Summary
The fungus Aspergillus fumigatus is ubiquitous in nature and the most common cause of invasive pulmonary aspergillosis (IPA) in patients with a compromised immune system. The development of IPA in patients under immunosuppressive treatment or in patients with primary immunodeficiency demonstrates the importance of the host immune response in controlling aspergillosis. However, study of the host-microbe interaction has been hampered by the lack of tools for their non-invasive assessment. We developed a methodology to study the response of the host's immune system against IPA longitudinally in vivo by using fluorine-19 magnetic resonance imaging (19F MRI). We showed the advantage of a perfluorocarbon-based contrast agent for the in vivo labeling of macrophages and dendritic cells, permitting quantification of pulmonary inflammation in different murine IPA models. Our findings reveal the potential of 19F MRI for the assessment of rapid kinetics of innate immune response against IPA and the permissive niche generated through immunosuppression.
Subject Areas: Infection Control in Health Technology, Medical Imaging, Mycology
Graphical Abstract
Highlights
-
•
Host-pathogen immune response is visualized in vivo and quantified against IPA
-
•
Modified PFC-based nanoparticles were used for in vivo labeling of immune cells
-
•
Clinical immunosuppression depict dynamic immune response upon fungal challenge
-
•
19F MRI showed follow-up of labeled immune cells in individual animals over time
Infection Control in Health Technology; Medical Imaging; Mycology
Introduction
Aspergillus fumigatus is an opportunistic, potentially life-threatening fungus, which thrives mainly on organic substrates like decaying vegetation in the soil or food. Although environmental exposure of humans to the airborne A. fumigatus conidia is common, host-pathogen interactions effectively eradicate conidia from the pulmonary region of healthy individuals (Margalit and Kavanagh, 2015, Mccormick et al., 2010). The key determinant of infection is thought to be the innate immune response. A. fumigatus conidia in the alveolar space of lungs trigger pathogen recognizing receptors (PRRs), driving the first responders of the immune system (Dagenais and Keller, 2009). Key cellular mediators of immunity include resident alveolar macrophages, monocytes and dendritic cells for the engulfment of conidia, and neutrophils for the destruction of hyphae using neutrophil extracellular traps (Khanna et al., 2016, Roilides et al., 1998, Sales-campos et al., 2013, Zhang et al., 2019).
Healthy individuals effectively clear A. fumigatus, whereas infection becomes life-threatening in immunocompromised patients. With an increasing number of immunocompromised patients from organ transplantation or cancer treatment, invasive pulmonary aspergillosis (IPA) is rapidly growing as a medical problem (Berenguer et al., 1995). The acute inflammation in the lungs of patients with IPA suggests an underlying malfunction, rather than absence, of essential host immune components as the causative factor (Krenke and Grabczak, 2011). The modus operandi for the clinical use of immunosuppressive drugs mainly includes cyclophosphamide (CY) (Emadi et al., 2009) and hydrocortisone acetate (HCA) (Garth and Steele, 2017, Shaikh et al., 2012) administered intravenously to the patients. In previous studies, it was shown that the pathophysiology of IPA and the immune response against the fungal infection differs for each compounds (Dagenais and Keller, 2009, Stephens-Romero et al., 2005). Corticosteroids treatment impairs phagocyte function, including an abnormality in cellular migration and production of the inflammatory cytokines, while leaving neutrophils intact and functional (Brummer et al., 2001, Kamberi et al., 2002, Nawada et al., 1996). The phagocytic defect permits infection growth, which in turn drives a massive recruitment of neutrophils to the site of infection, resulting in intensive tissue damage. By contrast, CY induces neutropenia and depletes other circulating white blood cells, while leaving the local innate immune response relatively intact. Here the neutropenia is thought to be critical in permitting hyphal growth and further invasion in the tissue (Jones et al., 2019, Kalleda et al., 2016). A key limitation of these conclusions, however, is the reliance on invasive methods that are restricted to single time point measurements, such as immunohistochemistry (Balloy et al., 2005, Wang et al., 2017). Knowledge of immune kinetics and longitudinal disease progression is currently lacking but is essential for understanding the dynamics of these processes (Kalleda et al., 2016). In vivo imaging techniques are potentially able to assess the host response against the infection longitudinally in individual animals.
Different imaging techniques have been used in preclinical models to characterize IPA, such as computed tomography, positron emission tomography, bioluminescence imaging, single photon emission tomography, proton magnetic resonance imaging (1H MRI), and fibered confocal fluorescence microscopy (Brock et al., 2008, Poelmans et al., 2016, Poelmans et al., 2018, Rolle et al., 2016, Vanherp et al., 2018, Wang et al., 2013). Although these approaches fulfill the non-invasive and longitudinal criteria required, they lack specific information on inflammatory processes occurring in the host. In other disease models, ex vivo and in vivo cell labeling approaches use 1H MRI contrast agents to visualize immune cells for studying various inflammatory processes (De Temmerman et al., 2014, Ho and Hitchens, 2004, Schwarz et al., 2012, Wu et al., 2006). However, 1H MRI contrast agents such as (super)paramagnetic nanoparticles generate unspecific signal voids, making it difficult to locate and quantify labeled cells in vivo. Fluorine contrast agents in combination with 19F MRI may provide an alternative, with specific and quantifiable contrast (Ebner et al., 2010, Srinivas et al., 2010a, Zhong et al., 2015).
19F MRI is an emerging non-invasive tool, which can be applied both for imaging of ex vivo contrast agent labeled cells after their transplantation and for in vivo labeling of cells after systemic administration of fluorinated contrast agents (Jacoby et al., 2014b, Srinivas et al., 2012, Srinivas et al., 2010b). Using fluorinated contrast agents such as perfluoro-15-crown-5-ether nanoparticles (PFCE-NPs) in combination with 19F MRI, one can generate highly specific MR contrast, owing to the lack of background signal. Overlaying the 19F MR image with a conventional 1H MR image provides the necessary anatomical background (Liang et al., 2018).
