated by an increase of hypoxia-inducible factor-1 activity in rat. Anesthesiology. (2011) 114:340–54. 10.1097/ALN.0b013e318206ff6f [DOI] [PubMed] [Google Scholar]
- 47.Butterick TA, Nixon JP, Billington CJ, Kotz CM. Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neurosci Lett. (2012) 524:30–4. 10.1016/j.neulet.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boss C, Roch C. Orexin research: patent news from 2016. Expert Opin Ther Pathol. (2017) 27:1123–33. 10.1080/13543776.2017.1344221 [DOI] [PubMed] [Google Scholar]
- 49.Graybill NL, Weissig V. A review of orexin's unprecedented potential as a novel, highly-specific treatment for various localized and metastatic cancers. SAGE Open Med. (2017) 5:2050312117735774. 10.1177/2050312117735774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu TR, Yang Y, Ward R, Gao L, Liu Y. Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell Signal. (2013) 25:2413–23. 10.1016/j.cellsig.2013.07.025 [DOI] [PubMed] [Google Scholar]
- 51.Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW. Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab. (2001) 86:4808–13. 10.1210/jcem.86.10.7921 [DOI] [PubMed] [Google Scholar]
- 52.Nakabayashi M, Suzuki T, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, et al. Orexin-A expression in human peripheral tissues. Mol Cell Endocrinol. (2003) 205:43–50. 10.1016/S0303-7207(03)00206-5 [DOI] [PubMed] [Google Scholar]
- 53.Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron. (1999) 24:941–51. 10.1016/S0896-6273(00)81041-7 [DOI] [PubMed] [Google Scholar]
- 54.Liguori G, Tafuri S, Miyoshi C, Yanagisawa M, Squillacioti C, De Pasquale V, et al. Localization of orexin B and orexin-2 receptor in the rat epididymis. Acta Histochem. (2018) 120:292–7. 10.1016/j.acthis.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 55.Liguori G, Pavone LM, Assisi L, Langella E, Tafuri S, Mirabella N, et al. Expression of orexin B and its receptor 2 in rat testis. Gen Comp Endocrinol. (2017) 242:66–73. 10.1016/j.ygcen.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 56.Valiante S, Liguori G, Tafuri S, Pavone LM, Campese R, Monaco R, et al. Expression and potential role of the peptide orexin-A in prostate cancer. Biochem Biophys Res Commun. (2015) 464:1290–6. 10.1016/j.bbrc.2015.07.124 [DOI] [PubMed] [Google Scholar]
- 57.Leonard CS, Kukkonen JP. Orexin/hypocretin receptor signalling: a functional perspective. Br J Pharmacol. (2014) 171:294–313. 10.1111/bph.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arihara Z, Takahashi K, Murakami O, Totsune K, Sone M, Satoh F, et al. Immunoreactive orexin-A in human plasma. Peptides. (2001) 22:139–42. 10.1016/S0196-9781(00)00369-7 [DOI] [PubMed] [Google Scholar]
- 59.Sakurai S, Nishijima T, Takahashi S, Yamauchi K, Arihara Z, Takahashi K. Clinical significance of daytime plasma orexin-A-like immunoreactivity concentrations in patients with obstructive sleep apnea hypopnea syndrome. Respiration. (2004) 71:380–4. 10.1159/000079643 [DOI] [PubMed] [Google Scholar]
- 60.Nicole P, Couvineau P, Jamin N, Voisin T, Couvineau A. Crucial role of the orexin-B C-terminus in the induction of OX1 receptor-mediated apoptosis: analysis by alanine scanning, molecular modelling and site-directed mutagenesis. Br J Pharmacol. (2015) 172:5211–23. 10.1111/bph.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sokolowska P, Urbanska A, Namiecinska M, Bieganska K, Zawilska JB. Orexins promote survival of rat cortical neurons. Neurosci Lett. (2012) 506:303–6. 10.1016/j.neulet.2011.11.028 [DOI] [PubMed] [Google Scholar]
- 62.Xiong X, White RE, Xu L, Yang L, Sun X, Zou B, et al. Mitigation of murine focal cerebral ischemia by the hypocretin/orexin system is associated with reduced inflammation. Stroke. (2013) 44:764–70. 10.1161/STROKEAHA.112.681700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duffy CM, Hofmeister JJ, Nixon JP, Butterick TA. High fat diet increases cognitive decline and neuroinflammation in a model of orexin loss. Neurobiol Learn Mem. (2019) 157:41–47. 10.1016/j.nlm.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mihara Y, Dohi K, Yofu S, Nakamachi T, Ohtaki H, Shioda S, et al. Expression and localization of the orexin-1 receptor (OX1R) after traumatic brain injury in mice. J Mol Neurosci. (2011) 43:162–8. 10.1007/s12031-010-9438-6 [DOI] [PubMed] [Google Scholar]
