Abstract
Antibiotics have components to inhibit infections against Staphylococcus aureus, but they depend on judicious use to minimize the incidence of resistance forms. Strategies to improve the current situation include research in finding a new antimicrobial from virgin coconut oil (VCO). The saturated fatty acid, lauric acid (LA) (C12) contain in VCO was reported to have antibacterial activities. This study developed antimicrobial of VCO as an antimicrobial and immunomodulatory agent. Staphylococcus aureus used in this study had been isolated and identified from the mastitis milk crossbreed Etawa goat from Riau, Indonesia. The susceptibility of S. aureus to VCO was tested using the broth dilution method. The inhibition mechanisms of S. aureus had been studied by scanning electron microscopy (SEM) after treatment with VCO, and potential of VCO, which is known in phagocytosis macrophage. In vitro test confirmed the inhibitory effect of VCO on the growth of S. aureus at the concentration of 200 μl (equal to 0.102 % LA). Based on the result of the phagocytosing assay, VCO could increase the ability of the macrophage cells to phagocyte S. aureus significantly at a concentration of 200 μL (equal to 0.102% LA). This study concluded that the VCO could inhibit the growth of S. aureus with destructive mechanisms of bacterial cell walls and increase the ability of the phagocytic immune cells.
Keywords: Microbiology, Staphylococcus aureus, Virgin coconut oil, Antimicrobial, Immunomodulator
Microbiology; Staphylococcus aureus; Virgin coconut oil; Antimicrobial; Immunomodulator
1. Introduction
Intramammary infections among small ruminants raised in Indonesia are not well documented in spite of research overseas reported that describe the susceptibility of cows and goats to Staphylococcal infections. Staphylococcus aureus is one of the bacteria caused clinical mastitis or subclinical mastitis in small ruminants as well as sheep and goats [1, 2, 3]. In dairy goats, S. aureus, as well as coagulase-negative staphylococci, are usually considered to have the higher pathogenicity of subclinical mastitis agents [4]. The subclinical mastitis in dairy cow and goat farms, causing significant economic clauses due to the reduction in milk production and poor milk quality [5].
Staphylococcus aureus has an inadequate response to various antibiotic therapy, reflected a severe problem in the world. This problem because of the far-ranging use of lactam drugs, multidrug-resistant S. aureus (especially the methicillin-resistant Staphylococcus aureus, MRSA) became a frightening new threat in the past decades [6, 7]. Methicillin-resistant Staphylococcus aureus can hydrolyze almost all kinds of lactams, and the drug-resistant strains spread quickly, causing high mortality rate in infected patients and the severe overdraft of medical resources due to its broad drug-resistant spectrum [8].
The risk of antibiotic residues in milk is also becoming a threat to consumer health. Negative consequences of antibiotics residues in dairy products and antibiotics resistance genes developed in bacteria following exposure to antimicrobial agents are well documented. Data on antibiotic resistance could also be used to characterize these opportunistic pathogens, which may further limit the risks associated with the consumption of contaminated milk and its products [9, 10].
As many natural products that possess antibacterial activity can resolve the problems of chemical and antibiotic resistance, they have the potential to be a choice [11, 12, 13, 14, 15]. The developments of alternatives to the existing antibiotics against MRSA infections are still of interest and need to be actualized [8, 16]. Chuzhou chrysanthemum oil (CCO) has been reported by Cui et al. [17] has antibacterial activity. The antibacterial mechanism of CCO affects through the Embden-Meyerhof-Parnas Pathway of S. aureus. Virgin coconut oil (VCO) has a functional ingredient such as caprylic acid (C8) 8%, capric acid (C10) 10%, lauric acid (LA) 48% and meristic acid 17%. Medium-chain fatty acids (MCFA) were found to be a potential antibacterial functional food [18]. Many recent studies indicate that exposure to LA was the most inhibitory to growth bacteria. Capric acid and caprylic acid can also inhibit the growth of bacteria [19, 20, 21].
Although there are many studies reported the potential of virgin coconut oil as antibacterial activity, there is need more investigations to support that the virgin coconut oil has the ability to inhibit the S. aureus isolated from mastitis milk goat and its potential as an immunomodulator.