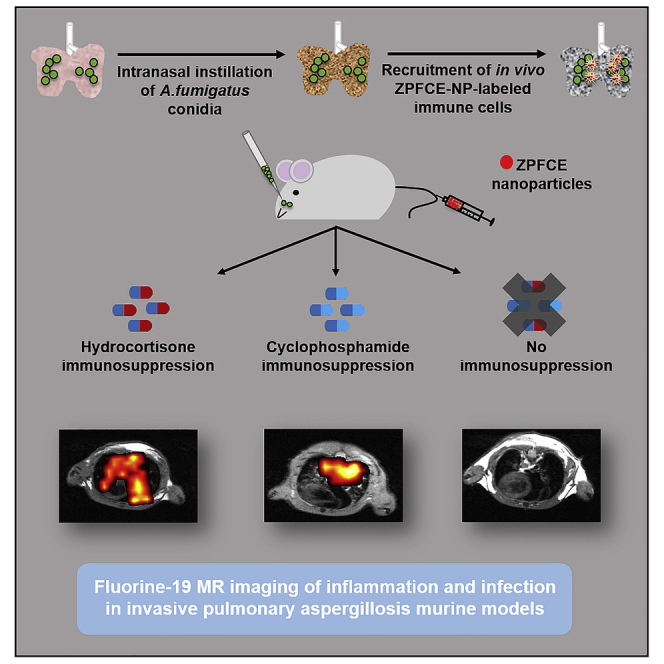
Here, we developed an imaging platform allowing non-invasive and longitudinal quantification of the degree of pulmonary inflammation in IPA murine models. We showed that the in vivo labeling of immune cells with newly developed zonyl perfluoro-15-crown-5-ether nanoparticles (ZPFCE-NPs) reveal underlying pathophysiological events during acute IPA using 19F MRI.
Results
Small-Sized Biocompatible ZPFCE-NPs Showed Efficient In Vitro Labeling of Murine Phagocytes
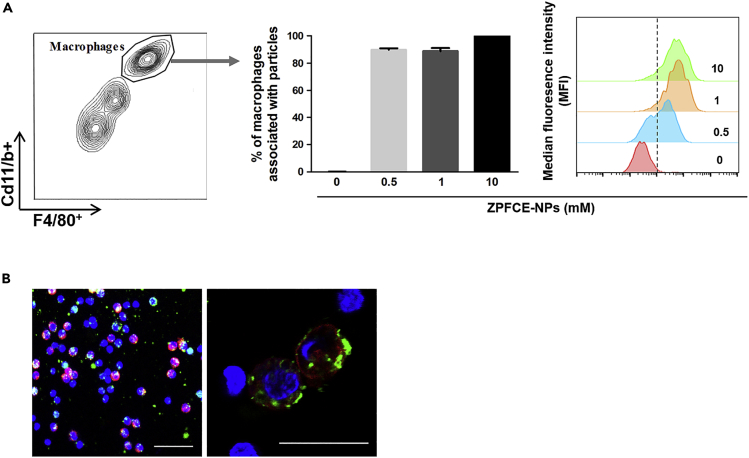
To monitor the immune responses in vivo, we sought to exploit the functional property of phagocytosis for immune cells labeling. ZPFCE-NPs label professional phagocytic cells owing to their small size (Waiczies et al., 2011). To validate the feasibility of this strategy, Macrophages, identified by their characteristic high surface expression of CD11b and F4/80, successfully phagocytosed ZPFCE-NPs in a dose-dependent manner (Figure 1). ZPFCE-NP labeling of macrophages showed similar labeling efficiencies for particle concentrations of 1 and 10 mM (Figure 1A). We used ZPFCE-NPs incorporated with Cholesteryl BODIPY FLC 12 green fluorescent dye. These nanoparticles did not show nanotoxicity in primary macrophages upon labeling with relatively high concentrations, affirming their suitability for in vivo applications (Figure S1).
Figure 1.
ZPFCE-NPs Allow Labeling of Macrophages
(A) Labeling of macrophages (positive cells for F4/80 and CD11b surface marker) with ZPFCE-NPs was measured in terms of percentage uptake at variable doses of particles. Median fluorescence intensities were measured from the gated ZPFCE-NP-labeled macrophages for each dose (mean ± SD).
(B) Confocal image showing macrophages stained by F4/80 surface (red) with the ZPFCE-NPs (green), magnified representative images show intracellular uptake of ZPFCE-NPs. Scale bar is 20μm.
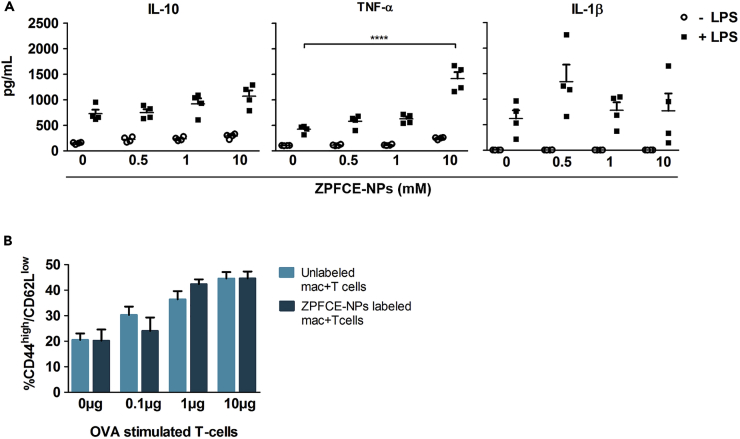
A key requirement for an in vivo labeling protocol is that the label does not interfere with the biological processes being measured. To investigate whether ZPFCE-NP labeling modulates macrophage function, we tested key innate and adaptive functions in vitro. Using cytokine secretion as a readout for innate functional activation of macrophages, we found no impact of ZPFCE-NPs on spontaneous or lipopolysaccharide (LPS)-induced immune activation at a dose of 1 mM and only a weak enhancement of LPS-induced TNF-alpha production at 10 mM (Figure 2A). For the adaptive immune system, we tested the biological effect of ZPFCE-NPs labeling on antigen-presentation by macrophages. C57BL/6 peritoneal macrophages were pre-labeled with ZPFCE-NPs and pulsed with OVA peptide (OVA323-339) before co-culture with OVA-reactive OT-II TCR transgenic CD4+ T cells. OT-II T cells showed efficient activation when primed with OVA-loaded macrophages, which was unaltered by the pre-loading with ZPFCE-NPs (Figure 2B). Together, these results demonstrate that 1 mM ZPFCE-NPs allow macrophage labeling without inducing biological alterations to either the innate or adaptive functions of macrophages.
Figure 2.
ZPFCE-NPs Allow Labeling of Murine Macrophages without Modulating Their Innate and Adaptive Immune Function
(A) Cytokine measurements performed on supernatants from ZPFCE-labeled macrophages in the presence and absence of LPS. Data represent mean ± SEM (n = 5, ****p < 0.0001).
(B) C57BL/6 peritoneal macrophages were pre-loaded with ZPFCE-NPs and pulsed with OVA peptide (OVA323-339) at different concentrations, before co-culture with OT-II TCR transgenic CD4+ T cells. After 3 days of co-culture, the cells were stained for lineage T cell markers in combination with T cell activation markers and analyzed by flow cytometry. The percentage of CD44highCD62Llow activated T cells across variable doses of the OT-II peptide, for ZPFCE-NP-labeled and unlabeled macrophages (mean ± SEM).