- 65.Nakamachi T, Endo S, Ohtaki H, Yin L, Kenji D, Kudo Y, et al. Orexin-1 receptor expression after global ischemia in mice. Regul Pept. (2005) 126:49–54. 10.1016/j.regpep.2004.08.037 [DOI] [PubMed] [Google Scholar]
- 66.Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, et al. Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neurosci Lett. (2015) 606:140–4. 10.1016/j.neulet.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. (2012) 43:3063–70. 10.1161/STROKEAHA.112.659656 [DOI] [PubMed] [Google Scholar]
- 68.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. (2014) 10:217–24. 10.1038/nrneurol.2014.38 [DOI] [PubMed] [Google Scholar]
- 69.Harada S, Fujita-Hamabe W, Tokuyama S. Effect of orexin-A on post-ischemic glucose intolerance and neuronal damage. J Pharmacol Sci. (2011) 115:155–63. 10.1254/jphs.10264FP [DOI] [PubMed] [Google Scholar]
- 70.Sokolowska P, Urbanska A, Bieganska K, Wagner W, Ciszewski W, Namiecinska M, et al. Orexins protect neuronal cell cultures against hypoxic stress: an involvement of Akt signaling. J Mol Neurosci. (2014) 52:48–55. 10.1007/s12031-013-0165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esmaeili-Mahani S, Vazifekhah S, Pasban-Aliabadi H, Abbasnejad M, Sheibani V. Protective effect of orexin-A on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells. Neurochem Int. (2013) 63:719–25. 10.1016/j.neuint.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 72.Feng Y, Liu T, Li XQ, Liu Y, Zhu XY, Jankovic J, et al. Neuroprotection by Orexin-A via HIF-1alpha induction in a cellular model of Parkinson's disease. Neurosci Lett. (2014) 579:35–40. 10.1016/j.neulet.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 73.Pasban-Aliabadi H, Esmaeili-Mahani S, Abbasnejad M. Orexin-A protects human neuroblastoma SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity: involvement of PKC and PI3K signaling pathways. Rejuvenation Res. (2017) 20:125–33. 10.1089/rej.2016.1836 [DOI] [PubMed] [Google Scholar]
- 74.Davies J, Chen J, Pink R, Carter D, Saunders N, Sotiriadis G, et al. Orexin receptors exert a neuroprotective effect in Alzheimer's disease (AD) via heterodimerization with GPR103. Sci Rep. (2015) 5:12584. 10.1038/srep12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bulbul M, Tan R, Gemici B, Ongut G, Izgut-Uysal VN. Effect of orexin-a on ischemia-reperfusion-induced gastric damage in rats. J Gastroenterol. (2008) 43:202–7. 10.1007/s00535-007-2148-3 [DOI] [PubMed] [Google Scholar]
- 76.Kitamura E, Hamada J, Kanazawa N, Yonekura J, Masuda R, Sakai F, et al. The effect of orexin-A on the pathological mechanism in the rat focal cerebral ischemia. Neurosci Res. (2010) 68:154–7. 10.1016/j.neures.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 77.Ogawa Y, Irukayama-Tomobe Y, Murakoshi N, Kiyama M, Ishikawa Y, Hosokawa N, et al. Peripherally administered orexin improves survival of mice with endotoxin shock. Elife. (2016) 5:e21055. 10.7554/eLife.21055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grossberg AJ, Zhu X, Leinninger GM, Levasseur PR, Braun TP, Myers MG, Jr, et al. Inflammation-induced lethargy is mediated by suppression of orexin neuron activity. J Neurosci. (2011) 31:11376–86. 10.1523/JNEUROSCI.2311-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Becquet L, Abad C, Leclercq M, Miel C, Jean L, Riou G, et al. Systemic administration of orexin A ameliorates established experimental autoimmune encephalomyelitis by diminishing neuroinflammation. J Neuroinflammation. (2019) 16:64. 10.1186/s12974-019-1447-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Messal N, Fernandez N, Dayot S, Gratio V, Nicole P, Prochasson C, et al. Ectopic expression of OX1R in ulcerative colitis mediates anti-inflammatory effect of orexin-A. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:3618–28. 10.1016/j.bbadis.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 81.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. (2007) 369:499–511. 10.1016/S0140-6736(07)60237-2 [DOI] [PubMed] [Google Scholar]