2. Materials and methods
2.1. Bacterial isolates
In this research, we used S. aureus isolated from subclinical mastitis milk's Peranakan Etawa (PE) or crossbred Etawa goats in Riau, Indonesia. Peranakan Etawa goat is descended originally from crossings between the Kacang goat (an indigenous breed of goat in Indonesia) with Etawa (Jamnapari India) goat. All samples were identified based on the Gram staining, fermentation on mannitol salt agar (MSA, Oxoid, England UK), catalase, and coagulase tests as described previously [22, 23]. The bacterial isolate was genetically confirmed as S. aureus by using the Polymerase Chain Reaction (PCR) amplification of the 23S rRNA gene [24], thermonuclease nuc gene [25], and coagulase (coa) gene [26].
The bacterial isolates were cultured on the Base Agar (BA; Oxoid, England UK) supplemented with 5% sheep blood. After incubation at 37 °C for 18–24 h, colonies were picked and resolved in 2 ml volume of sterile Todd Hewitt Broth (THB; Oxoid, England UK). The bacterial cell density was adjusted to 4 McFarland standard or approximately 109 colony-forming units CFU ml−1. The inoculate were then diluted ten-fold in sterile normal saline solution (NSS), giving a final cell density of approximately 108 CFU ml−1.
2.2. Agar dilution method
The procedure followed the international recommendations provided by the Clinical and Laboratory Standards Institute (CLSI) [27] in a modified tube dilution assay. Dilution tubes containing 1% Müeller Hinton Agar (MHA; Oxoid, England UK) were adjusted at a final volume of 7.5 mL. Staphylococcus aureus were striked on the MHA tubes containing VCO (50, 100, 150, 200, 250, 300, 350 and 400 μL, which 1 μL VCO equal to 0.00051 LA) and incubated for 16–18 h. After incubation, the growth of the bacteria on the tubes was recorded as densities of media with no visible growth of S. aureus in compare to the positive control tube.
2.3. Assessment of morphological alteration by scanning electron microscopy (SEM)
The alteration S. aureus after treatment with VCO was examined by SEM. The bacteria were cultured in THB and incubated at 37 °C for 24 h. The suspensions were spun at 3000 rpm for 15 min. A total of 250 μL VCO as a recommended dose was added to the pellet, centrifuged and incubated for 24 h at 37 °C. After incubation, the suspension was spun at 3000 rpm for 15 min. Pellet was dried according to Duke [28] at the critical point by using a Polaron CPD 7501 (VG Microtech, E Sussex, UK). The specimens were coated with a thin layer of gold by a Sputter Coater (SPI suppliers, PA, USA) prior to examination with SEM (JSM-6510LA, Japan).
2.4. Phagocytosing activity
In this study, we used male Balb/c mice with bodyweight 20 g of the pathogen-free strain, maintained at the UPHP LPPT UGM. This study meets the requirements of ethics for research in experimental animals and approved by the ethical committee clearance for preclinical research Gadjah Mada University with number: 333/KEC-LPPT/X/2015.
Mice were euthanized with pentobarbital 125 mg kg−1 intravenous. The skin over the abdomen was cleaned up, and 1 ml of Hanks Balanced Salt Solution (HBSS, Lonza, USA) was injected into the peritoneal cavity. The abdomen was massaged gently, and after about 1 min, the cell-rich fluid was removed with a capillary pipette and placed in a tube (Biologic, China). These fluid contain macrophage cells. The cells were centrifuged for 8 min, at 150 g, at room temperature. Total 2 ml HBSS was added to the pellet. The cells were thoroughly mixed by repeated pipetting [29].
A total of 108 bacteria mL−1 was suspended with macrophage cells (105 cells mL-1) and VCO with a ratio of 1:1:1 into 2 mL microtube. The suspension containing bacteria was stained with Safranin 10% (Merck, Germany) and incubated for 1 h at 37 °C. Phagocytosing activity were assayed by placing a drop of suspension on an object-glass. The slides were examined with immersion objective at a magnification of 100x, by using a Zeiss binocular microscope. Phagocytosing result was evaluated by counting the number of bacteria phagocytosed by 20 macrophages cells [30].
2.5. Data analysis
The susceptibility test was recorded descriptively as densities of media with no visible growth of S. aureus in compare to the control. The result of phagocytosing activity was analyzed using ANOVA single factor with considered significant at p = 0.05, and followed by the honestly significant difference (HSD) test (SPSS statistical software version 21.0; SPSS Inc., Chicago, IL, USA).