19F MRI Allows In Vivo Visualization and Quantification of Immune Cell Recruitment in A. fumigatus-Infected Lungs
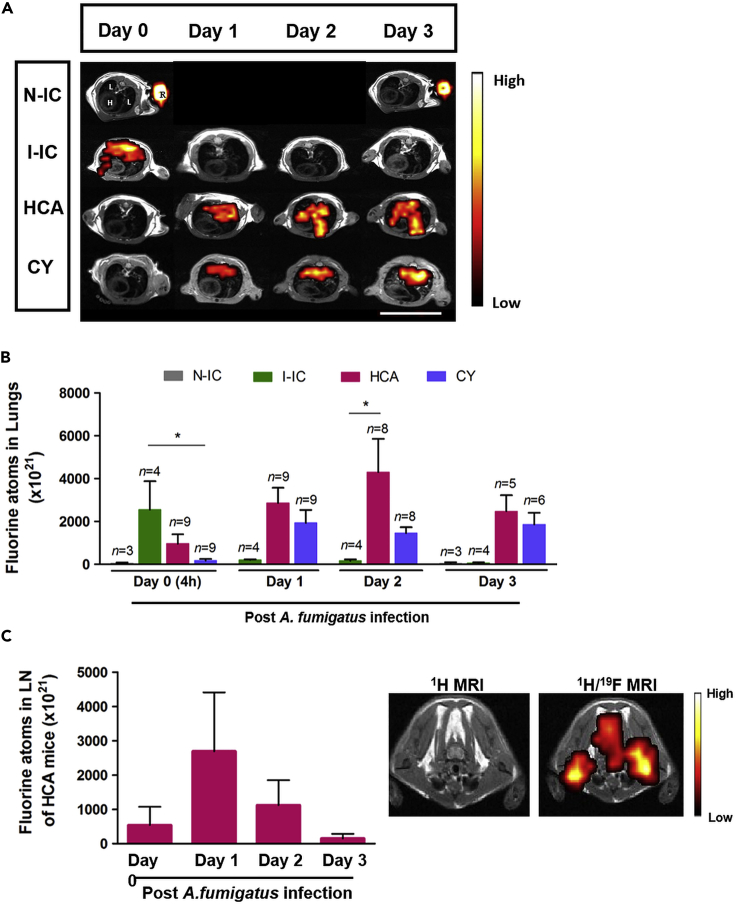
Having validated ZPFCE-NPs as an efficient and biologically neutral contrast agent for macrophages, we sought to assess the in vivo utility using IPA mouse models to apply our methodology as a proof of principle. To test the robustness of our immunomonitoring method, we used three models of pulmonary aspergillosis with immunocompetent and immunosuppressed mice, together with non-infected mice as control. CY or HCA immunosuppressive drugs were used to induce neutropenia and phagocytic dysfunction in mice, respectively. Immunocompetent mice infected with A. fumigatus demonstrated a large and rapid influx of macrophages into the lung within 4 h of infection (Figure 3A, second row and 3B). Inflammation was quickly resolved, with a return to near-baseline macrophage levels by 24 h (Figure 3B). By contrast, both forms of immunosuppression sharply reduced the immediate innate response to infection, with poor influx at 4 h (Figure 3A, third and fourth row and 3B). In both cases, this defect in the immediate response corrected with a more chronic inflammatory signal, with large macrophage influx out to at least 3 days post infection (Figure 3B), consistent with a model where the defective immediate response allowed infection to become invasive and chronic. We observed and quantified a higher fluorine MRI signal intensity in the HCA mice as compared with the CY mice groups at the site of inflammation post pulmonary infection. No detectable fluorine signal was observed from the non-infected immunocompetent mice (N-IC).
Figure 3.
19F MRI Identifies the Differential Local Immune Response to Infection by A. fumigatus in Immunocompromised Murine Hosts
(A) 19F MR images (fluorine signal was superimposed on anatomical 1H MR images) were obtained from HCA, CY, and infected-immunocompetent (I-IC) mice as well as non-infected control mice (N-IC) on day 0 (4 h post infection). All mice received systemic injection of ZPFCE-NPs on day 0 (4 h post infection) and day 1. All infected mice were imaged daily (1H and 19F MRI). The non-infected immunocompetent (N-IC) group was followed up on day 0 and day 3. Labels for different organs (image top left) include H, heart; L, lungs; and R, reference containing 30 mM ZPFCE-NP. Scale bar is 2.6 cm.
(B) Quantification of the 19F MR signal from the lung region was performed for all groups by comparing the signal intensity of the lung region with a reference (R in (A) top left image) containing 30 mM ZPFCE-NPs. Data shown as mean ± SEM (*p < 0.05).
(C) 19F MRI signal was observed from the lymph node region only for mice from the HCA group on day 1. Mean 19F MR signal intensity was quantified with respect to the 30 mM reference placed next to each animal. 19F MRI signal in lymph nodes is indicated as hot spots overlaid over the anatomical 1H MR image (right panel). Data shown as mean ± SEM (*p < 0.05).
Key differences were also observed between the immunosuppressed groups, with HCA-treated mice but not CY-treated mice, resulting in a transient flux of macrophages into the cervical lymph nodes on day 1 post infection (Figure 3C). This indicates that HCA allows macrophage mobilization but diverts recruitment into the draining lymph node rather than into the tissue.
To monitor progression of infection, we applied a cumulative scoring of 1H MR images based on the lung signal intensity in all murine groups. This shows the pathophysiological changes occurring over time following infection from day of infection (day 0) until day 3 (Figure S2A). We have observed high signal intensities in the CY group where infection was more profound compared with the HCA group (Figure S2B). Together, these results both validate 19F MRI as an in vivo monitoring tool for anti-microbe responses and indicate a critical window of response for the innate immune system against A. fumigatus invasive infection.