- 82.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. (2000) 6:991–7. 10.1038/79690 [DOI] [PubMed] [Google Scholar]
- 83.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. (2000) 27:469–74. 10.1016/S0896-6273(00)00058-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tafti M, Hor H, Dauvilliers Y, Lammers GJ, Overeem S, Mayer G, et al. DQB1 locus alone explains most of the risk and protection in narcolepsy with cataplexy in Europe. Sleep. (2014) 37:19–25. 10.5665/sleep.3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tafti M, Lammers GJ, Dauvilliers Y, Overeem S, Mayer G, Nowak J, et al. Narcolepsy-associated HLA class I alleles implicate cell-mediated cytotoxicity. Sleep. (2016) 39:581–7. 10.5665/sleep.5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ollila HM, Ravel JM, Han F, Faraco J, Lin L, Zheng X, et al. HLA-DPB1 and HLA class I confer risk of and protection from narcolepsy. Am J Hum Genet. (2015) 96:136–46. 10.1016/j.ajhg.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hallmayer J, Faraco J, Lin L, Hesselson S, Winkelmann J, Kawashima M, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. (2009) 41:708–11. 10.1038/ng.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han F, Faraco J, Dong XS, Ollila HM, Lin L, Li J, et al. Genome wide analysis of narcolepsy in China implicates novel immune loci and reveals changes in association prior to versus after the 2009 H1N1 influenza pandemic. PLoS Genet. (2013) 9:e1003880. 10.1371/journal.pgen.1003880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cvetkovic-Lopes V, Bayer L, Dorsaz S, Maret S, Pradervand S, Dauvilliers Y, et al. Elevated tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. (2010) 120:713–9. 10.1172/JCI41366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bergman P, Adori C, Vas S, Kai-Larsen Y, Sarkanen T, Cederlund A, et al. Narcolepsy patients have antibodies that stain distinct cell populations in rat brain and influence sleep patterns. Proc Natl Acad Sci USA. (2014) 111:E3735–44. 10.1073/pnas.1412189111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmed SS, Volkmuth W, Duca J, Corti L, Pallaoro M, Pezzicoli A, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med. (2015) 7:294ra105 10.1126/scitranslmed.aab2354 [DOI] [PubMed] [Google Scholar]
- 92.Dauvilliers Y, Arnulf I, Lecendreux M, Monaca Charley C, Franco P, Drouot X, et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain. (2013) 136:2486–96. 10.1093/brain/awt187 [DOI] [PubMed] [Google Scholar]
- 93.Szakacs A, Darin N, Hallbook T. Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology. (2013) 80:1315–21. 10.1212/WNL.0b013e31828ab26f [DOI] [PubMed] [Google Scholar]
- 94.Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS ONE. (2012) 7:e33536. 10.1371/journal.pone.0033536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han F, Lin L, Li J, Dong XS, Mignot E. Decreased incidence of childhood narcolepsy 2 years after the 2009 H1N1 winter flu pandemic. Ann Neurol. (2013) 73:560. 10.1002/ana.23799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bernard-Valnet R, Yshii L, Queriault C, Nguyen XH, Arthaud S, Rodrigues M, et al. CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice. Proc Natl Acad Sci USA. (2016) 113:10956–61. 10.1073/pnas.1603325113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. (2003) 38:715–30. 10.1016/S0896-6273(03)00330-1 [DOI] [PubMed] [Google Scholar]