3. Results
Staphylococcus aureus used in this study was isolated from the subclinical mastitis milk's Peranakan Etawa (PE) and confirmed based on biochemical and genotypic identification according to the previous study [22, 23].
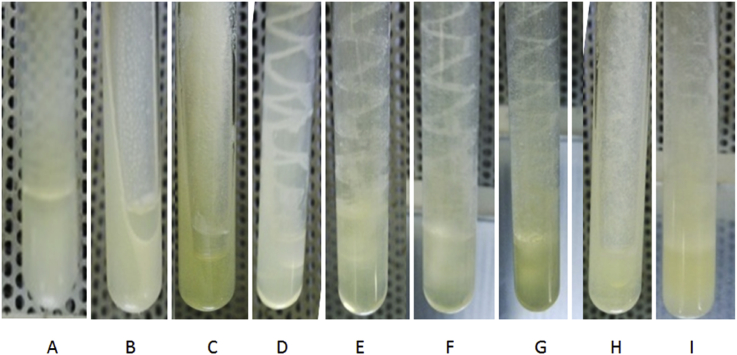
Virgin coconut oil was evaluated at different concentration levels (0, 50, 100,150, 200, 250, 300, 350, 400 μl) against S. aureus on the MHA dilution test. The result showed that VCO could inhibit the growth of S. aureus on the agar dilution media with a minimal concentration of 200 μl (equal to 0.102% LA) of media. Amoxicillin was used as an antibiotic control. The inhibition growth of S. aureus in the agar dilution media containing VCO was summarized in Fig. 1.
Fig. 1.
Müller Hinton Agar dilution test results of Staphylococcus aureus on the MHA containing VCO with different concentrations. A = MHA without VCO (clear), B = MHA containing VCO 100 μL (clear), C = MHA containing ampicillin after inoculation with S. aureus (no bacterial growth), D = MHA after inoculation with S. aureus (bacterial growth), E-G = MHA containing VCO 50 μL, 100 μL and 150 μL after inoculation with S. aureus (bacterial bacterial growth), H = MHA containing VCO 200 μL after inoculation with S. aureus (less bacterial growth), I = MHA containing VCO 400 μL after inoculation with S. aureus (no bacterial growth).
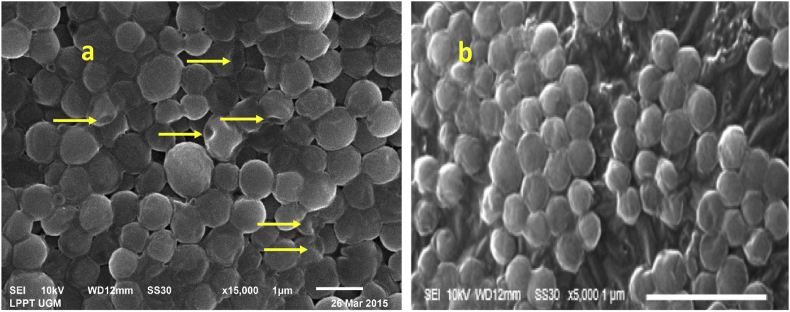
Scanning electron microscopy is a well-established basic method to observe the morphology of the material surface. Virgin coconut oil could damage the surface of the S. aureus cell walls. In this study, there were some leakage and change of the bactaeria cell walls following exposure to VCO. Treatment by VCO yielded bacteria with surface depressions and visualized the holes in the bacteria cell walls (Fig. 2a). This finding was different from the control of S. aureus without treatment (Fig. 2b).
Fig. 2.
Morphology of Staphylococcus aureus after treatment with 250 μL VCO (equal to 0.1275% LA) (a), compare to control of Staphylococcus aureus without treatment (b), examinated with Scanning electron Microscopy (SEM) 15000 x magnification. Yellow arrows indicate holes in the bacterial wall.
Table 1 shows the results of the phagocytic activities of mice peritoneal macrophage cells against S. aureus after treatment with VCO. The number of phagocyted S. aureus in the macrophage cells at any given 50, 100, 150, 200 μL VCO were 24.7 ± 14.14, 26.1 ± 12.94, 42.7 ± 18.31, 49.1 ± 32.29 bacteria cell−1, respectively. There were significant (P < 0.05) increased comparing to untreated VCO (23.1 ± 7.52 bacteria cell−1).
Table 1.