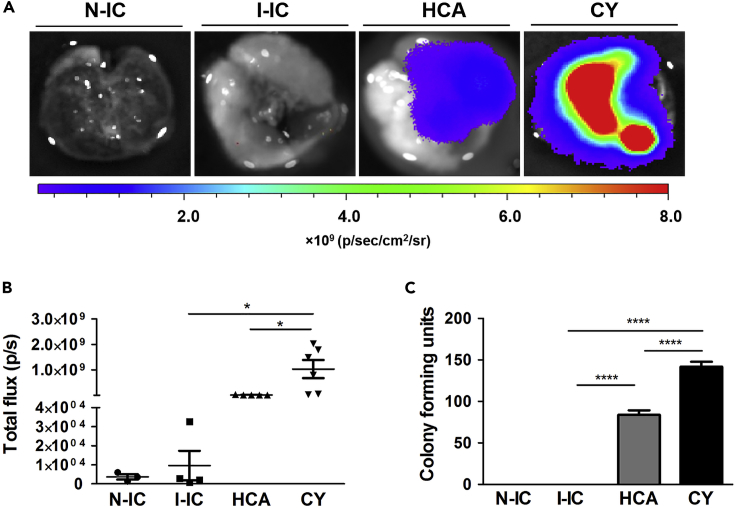
Distinctive Fungal Burden Depicted by Bioluminescent Imaging and Colony-Forming Units Confirms Infection
To affirm infection and viable pulmonary fungal load with inflammatory processes that we have monitored by 19F MRI, we have examined the Fluc+ A. fumigatus infection 3 days after infection by using ex vivo bioluminescent imaging (BLI). After D-luciferin administration in the lungs, CY-treated mice showed higher BLI signal intensity compared with the hydrocortisone-treated mice (Figure 4A). No detectable bioluminescence signal was observed from the lungs in the two control groups, infected immunocompetent and non-infected mice. Quantification of BLI signal also showed significantly high fungal infection in the CY-treated group compared with the hydrocortisone-treated group and infected-immunocompetent group (Figure 4B). This indicates strong invasion of fungi in the lungs of the CY group owing to lack of an efficient immune response compared with the HCA group, where the immune response prevents the growth of A. fumigatus.
Figure 4.
Visualization and Quantification of Fungal Load Reveals the Impaired Immune Response against A. fumigatus Invasion in Immunocompromised Mice
(A) Ex vivo bioluminescent imaging (BLI) was performed to visualize firefly luciferase (Fluc)-expressing A. fumigatus in the lungs 3 days after the infection. After the endpoint 19F MRI experiment, animals were sacrificed and D-luciferin was administered into the excised lungs of murine groups of mice. Lungs were imaged immediately after D-luciferin administration. The scale bar represents BLI signal intensity in photons flux/second. The color-coded BLI images are overlaid onto the photographic images of lungs. Intensity thresholds for all BLI images were kept the same.
(B) BLI signal intensity from all murine groups was measured as total flux after assigning identical regions of interest on the BLI images of lungs.
(C) Lungs were isolated 3 days after infection from all mice groups. Colony-forming units (CFUs) were manually counted from lung homogenates 24 h after incubation at 37°C. No fungi were observed in the two control groups (N-IC and I-IC). Data are represented as mean ± SEM (*p < 0.05, ****p < 0.0001). HCA n = 5, CY n = 6, I-IC n = 4, N-IC n = 3.
For the quantification of pulmonary fungal load, we performed colony-forming unit (CFU) counting on the cultured lung homogenates from all mice groups. We observed a significant increase in the A. fumigatus burden in the lungs of the CY group when compared with the HCA on day 3 (Figure 4C). In contrast, N-IC and I-IC groups did not show any fungal growth. These results together with 19F MRI suggest the early immune activation in infected mice as a critical aspect for the control of potentially invasive A. fumigatus progression.
Validation of ZPFCE-NP-Labeled Immune Cell Recruitment in the Lungs and Lymph Nodes by Histology and Immunofluorescence Imaging
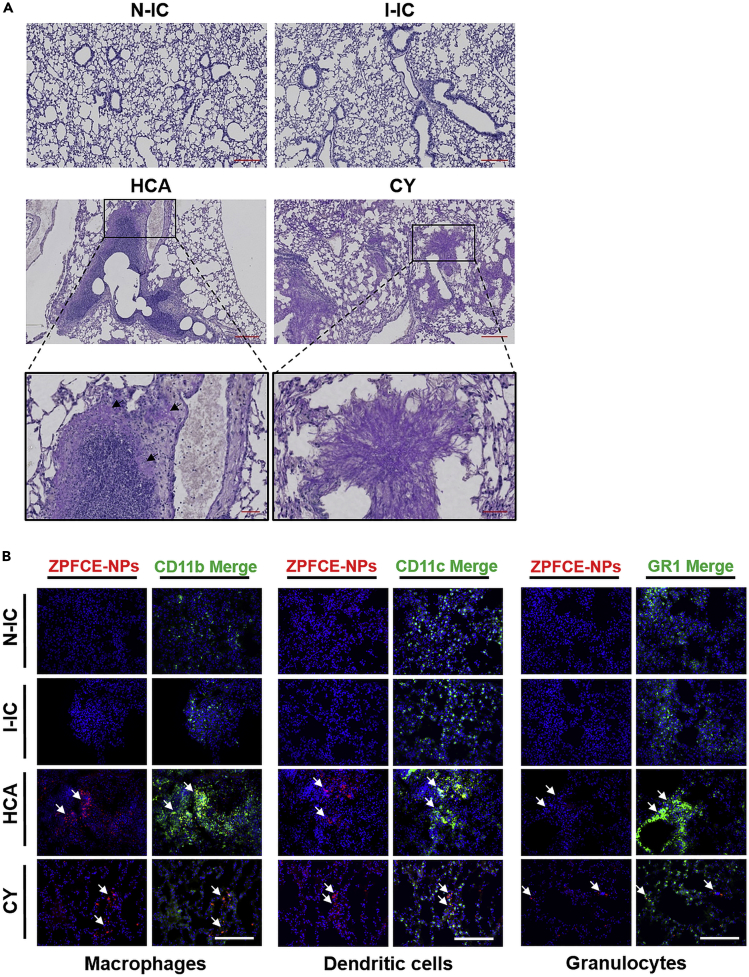
To validate our imaging results, we performed periodic acid-Schiff (PAS) staining and immunofluorescence after sacrificing the animals 3 days after infection. Similar to 19F MRI, we observed high pulmonary inflammation in the lung tissue of the HCA group upon A. fumigatus challenge with minimal fungal invasion. In contrast, the CY group, showed as expected fungal growth and hyphal growth formation with invasion in nearby tissues and no visible inflammation (Figure 5A). The two control N-IC and I-IC mice groups showed normal lung tissue morphology with no fungal infection on day 3, validating the 19F MRI findings.
Figure 5.