- 98.Hondo M, Nagai K, Ohno K, Kisanuki Y, Willie JT, Watanabe T, et al. Histamine-1 receptor is not required as a downstream effector of orexin-2 receptor in maintenance of basal sleep/wake states. Acta Physiol. (2010) 198:287–94. 10.1111/j.1748-1716.2009.02032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dugovic C, Shelton JE, Yun S, Bonaventure P, Shireman BT, Lovenberg TW. Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Front Neurosci. (2014) 8:28. 10.3389/fnins.2014.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Irukayama-Tomobe Y, Ogawa Y, Tominaga H, Ishikawa Y, Hosokawa N, Ambai S, et al. Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci USA. (2017) 114:5731–6. 10.1073/pnas.1700499114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. (2005) 191:318–25. 10.1016/j.expneurol.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 102.Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. PPARgamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology. (2012) 153:329–38. 10.1210/en.2011-1502 [DOI] [PubMed] [Google Scholar]
- 103.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. (2012) 122:153–62. 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cai D Neuroinflammation in overnutrition-induced diseases Vitam Horm. (2013) 91:195–218. 10.1016/B978-0-12-407766-9.00008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. (2013) 37:839–49. 10.1111/ejn.12088 [DOI] [PubMed] [Google Scholar]
- 106.Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, et al. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J Neurochem. (2003) 84:982–96. 10.1046/j.1471-4159.2003.01606.x [DOI] [PubMed] [Google Scholar]
- 107.Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS ONE. (2009) 4:e5045. 10.1371/journal.pone.0005045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Z, Liu D, Wang F, Liu S, Zhao S, Ling EA, Hao A. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-κB signalling. Br J Nutr. (2012) 107:229–41. 10.1017/S0007114511002868 [DOI] [PubMed] [Google Scholar]
- 109.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. (2014) 9:2124–38. 10.1016/j.celrep.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duffy CM, Nixon JP, Butterick TA. Orexin A attenuates palmitic acid-induced hypothalamic cell death. Mol Cell Neurosci. (2016) 75:93–100. 10.1016/j.mcn.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. (2001) 3:75–80. 10.3233/JAD-2001-3111 [DOI] [PubMed] [Google Scholar]
- 112.Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJ, van Gool WA, Hoozemans JJ. The significance of neuroinflammation in understanding Alzheimer's disease. J Neural Transm. (2006) 113:1685–95. 10.1007/s00702-006-0575-6 [DOI] [PubMed] [Google Scholar]
- 113.Minter MR, Taylor JM, Crack PJ. The contribution of neuroinflammation to amyloid toxicity in Alzheimer's disease. J Neurochem. (2016) 136:457–74. 10.1111/jnc.13411 [DOI] [PubMed] [Google Scholar]
- 114.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. (2013) 342:373–7. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tononi G. Slow wave homeostasis and synaptic plasticity. J Clin Sleep Med. (2009) 5:S16–9. [PMC free article] [PubMed] [Google Scholar]
- 116.Vyazovskiy VV, Faraguna U. Sleep and synaptic homeostasis. Curr Top Behav Neurosci. (2015) 25:91–121. 10.1007/7854_2014_301 [DOI] [PubMed] [Google Scholar]
- 117.Carroll JE, Cole SW, Seeman TE, Breen EC, Witarama T, Arevalo JM, et al. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav Immun. (2016) 51:223–9. 10.1016/j.bbi.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiol Aging. (1982) 3:361–70. 10.1016/0197-4580(82)90024-0 [DOI] [PubMed] [Google Scholar]
- 119.Bliwise DL. Sleep disorders in Alzheimer's disease and other dementias. Clin Cornerstone. (2004) 6 (Suppl. 1):S16–28. 10.1016/S1098-3597(04)90014-2 [DOI] [PubMed] [Google Scholar]
- 120.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. (2006) 57:139–66. 10.1146/annurev.psych.56.091103.070307 [DOI] [PubMed] [Google Scholar]
- 121.Bliwise DL, Mercaldo ND, Avidan AY, Boeve BF, Greer SA, Kukull WA. Sleep disturbance in dementia with Lewy bodies and Alzheimer's disease: a multicenter analysis. Dement Geriatr Cogn Disord. (2011) 31:239–46. 10.1159/000326238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duncan MJ, Farlow H, Tirumalaraju C, Yun DH, Wang C, Howard JA, et al. Effects of the dual orexin receptor antagonist DORA-22 on sleep in 5XFAD mice. Alzheimers Dement. (2019) 5:70–80. 10.1016/j.trci.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dendrou CA, Fugger L. Immunomodulation in multiple sclerosis: promises and pitfalls. Curr Opin Immunol. (2017) 49:37–43. 10.1016/j.coi.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 124.Zadeh R, Askari M, Azadani NN, Ataei A, Ghadimi K, Tavoosi N, et al. Mechanism and adverse effects of multiple sclerosis drugs: a review article. Part 1. Int J Physiol Pathophysiol Pharmacol. (2019) 11:95–104. [PMC free article] [PubMed] [Google Scholar]