The phagocytic activities of mice peritoneal macrophage cells against S. aureus after treatment with VCO.
| Concentration VCO (μL) (1 μL equal to 0.00051% LA) | Number of phagocyted Staphylococcus aureus (bacteria cell−1) |
|---|---|
| 0 | 23.1 ± 7.52b |
| 50 | 24.7 ± 14.14ab |
| 100 | 26.1 ± 12.94ab |
| 150 | 42.7 ± 18.31ab |
| 200 | 49.1 ± 32.29a |
| 250 | 19.4 ± 16.19b |
Note: Different notations indicate significant differences at the level of 5% (p < 0.05).
4. Discussion
Our finding resulted that the addition of VCO to MHA could inhibit the growth of S. aureus. The mechanism of killing or inhibition of organisms had many variable aspects and complete process [19]. Sihombing et al. [31] reported VCO with a dose equal to 0.5% LA inhibited the growth of S. aureus. The amount of high LA (40–60%) mainly in the form of free fatty acids and monoglycerides in coconut oil has antibacterial activity, antiviral, antifungal, antiprotozoal, and also can enhance the immune system. Lauric acid is an MCFA, which has the additional beneficial function of being formed into monolaurin in the human or animal body [32]. Some studies had also shown some antimicrobial effects of free LA. This component did not cause resistance problem, so far increased the use of coconut oil as an alternative treatment in humans. Several health benefits have been attributed to this oil. These include benefits in skincare, haircare, stress relief, weight loss, cholesterol level maintenance, immunomodulatory effects, cardiovascular uses, and more recently, in Alzheimer's disease [33]. Huang et al. [34] evaluated the antimicrobial property of LA against Propionibacterium acnes both in vitro and in vivo. Incubation of the skin bacteria P. acnes, S. aureus and Staphylococcus epidermidis with LA yielded minimal inhibitory concentration (MIC) values against the bacterial growth over 15 times lower than those of benzoyl peroxide (BPO). The lower MIC values of LA indicated stronger antimicrobial properties than that of BPO.
Observations by using SEM, revealed that VCO could damage the S. aureus through the mechanisms of the bacterial cell wall surface. Medium-chain fatty acids (MCFA) of VCO, especially LA, has a composition similar to bacterial peptidoglycan cell wall constituent. LA was initially covered the entire surface of the cell wall, penetrate and destroyed the bacteria. As seen in Fig. 2a, the S. aureus visualized to be damaged and there were some holes in the surface of cell walls after treatment with VCO. Monoglycerides and MCFA in VCO break up the bacterial membrane with the aid of gastrointestinal lipase enzyme. The working system of the MCFA, especially LA, has been changing the permeability of the bacterial cell wall, disrupting metabolism, inhibiting the essential nutrients needed by bacteria or linkage with carbohydrate metabolism. Monoglyceride monolaurin has been functioning as an antiviral, antibacterial and antiprotozoa. Essential oils and their components have activity against the bacteria with a variety of targets, particularly the membrane and cytoplasm, and in some cases, they completely change the morphology of the cells [35, 36].
Shilling et al. [20] reported 2 mg mL−1 of LA in the VCO could disturb of both the cell membrane and cytoplasm of bacterial cells, under the transmission electron microscopy (TEM) examination. In vitro study activities of VCO against bacterial showed that LA and monolaurin in combination with lactic acid could damage the cytoplasm and membrane cells of the bacterium by using SEM and TEM as well [19]. The antibacterial activity of essential oils from S. officinalis has been reported by several authors. Four essential oil samples from this plant were tested against Pseudomonas aeruginosa, Escherichia coli, Listeria monocytogenes, and S. aureus [11, 15].
Adhesions between bacteria due administration of VCO, facilitating the process of phagocytosis, in which the bacterial cells are closer to become more phagocyte by macrophage cells. Lauric acid might show antimicrobial activity by disruption of the cell membrane, inactivation of enzymes, and denaturation of cell proteins similar to other long-chain fatty acids. Monoglycerides and the free fatty acid act as an antibacterial. Lauric acid has a role either as bacteriostatic or bacteriocidal [19, 36].