Histological Imaging Elucidates Diverse Infection and Inflammation Patterns in Immunocompromised Groups
(A) Representative light microscopy of PAS-stained images showing histopathology from the PFA fixed lung sections of different mice groups on day 3 post infection. Excessive immune cell infiltration near the bronchi and bronchiole upon pulmonary A. fumigatus infection in HCA mice showing profound inflammation-induced tissue destruction. Mild fungal infection observed in HCA mice (black arrows, HCA magnified image). Fungal dissemination shown in the CY mice resulted in compression of lung tissue with massive hyphal growth (magnified CY image). Scale bar is 200 μm for the first and second rows and 50 μm for magnified images (third row).
(B) Immunofluorescence microscopy performed on fresh-frozen lung tissues showing the ZPFCE-NP-labeled macrophages (anti CD11b) and dendritic cells (anti CD11c) recruited to the bronchioles (white arrows) in the lungs of HCA and CY mice. Granulocytes (anti-GR1) were also found to be accumulated (white arrows) in high numbers in the HCA group compared with other groups. Scale bar is 100 μm, and staining represented as Blue = DAPI, Red = ZPFCE-NPs.
Immunofluorescence images also showed the presence of stringent inflammation resulting in higher influx of ZPFCE-NP-labeled macrophages and dendritic cells in the lungs of the HCA group in contrast to the CY group (Figure 5B, third and fourth row). Additionally, elevated recruitment of granulocytes was observed near the airways of the HCA group compared with other groups. The N-IC and I-IC groups showed no visible inflammation or infection similar to 19F MRI (Figure 5B, first and second row). We also noticed differences in white blood cell counts, analyzed individually from the peripheral blood of different groups on day 3, indicating severe inflammation in the HCA group reflected by an increase in the number of neutrophils and lymphocytes in the blood in contrast to the CY group (Figure S3). The non-infected model showed similar levels of neutrophils and lymphocytes as the I-IC model where immune cells were in the normal range. Notably, the cervical lymph nodes of the HCA group showed high ZPFCE-NPs accumulation (Figure S4). ZPFCE-NPs were also visualized in the OCT (optimum cutting temperature)-embedded lungs on day 3 by ex vivo fluorescence imaging illustrating high fluorescent signal observed both in the lungs and cervical lymph nodes, only in the HCA group (Figure S5). Briefly, these results strongly support the 19F MRI findings, demonstrating the feasibility of our established methodology for non-invasive monitoring of infection.
Discussion
With the increased number of immunocompromised patients, it becomes more important to closely monitor those patients, diagnose, and follow up IPA. Among the profound number of clinical IPA cases, 90% are caused by A. fumigatus (Lin et al., 2001, Massam et al., 2011). For a better understanding of aspergillosis and for testing of novel antifungal compounds, preclinical animal models are essential. Although methods for monitoring the dynamics of the immune cells upon A. fumigatus infection have been developed recently (Kalleda et al., 2016), these studies were not able to assess the interaction with the host's immune system longitudinally in vivo. Here, we have developed an approach that allows the non-invasive, dynamic monitoring of both inflammatory processes and the infection in three different animal models of IPA.
Several cell-labeling approaches have been established to non-invasively visualize the mechanisms of immune reactions involved in various diseases by tracking the loci of inflammatory immune cells using 19F MRI (Ebner et al., 2010, Flogel et al., 2008, Jacoby et al., 2014a, Stoll et al., 2012). In this study, we focused on the quantification and localization of inflammation in IPA murine models of immune impairments induced by clinically used immunosuppressive drugs (Balloy et al., 2005, Stergiopoulou et al., 2007, Woodruff and Hebert, 2002). PFCs have been used and tested as blood substitutes and thus proven to be safe in humans (Janjic and Ahrens, 2009, Ruiz-Cabello et al., 2011). Here, we synthesized ZPFCE-NPs and studied their potential for the labeling of phagocytic immune cells. It has been shown that labeling with PFCE particles of different sizes could potentially modulate the immune function of dendritic cells (Waiczies et al., 2011). In this study, we showed the biological compatibility of ZPFCE-NPs for in vivo studies, where labeled macrophages retained their antigen processing and T cell activation capacity. Compared with most other PFCs, ZPFCE-NPs have a reduced size of ∼280 nm (Dewitte et al., 2013).In our in vivo study, we have used 19F MRI to quantify inflammation non-invasively and longitudinally after systemic injections of ZPFCE-NPs. The 19F MRI signal detected in inflamed areas corresponds to the infiltration of labeled phagocytic cells in the region of interest (Flogel et al., 2008, van Heeswijk et al., 2015). We observed high 19F MRI signal intensities in the lungs of the infected immunocompetent (I-IC) mice already 4 h after the fungal infection, which was completely cleared after 24 h, confirming the expected eradication of A. fumigatus conidia by the immune system. In the hydrocortisone (HCA)-treated mice, the exacerbated intrusive recruitment of immune cells resulted in the labeling of not only tissue-resident macrophages but also dendritic cells (Temme et al., 2012). Inflammation was found to be less pronounced in the CY-treated mice, with increased fungal invasion in the lungs. We did not observe any detectable fluorine signal in the lungs from the non-infected immunocompetent (N-IC) mice that have received ZPFCE-NPs, indicating absence of any inflammation in the lungs. Only in HCA mice, elevated accumulation of ZPFCE-NPs was observed in the nearby cervical lymph nodes to 3 days after infection as shown in the 19F MR images.
In addition to 19F MRI, in vivo 1H MRI in these mouse models was able to document the lesion development occurring in the lungs upon fungal conidia challenge. As shown by our quantitative image analysis, we observed high lung lesion formation in CY mice two and three days after infection, which is consistent with profound fungal hyphal growth and invasion in lungs as reported before (Poelmans et al., 2016). 1H MRI indicated only weak lesion formation in the infected HCA-treated and I-IC groups. These results endorse the fact that the administration of CY as a frequently used immunosuppressive drug can lead to lethal IPA with mild initial inflammation in the lungs as indicated by 19F MRI. On the contrary, hydrocortisone-based immunosuppression leads to less lesion formation by A. fumigatus but triggers acute inflammation leading to potentially lethal tissue destruction (Kalleda et al., 2016). The functional immune system of the infected-immunocompetent mice resulted in the rapid initial immune reaction and complete clearance of the fungi as shown in the 1H MR images and also confirmed by histology. By using 19F MRI, we were able to monitor the intricate dynamic profile of the host-pathogen interaction in pulmonary A. fumigatus infection in vivo. We documented the differences between immunocompetent hosts and animals treated with two different immunosuppressive compounds. With clinically safe contrast agents like perfluorocarbon (PFC)-based fluorinated nanoparticles, 19F MRI not only proved to be a powerful imaging modality for numerous preclinical studies but also showed potential for translation to humans. For immune cell imaging, 19F MRI has developed into a robust method for the follow-up of phagocytic cells with less success in the tracking of non-phagocytic cells owing to the sensitivity issues of 19F MRI (Saini et al., 2019).