- 125.Zadeh R, Ghadimi K, Ataei A, Askari M, Sheikhinia N, Tavoosi N, et al. Mechanism and adverse effects of multiple sclerosis drugs: a review article. Part 2. Int J Physiol Pathophysiol Pharmacol. (2019) 11:105–114. [PMC free article] [PubMed] [Google Scholar]
- 126.Fatemi I, Shamsizadeh A, Roohbakhsh A, Ayoobi F, Sanati MH, Motevalian M. Increase in mRNA level of orexin1 and 2 receptors following induction of experimental autoimmune encephalomyelitis in mice. Iran J Allergy Asthma Immunol. (2016) 15:20–6. [PubMed] [Google Scholar]
- 127.Fatemi I, Shamsizadeh A, Ayoobi F, Taghipour Z, Sanati MH, Roohbakhsh A, et al. Role of orexin-A in experimental autoimmune encephalomyelitis. J Neuroimmunol. (2016) 291:101–9. 10.1016/j.jneuroim.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 128.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. (1999) 289:219–23. [PubMed] [Google Scholar]
- 129.Oligschlaeger Y, Yadati T, Houben T, Condello Olivan CM, Shiri-Sverdlov R. Inflammatory bowel disease: a stressed “Gut/Feeling”. Cells. (2019) 8:E659. 10.3390/cells8070659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. (2011) 365:1713–25. 10.1056/NEJMra1102942 [DOI] [PubMed] [Google Scholar]
- 131.Domenech E, Manosa M, Cabre E. An overview of the natural history of inflammatory bowel diseases. Dig Dis. (2014) 32:320–7. 10.1159/000358131 [DOI] [PubMed] [Google Scholar]
- 132.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2018) 390:2769–78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 133.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. (2016) 13:13–27. 10.1038/nrgastro.2015.186 [DOI] [PubMed] [Google Scholar]
- 134.Hindryckx P, Jairath V, D'Haens G. Acute severe ulcerative colitis: from pathophysiology to clinical management. Nat Rev Gastroenterol Hepatol. (2016) 13:654–64. 10.1038/nrgastro.2016.116 [DOI] [PubMed] [Google Scholar]
- 135.Thomas S, Baumgart DC. Targeting leukocyte migration and adhesion in Crohn's disease and ulcerative colitis. Inflammopharmacology. (2012) 20:1–18. 10.1007/s10787-011-0104-6 [DOI] [PubMed] [Google Scholar]
- 136.Ashton JC. Cannabinoids for the treatment of inflammation. Curr Opin Investig Drugs. (2007) 8:373–84. [PubMed] [Google Scholar]
- 137.Zhang M, Venable JD, Thurmond RL. The histamine H4 receptor in autoimmune disease. Expert Opin Investig Drugs. (2006) 15:1443–52. 10.1517/13543784.15.11.1443 [DOI] [PubMed] [Google Scholar]
- 138.Waschek JA. VIP and PACAP: neuropeptide modulators of CNS inflammation, injury, and repair. Br J Pharmacol. (2013) 169:512–23. 10.1111/bph.12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Voisin T, El Firar A, Fasseu M, Rouyer-Fessard C, Descatoire V, Walker F, et al. Aberrant expression of OX1 receptors for orexins in colon cancers and liver metastases: an openable gate to apoptosis. Cancer Res. (2011) 71:3341–51. 10.1158/0008-5472.CAN-10-3473 [DOI] [PubMed] [Google Scholar]
- 140.Zhang D, Ren YB, Wei K, Hong J, Yang YT, Wu LJ, et al. Herb-partitioned moxibustion alleviates colon injuries in ulcerative colitis rats. World J Gastroenterol. (2018) 24:3384–97. 10.3748/wjg.v24.i30.3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martinez-Orozco FJ, Vicario JL, Villalibre-Valderrey I, De Andres C, Fernandez-Arquero M, Peraita-Adrados R. Narcolepsy with cataplexy and comorbid immunopathological diseases. J Sleep Res. (2014) 23:414–9. 10.1111/jsr.12143 [DOI] [PubMed] [Google Scholar]