Cells evolved a whole host of mechanisms for ingesting particles and fluids. These vary from receptor-mediated endocytosis (absorption of small particles into clathrin-coated vesicles) to pinocytosis (the uptake of soluble material), to phagocytosis. Phagocytosis is the mechanism by which relatively large (>0.5 mm) particles, such as bacteria, dead cells, or polystyrene beads [34]. The mechanisms that lead to high numbers of S. aureus phagocytosed by macrophages possibly because of LA contained in the VCO helps macrophages in recognizing bacteria. Staphylococcus aureus infection activated the most highly conserved unfolded protein response sensor, inositol-requiring enzyme 1α (IRE1α). The macrophage IRE1-dependent bactericidal activity required reactive oxygen species (ROS). Response sensor, inositol-requiring enzyme (IRE1)-mediated persistent ROS generation might act as a fail-safe mechanism to kill bacterial pathogens that evade the initial macrophage oxidative burst [37]. Macrophages contain bactericidal ingredients to kill bacteria. Phagosome membrane secreted enzymes that form oxidants such as hydrogen peroxide (H2O2), superoxide (O2-) and hydroxyl ions (OH−). Myeloperoxidase has been one of the lysosomal enzymes that can catalyze the reaction between H2O2 and chloride ions to form hypochlorite is bactericidal [38].
Medium-chain fatty acids contained in VCO might have a role in enhancing phagocytic function. Essential fatty acids contained in the VCO thought to play a role in improving the ability of macrophage cell phagocytosis. Phagocytosis process has been influenced by the physical and chemical state of phagocytes and foreign particles. Called humoral components of the complement opsonin is major factor phagocytosis. Opsonin is a component of blood serum, blanketing the surface of the foreign agent. Several factors promote avoidance of phagocytosis by PMN leucocytes. Clumping factor A (ClfA) was a fibrinogen-binding surface protein of S. aureus that is an important virulence factor in several infection models. Structural and functional analysis has identified four distinct classes of surface proteins, of which microbial surface component recognizing adhesive matrix molecules (MSCRAMMs) are the largest class. These surface proteins have numerous functions, including adhesion to and invasion of host cells and tissues, evasion of immune responses and biofilm formation. Thus, cell wall-anchored proteins are essential virulence factors for the survival of S. aureus in the commensal state and during invasive infections, and targeting them with vaccines could combat S. aureus infections [39].
5. Conclusion
The results presented in this study concluded that the VCO can inhibit the growth of S. aureus and increase the ability of phagocytic immune cells against S. aureus originated from PE goat milk. Virgin coconut oil might be used as an alternative to antibiotics and used as a modulator of the cellular immune system.
Declarations
Author contribution statement
Desy Cahya Widianingrum: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Cuk Tri Noviandi: Analyzed and interpreted the data.
Siti Isrina Oktavia Salasia: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Ministry of Research, Technology and Higher Education of the Republic of Indonesia, No. 280/LPPM/2015 and No. 1703/UN1/DITLIT/DIT-LIT/LT/2018.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Almeida L.M.D., De Almeida M.Z.P., Mendonça C.L.D., Mamizuka E.M. Comparative analysis of agr groups and virulence genes among subclinical and clinical mastitis Staphylococcus aureus isolates from sheep flocks of the Northeast of Brazil. Braz. J. Microbiol. 2013;44(2):493–498. doi: 10.1590/S1517-83822013000200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purnomo A., Khusnan Hartatik, Salasia S.I.O., Soegiyono Isolation and characterization of Staphylococcus aureus of milk of Etawa crossbred Goat. Media Ked. Hewan. 2006;22(3):142–147. http://journal.unair.ac.id/download-fullpapers-MKH-22-3-26.pdf [Google Scholar]
- 3.Windria S., Widianingrum D.C., Salasia S.I.O. Identification of Staphylococcus aureus and coagulase negative Staphylococci isolates from mastitis milk of Etawa Crossbred Goat. Res. J. Microbiol. 2016;11:11–19. [Google Scholar]