In summary, we demonstrated the potential of 19F MRI and perfluorocarbon-based ZPFCE-NPs by successful tracking and quantifying the fluorine signal generated by innate immune cells, macrophages, and dendritic cells in the lungs corresponding to the intensity of local inflammation. By being able to monitor both the infection and immune reaction in live animals over time, it is possible to make treatment decisions rapidly and almost in real time. In the future, this will help in testing transgenic fungal strains, novel antifungal drugs, or new approaches to influence the immune system. Overall, it will provide an emerging 19F MRI platform for studying not only basic mechanisms of fungal infections but also advanced immune cell therapies in patients (Ahrens et al., 2014, Amiri et al., 2015, Fink et al., 2018, Hertlein et al., 2013).
Limitations of the Study
Although the PFC-based fluorinated nanoparticles provided specific contrast for immune cell imaging in the preclinical aspergillosis models, 19F MRI is still limited to applications where large cell numbers accumulate locally. For less severe models of inflammation, the sensitivity of 19F MRI needs to be improved by using either contrast agents with higher fluorine load or improved hardware (Khalil et al., 2019) and/or image processing approaches (Liang et al., 2017). In addition, in vivo cell labeling was restricted to phagocytic cells like macrophages. Animal models used in this study were already described by invasive methods. In the future, a comparative experiment using a PRR knockout mouse strain instilled with a transgenic fungus lacking a key PAMP (e.g., melanin or galactosaminoglycan) can be used as a suitable model to study the immune response in differently modulated transgenic animals.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors acknowledge Dr. Bala Attili (Laboratory of Radiopharmaceutical Research, KU Leuven, Belgium) for providing help with the formulation of HCA treatment and Dr. Matthias Brock (University of Nottingham, UK) for providing the Fluc+ A. fumigatus strain 2/7/1. Use of the strain was granted by the Leibniz Institute for Natural Product Research and Infection Biology (Hans Knöll Institute, Jena, Germany). We are grateful for the financial support by the following funding agencies: the European Commission Marie Curie (ITN) BetaTrain (289932), the Research Foundation Flanders (FWO, G.0A75.14, G.0B28.14, and G.069115N), the Agentschap voor Innovatie door Wetenschap en Technologie for the SBO NanoCoMIT (IWT SBO 140061), the European ERA-NET project “CryptoView” (third call of the FP7 programme Infect-ERA), and KU Leuven for PF 10/017 (IMIR).
Author Contributions
Conceptualization, S.S. and U.H.; Methodology, S.S., H.K., J.P., J.L.D., B.B.M., S.J.S., S.L., R.V., I.L., and G.V.V.; Investigation, S.S., J.P., B.B.M., and H.K.; Writing - Original Draft, S.S.; Writing - Review & Editing, S.S., J.P., A.L., C.G., S.C.D., and U.H.; Funding Acquisition, U.H., S.C.D., H.K., G.V.V., I.L., and K.L.; Supervision, H.K. and U.H.
Declaration of Interests
The authors declare no competing interests.
Published: October 25, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.09.022.
Supplemental Information
References
- Ahrens E.T., Helfer B.M., O’Hanlon C.F., Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn. Reson. Med. 2014;72:1696–1701. doi: 10.1002/mrm.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri H., Srinivas M., Veltien A., van Uden M.J., de Vries I.J.M., Heerschap A. Cell tracking using 19F magnetic resonance imaging: technical aspects and challenges towards clinical applications. Eur. Radiol. 2015;25:726–735. doi: 10.1007/s00330-014-3474-5. [DOI] [PubMed] [Google Scholar]
- Balloy V., Huerre M., Latgé J.-P., Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect. Immun. 2005;73:494–503. doi: 10.1128/IAI.73.1.494-503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenguer J., Allende M.C., Lee J.W., Garrett K., Lyman C., Ali N.M., Bacher J., Pizzo P.A., Walsh T.J. Pathogenesis of pulmonary aspergillosis: Granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am. J. Respir. Crit. Care Med. 1995;152:1079–1086. doi: 10.1164/ajrccm.152.3.7663787. [DOI] [PubMed] [Google Scholar]
- Brock M., Jouvion G., Droin-Bergère S., Dussurget O., Nicola M.A., Ibrahim-Granet O. Bioluminescent Aspergillus fumigatus, a new tool for drug efficiency testing and in vivo monitoring of invasive aspergillosis. Appl. Environ. Microbiol. 2008;74:7023–7035. doi: 10.1128/AEM.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Maqbool A., Stevens D.A. In vivo GM-CSF prevents dexamethasone suppression of killing of Aspergillus fumigatus conidia by bronchoalveolar macrophages. J. Leukoc. Biol. 2001;70:868–872. [PubMed] [Google Scholar]
- Dagenais T.R.T., Keller N.P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Temmerman M.-L., Soenen S.J., Symens N., Lucas B., Vandenbroucke R.E., Libert C., Demeester J., De Smedt S.C., Himmelreich U., Rejman J. Magnetic layer-by-layer coated particles for efficient MRI of dendritic cells and mesenchymal stem cells. Nanomedicine. 2014;9:1363–1376. doi: 10.2217/nnm.13.88. [DOI] [PubMed] [Google Scholar]
- Dewitte H., Geers B., Liang S., Himmelreich U., Demeester J., De Smedt S.C., Lentacker I. Design and evaluation of theranostic perfluorocarbon particles for simultaneous antigen-loading and19F-MRI tracking of dendritic cells. J. Control Release. 2013;169:141–149. doi: 10.1016/j.jconrel.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Ebner B., Behm P., Jacoby C., Burghoff S., French B.A., Schrader J., Flögel U. Early assessment of pulmonary inflammation by 19F MRI in vivo. Circ. Cardiovasc. Imaging. 2010;3:202–210. doi: 10.1161/CIRCIMAGING.109.902312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi A., Jones R.J., Brodsky R.A. Cyclophosphamide and cancer: golden anniversary. Nat. Rev. Clin. Oncol. 