- 142.Tunisi L, Forte N, Fernandez-Rilo AC, Mavaro I, Capasso R, D'Angelo L, et al. Orexin-A prevents lipopolysaccharide-induced neuroinflammation at the level of the intestinal barrier. Front Endocrinol. (2019) 10:219. 10.3389/fendo.2019.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dayot S, Speisky D, Couvelard A, Bourgoin P, Gratio V, Cros J, et al. In vitro, in vivo and ex vivo demonstration of the antitumoral role of hypocretin-1/orexin-A and almorexant in pancreatic ductal adenocarcinoma. Oncotarget. (2018) 9:6952–67. 10.18632/oncotarget.24084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Voisin T, El Firar A, Rouyer-Fessard C, Gratio V, Laburthe M. A hallmark of immunoreceptor, the tyrosine-based inhibitory motif ITIM, is present in the G protein-coupled receptor OX1R for orexins and drives apoptosis: a novel mechanism. FASEB J. (2008) 22:1993–2002. 10.1096/fj.07-098723 [DOI] [PubMed] [Google Scholar]
- 145.El Firar A, Voisin T, Rouyer-Fessard C, Ostuni MA, Couvineau A, Laburthe M. Discovery of a functional immunoreceptor tyrosine-based switch motif in a 7-transmembrane-spanning receptor: role in the orexin receptor OX1R-driven apoptosis. FASEB J. (2009) 23:4069–80. 10.1096/fj.09-131367 [DOI] [PubMed] [Google Scholar]
- 146.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign guidelines committee including the pediatric, surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. (2013) 39:165–228. 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hattori Y, Hattori K, Suzuki T, Matsuda N. Recent advances in the pathophysiology and molecular basis of sepsis-associated organ dysfunction: novel therapeutic implications and challenges. Pharmacol Ther. (2017) 177:56–66. 10.1016/j.pharmthera.2017.02.040 [DOI] [PubMed] [Google Scholar]
- 148.Rello J, Valenzuela-Sanchez F, Ruiz-Rodriguez M, Moyano S. Sepsis: a review of advances in management. Adv Ther. (2017) 34:2393–2411. 10.1007/s12325-017-0622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li X, Su J, Cui X, Li Y, Barochia A, Eichacker PQ. Can we predict the effects of NF-kappaB inhibition in sepsis? Studies with parthenolide and ethyl pyruvate. Expert Opin Investig Drugs. (2009) 18:1047–60. 10.1517/13543780903018880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med. (1997) 25:1298–307. 10.1097/00003246-199708000-00015 [DOI] [PubMed] [Google Scholar]
- 151.Cristofaro P, Opal SM. The Toll-like receptors and their role in septic shock. Expert Opin Ther Targets. (2003) 7:603–12. 10.1517/14728222.7.5.603 [DOI] [PubMed] [Google Scholar]
- 152.Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. (2013) 93:329–42. 10.1189/jlb.0912437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pinheiro da Silva F, Machado MC, Velasco IT. Neuropeptides in sepsis: from brain pathology to systemic inflammation. Peptides. (2013) 44:135–8. 10.1016/j.peptides.2013.03.029 [DOI] [PubMed] [Google Scholar]
- 154.Alberelli MA, De Candia E. Functional role of protease activated receptors in vascular biology. Vascul Pharmacol. (2014) 62:72–81. 10.1016/j.vph.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 155.Kanashiro A, Sonego F, Ferreira RG, Castanheira FV, Leite CA, Borges VF, et al. Therapeutic potential and limitations of cholinergic anti-inflammatory pathway in sepsis. Pharmacol Res. (2017) 117:1–8. 10.1016/j.phrs.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang L, Qiu C, Yang L, Zhang Z, Zhang Q, Wang B, et al. GPR18 expression on PMNs as biomarker for outcome in patient with sepsis. Life Sci. (2019) 217:49–56. 10.1016/j.lfs.2018.11.061 [DOI] [PubMed] [Google Scholar]
- 157.Seemann S, Zohles F, Lupp A. Comprehensive comparison of three different animal models for systemic inflammation. J Biomed Sci. (2017) 24:60. 10.1186/s12929-017-0370-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Deutschman CS, Raj NR, McGuire EO, Kelz MB. Orexinergic activity modulates altered vital signs and pituitary hormone secretion in experimental sepsis. Crit Care Med. (2013) 41:e368–75. 10.1097/CCM.0b013e31828e9843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hirota K. Sepsis and the orexin system. J Anesth. (2016) 30:919–22. 10.1007/s00540-016-2246-6 [DOI] [PubMed] [Google Scholar]
- 160.Takekawa D, Kushikata T, Akaishi M, Nikaido Y, Hirota K. Influence of orexinergic system on survival in septic rats. Neuropsychobiology. (2019) 77:45–8. 10.1159/000493739 [DOI] [PubMed] [Google Scholar]