- 4.Zhao Y., Liu H., Zhao X., Gao Y., Zhang M., Chen D. Prevalence and pathogens of subclinical mastitis in dairy goats in China. Trop. Anim. Health Prod. 2015;47(2):429–435. doi: 10.1007/s11250-014-0742-y. [DOI] [PubMed] [Google Scholar]
- 5.Sharma N., Rho G.J., Hong Y.H., Kang T.Y., Lee H.K., Hur T., Jeong D.K. Bovine mastitis: an Asian perspective. J. Anim. Vet. Adv. 2012;7(6):454–476. [Google Scholar]
- 6.Kuroda M., Ohta T., Uchiyama I., Baba T., Yuzawa H., Kobayashi I., Cui L., Oguchi A., Aoki K.I., Nagai Y., Lian J. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. The Lancet. 2001;357(9264):1225–1240. doi: 10.1016/s0140-6736(00)04403-2. [DOI] [PubMed] [Google Scholar]
- 7.Laupland K.B., Church D.L., Mucenski M., Sutherland L.R., Davies H.D. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J. Infect. Dis. 2003;187(9):1452–1459. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]
- 8.Hu W., Li L.C., Dai J., Cui H., Lin L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA) Ind. Crop. Prod. 2019;130:34–41. [Google Scholar]
- 9.Alian F., Rahimi E., Shakerian A., Momtaz H., Riahi M., Momeni M. Antimicrobial resistance of Staphylococcus aureus isolated from bovine, sheep and goat raw milk. Glob. Vet. 2012;8(2):111–114. [Google Scholar]
- 10.Widianingrum D.C., Windria S., Salasia S.I.O. Antibiotic resistance and methicillin resistant Staphylococcus aureus isolated from bovine, Crossbred Etawa goat and human. Asian J. Anim. Vet. Adv. 2016;11:122–129. [Google Scholar]
- 11.Sharifi-Rad M., Ozcelik B., Altın G., Daşkaya-Dikmen C., Martorell M., Ramirez-Alarcon K., Alarcón-Zapata P., Morais-Braga M.F.B., Carneiro J.N., Leal A.L.A.B., Coutinho H.D.M., Gyawali R., Tahergorabi R., Ibrahim S.A., Sahrifi-Rad R., Sharopov F., Salehi B., Contreras M.D.M., Segura-Carretero A., Sen S., Acharya K., Sharifi-Rad J. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018;80:242–263. [Google Scholar]
- 12.Mishra A.P., Saklani S., Salehi B., Parcha V., Sharifi-Rad M., Milella L., Iriti M., Sharifi-Rad J., Srivastava M. Satyrium nepalense, a high altitude medicinal orchid of Indian Himalayan region: chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. 2018;64(8):35–43. [PubMed] [Google Scholar]
- 13.Salehi B., Valussi M., Jugran A.K., Martorell M., Ramírez-Alarcóne K., Stojanović-Radić Z.Z., Antolak H., Kręgiel D., Mileski K.S., Sharifi-Rad M., Setzer W.N., Cádiz-Gurrea M.D.L.L., Segura-Carretero A., Şener B., Sharifi-Rad J. Nepeta species: from farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018;80:104–122. [Google Scholar]
- 14.Lin L., Mao X., Sun Y., Cui H. Antibacterial mechanism of artemisinin/beta-cyclodextrins against methicillin-resistant Staphylococcus aureus (MRSA) Microb. Pathog. 2018;118:66–73. doi: 10.1016/j.micpath.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Lin L., Gu Y., Cui H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf. 2019;19:86–93. [Google Scholar]
- 16.Lee B.Y., Singh A., David M.Z., Bartsch S.M., Slayton R.B., Huang S.S., Zimmer S.M., Potter M.A., Macal C.M., Lauderdale D.S., Miller L.G., Daum R.S. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) Clin. Microbiol. Infect. 2013;19(6):528–536. doi: 10.1111/j.1469-0691.2012.03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui H., Bai M., Sun Y., Abdel-Samie M.A., Lin L. Antibacterial activity and mechanism of Chuzhou chrysanthemum essential oil. J. Funct. Foods. 2018;48:159–166. [Google Scholar]
- 18.Zentek J., Ferrara F., Pieper R., Tedin L., Meyer W., Vahjen W. Effects of dietary combinations of organic acids and medium chain fatty acids on the gastrointestinal microbial ecology and bacterial metabolites in the digestive tract of weaning piglets. J. Anim. Sci. 2013;91(7):3200–3210. doi: 10.2527/jas.2012-5673. [DOI] [PubMed] [Google Scholar]
- 19.Tangwatcharin P., Khopaibool P. Activity of virgin coconut oil, lauric acid or monolaurin in combination with lactic acid against Staphylococcus aureus. Southeast Asian J. Trop. Med. Public Health. 2012;43(4):969–985. https://www.ncbi.nlm.nih.gov/pubmed/23077821 [PubMed] [Google Scholar]
- 20.Shilling M., Matt L., Rubin E., Visitacion M.P., Haller N.A., Grey S.F., Woolverton C.J. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J. Med. Food. 2013;16(12):1079–1085. doi: 10.1089/jmf.2012.0303. [DOI] [PubMed] [Google Scholar]