2009;6:638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- Fink C., Gaudet J.M., Fox M.S., Bhatt S., Viswanathan S., Smith M., Chin J., Foster P.J., Dekaban G.A. 19F-perfluorocarbon-labeled human peripheral blood mononuclear cells can be detected in vivo using clinical MRI parameters in a therapeutic cell setting. Sci. Rep. 2018;8:590. doi: 10.1038/s41598-017-19031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flogel U., Ding Z., Hardung H., Jander S., Reichmann G., Jacoby C., Schubert R., Schrader J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118:140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garth J.M., Steele C. Innate lung defense during invasive aspergillosis: new mechanisms. J. Innate Immun. 2017;9:271–280. doi: 10.1159/000455125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertlein T., Sturm V., Jakob P., Ohlsen K. 19F magnetic resonance imaging of perfluorocarbons for the evaluation of response to antibiotic therapy in a staphylococcus aureus infection model. PLoS One. 2013;8:e64440. doi: 10.1371/journal.pone.0064440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C., Hitchens T.K. A non-invasive approach to detecting organ rejection by MRI: monitoring the accumulation of immune cells at the transplanted organ. Curr. Pharm. Biotechnol. 2004;5:551–566. doi: 10.2174/1389201043376535. [DOI] [PubMed] [Google Scholar]
- Jacoby C., Borg N., Heusch P., Sauter M., Bönner F., Kandolf R., Klingel K., Schrader J., Flögel U. Visualization of immune cell infiltration in experimental viral myocarditis by 19F MRI in vivo. Magn. Reson. Mater. Phys. Biol. Med. 2014;27:101–106. doi: 10.1007/s10334-013-0391-6. [DOI] [PubMed] [Google Scholar]
- Jacoby C., Temme S., Mayenfels F., Benoit N., Krafft M.P., Schubert R., Schrader J., Flögel U. Probing different perfluorocarbons for in vivo inflammation imaging by19F MRI: image reconstruction, biological half-lives and sensitivity. NMR Biomed. 2014;27:261–271. doi: 10.1002/nbm.3059. [DOI] [PubMed] [Google Scholar]
- Janjic J.M., Ahrens E.T. Fluorine-containing nanoemulsions for MRI cell tracking. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:492–501. doi: 10.1002/wnan.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.N., Ellett F., Robertson A.L., Forrest K.M., Judice K., Balkovec J.M., Springer M., Markmann J.F., Vyas J.M., Warren H.S., Irimia D. Bifunctional small molecules enhance neutrophil activities against Aspergillus fumigatus in vivo and in vitro. Front. Immunol. 2019;10:644. doi: 10.3389/fimmu.2019.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalleda N., Amich J., Arslan B., Poreddy S., Mattenheimer K., Mokhtari Z., Einsele H., Brock M., Heinze K.G., Beilhack A. Dynamic immune cell recruitment after murine pulmonary Aspergillus fumigatus infection under different immunosuppressive regimens. Front. Microbiol. 2016;7:1–15. doi: 10.3389/fmicb.2016.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamberi M., Brummer E., Stevens D.A. Regulation of bronchoalveolar macrophage proinflammatory cytokine production by dexamethasone and granulocyte-macrophage colony-stimulating factor after stimulation by Aspergillus conidia or lipopolysaccharide. Cytokine. 2002;19:14–20. doi: 10.1006/cyto.2002.1049. [DOI] [PubMed] [Google Scholar]
- Khalil A.A., Mueller S., Foddis M., Mosch L., Lips J., Przesdzing I., Temme S., Flögel U., Dirnagl U., Boehm-Sturm P. Longitudinal 19F magnetic resonance imaging of brain oxygenation in a mouse model of vascular cognitive impairment using a cryogenic radiofrequency coil. MAGMA. 2019;32:105–114. doi: 10.1007/s10334-018-0712-x. [DOI] [PubMed] [Google Scholar]
- Khanna N., Stuehler C., Lünemann A., Wójtowicz A., Bochud P., Leibundgut-Landmann S. Host response to fungal infections – how immunology and host genetics could help to identify and treat patients at risk. Swiss Med. Wkly. 2016;146:w14350. doi: 10.4414/smw.2016.14350. [DOI] [PubMed] [Google Scholar]
- Krenke R., Grabczak E.M. Tracheobronchial manifestations of Aspergillus infections. ScientificWorldJournal. 2011;11:2310–2329. doi: 10.1100/2011/865239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Dresselaers T., Louchami K., Zhu C., Liu Y., Himmelreich U. Comparison of different compressed sensing algorithms for low SNR19F MRI applications—imaging of transplanted pancreatic islets and cells labeled with perfluorocarbons. NMR Biomed. 2017;30:e3776. doi: 10.1002/nbm.3776. [DOI] [PubMed] [Google Scholar]
- Liang S., Louchami K., Holvoet B., Verbeke R., Deroose C.M., Manshian B., Soenen S.J., Lentacker I., Himmelreich U. Tri-modal in vivo imaging of pancreatic islets transplanted subcutaneously in mice. Mol. Imaging Biol. 2018;20:940–951. doi: 10.1007/s11307-018-1192-0. [DOI] [PubMed] [Google Scholar]
- Lin S.-J., Schranz J., Teutsch S.M. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 2001;32:358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- Margalit A., Kavanagh K. The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol. Rev. 2015;39:670–687. doi: 10.1093/femsre/fuv018. [DOI] [PubMed] [Google Scholar]
- Massam J., Bitnun A., Solomon M., Somers G.R., Guerguerian A.M., Van Wylick R., Waters V. Invasive aspergillosis in cystic fibrosis: a fatal case in an adolescent and review of the literature. Pediatr. Infect. Dis. J. 2011;30:178–180. doi: 10.1097/INF.0b013e3181f63c90. [DOI] [PubMed] [Google Scholar]
- Mccormick A., Loeffler J., Ebel F. Aspergillus fumigatus: contours of an opportunistic human pathogen. Cell. Microbiol. 2010;12:1535–1543. doi: 10.1111/j.1462-5822.2010.01517.x. [DOI] [PubMed] [Google Scholar]
- Nawada R., Amitani R., Tanaka E., Niimi A., Suzuki K., Murayama T., Kuze F. Murine model of invasive pulmonary aspergillosis following an earlier stage, noninvasive Aspergillus infection. J. Clin. Microbiol. 1996;34:1433–1439. doi: 10.1128/jcm.34.6.1433-1439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmans J., Hillen A., Vanherp L., Govaerts K., Maertens J., Dresselaers T., Himmelreich U., Lagrou K., Vande Velde G. Longitudinal, in vivo assessment of invasive pulmonary aspergillosis in mice by computed tomography and magnetic resonance imaging. Lab. Invest. 2016;96:692–704. doi: 10.1038/labinvest.2016.45. [DOI] [PubMed] [Google Scholar]
- Poelmans J., Himmelreich U., Vanherp L., Zhai L., Hillen A., Holvoet B., Belderbos S., Brock M., Maertens J., Velde G.V., Lagrou K. A multimodal imaging approach enables in vivo assessment of antifungal treatment in a mouse model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.00240-18. e00240–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roilides E., Katsifa H., Walsh T.J. Pulmonary host defences against Aspergillus fumigatus. Res. Immunol. 1998;149:454–465. doi: 10.1016/s0923-2494(98)80769-4. discussion 523-4. [DOI] [PubMed] [Google Scholar]
- Rolle A.-M., Hasenberg M., Thornton C.R., Solouk-Saran D., Männ L., Weski J., Maurer A., Fischer E., Spycher P.R., Schibli R. ImmunoPET/MR imaging allows specific detection of Aspergillus fumigatus lung infection in vivo. Proc. Natl. Acad. Sci. U S A. 2016;113:E1026–E1033. doi: 10.1073/pnas.1518836113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Cabello J., Barnett B.P., Bottomley P.A., Bulte J.W.M. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011;24:114–129. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S., Korf H., Liang S., Verbeke R., Manshian B., Raemdonck K., Lentacker I., Gysemans C., De Smedt S.C., Himmelreich U. Challenges for labeling and longitudinal tracking of adoptively transferred autoreactive T lymphocytes in an experimental type-1 diabetes model. MAGMA. 2019;32:295–305. doi: 10.1007/s10334-018-0720-x. [DOI] [PubMed] [Google Scholar]
- Sales-campos H., Tonani L., Regina M., Kress V.Z. The immune interplay between the host and the pathogen in Aspergillus fumigatus lung infection 2. Aspergillus fumigatus virulence factors. Biomed. Res. Int. 2013;2013:1–14. doi: 10.1155/2013/693023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Wong J.E., Bornemann J., Hodenius M., Himmelreich U., Richtering W., Hoehn M., Zenke M., Hieronymus T. Polyelectrolyte coating of iron oxide nanoparticles for MRI-based cell tracking. Nanomedicine. 2012;8:682–691. doi: 10.1016/j.nano.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Shaikh S., Verma H., Yadav N., Jauhari M., Bullangowda J. Applications of steroid in clinical practice: a review. ISRN Anesthesiol. 2012;2012:1–11. [Google Scholar]
- Srinivas M., Boehm-Sturm P., Figdor C.G., de Vries I.J., Hoehn M. Labeling cells for in vivo tracking using19F MRI. Biomaterials. 2012;33:8830–8840. doi: 10.1016/j.biomaterials.2012.08.048. [DOI] [PubMed] [Google Scholar]
- Srinivas M., Cruz L.J., Bonetto F., Heerschap A., Figdor C.G., de Vries I.J.M. Customizable, multi-functional fluorocarbon nanoparticles for quantitative in vivo imaging using19F MRI and optical imaging. Biomaterials. 2010;31:7070–7077. doi: 10.1016/j.biomaterials.2010.05.069. [DOI] [PubMed] [Google Scholar]
- Srinivas M., Heerschap A., Ahrens E.T., Figdor C.G., de Vries I.J.M. 19F MRI for quantitative in vivo cell tracking. Trends Biotechnol. 2010;28:363–370. doi: 10.1016/j.tibtech.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens-Romero S.D., Mednick A.J., Feldmesser M. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect. Immun. 2005;73:114–125. doi: 10.1128/IAI.73.1.114-125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulou T., Meletiadis J., Roilides E., Kleiner D.E., Schaufele R., Roden M., Harrington S., Dad L., Segal B., Walsh T.J. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am. J. Clin. Pathol. 2007;127:349–355. doi: 10.1309/UJRV9DLC11RM3G8R. [DOI] [PubMed] [Google Scholar]
- Stoll G., Basse-Lüsebrink T., Weise G., Jakob P. Visualization of inflammation using 19F-magnetic resonance imaging and perfluorocarbons. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012;4:438–447. doi: 10.1002/wnan.1168. [DOI] [PubMed] [Google Scholar]
- Temme S., Bönner F., Schrader J., Flögel U. 19F magnetic resonance imaging of endogenous macrophages in inflammation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012;4:329–343. doi: 10.1002/wnan.1163. [DOI] [PubMed] [Google Scholar]
- van Heeswijk R.B., Pellegrin M., Flögel U., Gonzales C., Aubert J.-F., Mazzolai L., Schwitter J., Stuber M. Fluorine MR imaging of inflammation in atherosclerotic plaque in vivo. Radiology. 2015;275:421–429. doi: 10.1148/radiol.14141371. [DOI] [PubMed] [Google Scholar]
- Vanherp L., Poelmans J., Hillen A., Govaerts K., Belderbos S., Buelens T., Lagrou K., Himmelreich U., Vande Velde G. Bronchoscopic fibered confocal fluorescence microscopy for longitudinal in vivo assessment of pulmonary fungal infections in free-breathing mice. Sci. Rep. 2018;8:3009. doi: 10.1038/s41598-018-20545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiczies H., Lepore S., Janitzek N., Hagen U., Seifert F., Ittermann B., Purfürst B., Pezzutto A., Paul F., Niendorf T., Waiczies S. Perfluorocarbon particle size influences magnetic resonance signal and immunological properties of dendritic cells. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0021981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Zhang C., Jiang Y., Kou C., Kong Q., Long N., Lu L., Sang H. Innate and adaptive immune response to chronic pulmonary infection of hyphae of Aspergillus fumigatus in a new murine model. J. Med. Microbiol. 2017;66:1400–1408. doi: 10.1099/jmm.0.000590. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen L., Liu X., Cheng D., Liu G., Liu Y., Dou S., Hnatowich D.J., Rusckowski M. Detection of Aspergillus fumigatus pulmonary fungal infections in mice with99mTc-labeled MORF oligomers targeting ribosomal RNA. Nucl. Med. Biol. 2013;40:89–96. doi: 10.1016/j.nucmedbio.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff C.A., Hebert A.A. Neonatal primary cutaneous aspergillosis: case report and review of the literature. Pediatr. Dermatol. 2002;19:439–444. doi: 10.1046/j.1525-1470.2002.00203.x. [DOI] [PubMed] [Google Scholar]
- Wu Y.L., Ye Q., Foley L.M., Hitchens T.K., Sato K., Williams J.B., Ho C. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc. Natl. Acad. Sci. U S A. 2006;103:1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., He D., Gao S., Wei Y., Wang L. Aspergillus fumigatus enhances human NK cell activity by regulating M1 macrophage polarization. Mol. Med. Rep. 2019;20:1241–1249. doi: 10.3892/mmr.2019.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Narsinh K., Morel P.A., Xu H., Ahrens E.T. In vivo quantification of inflammation in experimental autoimmune encephalomyelitis rats using fluorine-19 magnetic resonance imaging reveals immune cell recruitment outside the nervous system. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0140238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.