- 21.Yassen L.T., Khelkal I.N. Effect of some fatty acids on virulence factors of Proteus mirabilis. Int. J. Adv. Biol. Res. 2015;5(2):108–117. http://www.scienceandnature.org/IJABR_Vol5(2)2015/IJABR_V5(2)15-3.pdf [Google Scholar]
- 22.Salasia S.I.O., Khusnan Z., Lämmler C., Zschock M. Comparative studies on pheno- and genotypic properties of Staphylococcus aureus isolated from bovine subclinical mastitis in central Java in Indonesia and Hesse in Germany. J. Vet. Sci. 2004;5:103–109. https://www.ncbi.nlm.nih.gov/pubmed/15192336 [PubMed] [Google Scholar]
- 23.Salasia S.I.O., Tato S., Sugiyono N., Ariyanti D., Prabawati F. Genotypic characterization of Staphylococcus aureus isolated from bovines, humans and food in Indonesia. J. Vet. Sci. 2011;12:353–361. doi: 10.4142/jvs.2011.12.4.353. https://www.ncbi.nlm.nih.gov/pubmed/22127020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straub J.A., Hertel C., Hammers W.P. A 23S rRNA- targeted polymerase chain reaction base system for detection of staphylococcus aureus in meat starter cultures and dairy products. J. Food Prot. 1999;62:1150–1156. doi: 10.4315/0362-028x-62.10.1150. https://www.ncbi.nlm.nih.gov/pubmed/10528718 [DOI] [PubMed] [Google Scholar]
- 25.Brakstad O.G., Aasbakk K., Maeland J.A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 1992;30:1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC265359/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hookey J.V., Richardson J.F., Cookson B.D. Molecular typing of Staphylococcus aureus based on PCR restriction fragment length polymorphism and DNA sequence analysis of the coagulase gene. J. Clin. Microbiol. 1998;36:1083–1089. doi: 10.1128/jcm.36.4.1083-1089.1998. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC104694/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute (CLSI) 2012. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Ninth Edition, M07-A9. 32(2)https://www.researchgate.net/file.PostFileLoader.html?id=564ceedf5e9d97daf08b45a2&assetKey=AS%3A297254750572544%401447882463055 [Google Scholar]
- 28.Duke P.J. Plenum Press; New York: 1990. Modern Microscopies: Techniques and Applications. [Google Scholar]
- 29.Zhang X., Goncalves R., Mosser D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Im. 2008 doi: 10.1002/0471142735.im1401s83. Chapter 14: Unit 14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Furth R., Cohn Z.A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968;128(3):415–435. doi: 10.1084/jem.128.3.415. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2138527/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sihombing N.T., Silalahi J., Suryanto D. Antibacterial activity of aqueous garlic (Allium sativum) extracts and virgin coconut oil and their combination against Bacillus cereus ATCC 14579 and Escherichia coli ATCC 8939. Int. J. Pharm. Tech. Res. 2014;6(5):2774–2782. https://scinapse.io/papers/2283119977 [Google Scholar]
- 32.Enig Mary G. 2019. Coconut: in Support of Good Health in the 21st Century.http://coconutoil.com/coconut_oil_21st_century Full story: [Google Scholar]
- 33.Kappally S., Shirwaikar A., Shirwaikar A. Coconut oil–a review of potential applications. Hygeia J. Drugs Med. 2015;7(2):34–41. [Google Scholar]
- 34.Huang W.C., Tsai T.H., Chuang L.T., Li Y.Y., Zouboulis C.C., Tsai P.J. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: a comparative study with lauric acid. J. Dermatol. Sci. 2014;73(3):232–240. doi: 10.1016/j.jdermsci.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Flannagan R.S., Jaumouillé V., Grinstein S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 36.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6(12):1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abuaita B.H., Burkholder K.M., Boles B.R., O’Riordan M.X. The endoplasmic reticulum stress sensor inositol-requiring enzyme 1α augments bacterial killing through sustained oxidant production. mBio. 2015;6(4):e00705–e00715. doi: 10.1128/mBio.00705-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurst J.K. What really happens in the neutrophil phagosome? Free Radical Biol. Med. 2012;53(3):508–520. doi: 10.1016/j.freeradbiomed.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster T.J., Geoghegan J.A., Ganesh V.K., Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014;12(1):49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]