Abstract
The recruitment of neutrophils to sites of inflammatory insult is a hallmark of the innate immune response. Neutrophil recruitment is regulated by a multistep process that includes cell rolling, activation, adhesion, and transmigration through the endothelium commonly referred to as the leukocyte adhesion cascade. After selectin-mediated braking, neutrophils migrate along the activated vascular endothelium on which ligands, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), are expressed. Previous studies have shown that two cells that commonly home from blood vessel to tissue—T cells and hematopoietic stem and progenitor cells—use the integrin lymphocyte functional antigen-1 (LFA-1) to migrate against the direction of shear flow once adherent on ICAM-1 surfaces. Like T cells and hematopoietic stem and progenitor cells, neutrophils express LFA-1, but they also express macrophage-1 antigen (Mac-1), which binds to ICAM-1. Previous reports have shown that neutrophils will not migrate against the direction of flow on ICAM-1, but we hypothesized this was due to the influence of Mac-1. Here, we report that both the HL-60 neutrophil-like cell line and primary human neutrophils can migrate against the direction of fluid flow on ICAM-1 surfaces via LFA-1 if Mac-1 is blocked; otherwise, they migrate downstream. We demonstrate this both on ICAM-1 surfaces and on activated endothelium. In sum, both LFA-1 and Mac-1 binding ICAM-1 play a critical role in determining the direction of neutrophil migration along the endothelium, and their interaction may play an important role in controlling neutrophil trafficking during inflammation.
Significance
Amoeboid cells of the immune system, most notably CD4+ T cells, can crawl upstream under flow on surfaces that bear intercellular adhesion molecule-1 (ICAM-1). The upstream migration is mediated by the binding of an integrin, lymphocyte functional antigen-1 (LFA-1), to ICAM-1. It had been reported that neutrophils, which also bear LFA-1, are unable to crawl upstream, but we hypothesized that this was because they had two competing receptors for ICAM-1, LFA-1 and macrophage-1 antigen (Mac-1). When we blocked Mac-1 with an antibody, neutrophils revert phenotype and crawl upstream. The identification that neutrophils share a common mechanism to crawl upstream with other amoeboid cells will lead to an examination both of the physiological significance and biophysical mechanisms that underlie upstream migration.
Introduction
Immune cell recruitment to sites of inflammation is a hallmark of an effective immune response. Neutrophils are the so-called “first responders of the immune system” and are the first cell type to respond to an inflammatory insult. Leukocyte recruitment occurs through the leukocyte adhesion cascade, which consists of several steps. Initially, neutrophils are slowed by selectin-mediated interactions, which allows for chemokine-induced activation of integrins, firm adhesion, and transmigration across the endothelial layer (1, 2). At the outset of the adhesion cascade, human neutrophils bind and roll on P- and L-selectins through a specific sialyl-Lewis-x-decorated O-glycan on P-selectin glycoprotein ligand-1 (3, 4, 5). Afterwards, a variety of sialyl-Lewis-x-decorated glycoproteins (6, 7) and glycolipids (8, 9) are utilized to bind to endothelial E-selectin to slow the neutrophil enough to interact with chemokines that are either expressed on the endothelial surface or in solution. Chemokine stimulation by CXCL12 or CXCL8 (IL-8) leads to intracellular signaling cascades activated through their cognate cell-surface receptors, CXCR1, 2, or 4 (10, 11, 12). Chemokine ligation or shear forces induce activation of integrins; the three integrins involved include macrophage-1 antigen (Mac-1, αMβ2), lymphocyte functional antigen-1 (LFA-1, αLβ2), and very late antigen-4 (VLA-4, α4β1) These integrins can adopt high affinity states that can bind to their cognate ligands, intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), on the endothelial surface (13, 14). After adhesion, leukocytes become motile and cross the endothelium to reach the basal lamina and the site of infection (15).
Our laboratory has recently been studying a unique type of motility displayed by certain immune cells in which these cells are able to efficiently crawl against the direction of shear flow, depending on which CAM the cell engages. This motility would be displayed in blood vessels on the endothelial apical surface after leukocyte adhesion but before diapedesis. The Theodoly laboratory and our laboratory established that CD4+ T lymphocytes crawl upstream on ICAM-1 surfaces and downstream on VCAM-1 surfaces and our laboratory demonstrated that T cells crawling upstream are able transmigrate faster through the endothelium (16, 17, 18, 19, 20). It has since been shown that marginal zone B cells will crawl upstream on ICAM-1, against the blood flow, to leave the marginal zone and enter the follicle in the spleen (21, 22). Our laboratory recently showed that hematopoietic stem and progenitor cells (HSPCs), although homing to the bone marrow post-transplantation, can migrate upstream on pure ICAM-1 surfaces, mixtures of ICAM-1 and VCAM-1, and stimulated human umbilical vein endothelial cells (HUVECs), but crawl primarily downstream on surfaces of VCAM-1 only (23). Interestingly, upstream migration is not observed in all immune cells, as it has been previously reported that human neutrophils do not migrate upstream (17). This is surprising because neutrophils carry LFA-1, which is critical for upstream migration (24, 25). However, neutrophils also carry another integrin, Mac-1, which can compete with LFA-1 for binding to ICAM-1 (26, 27). Therefore, we hypothesized that blocking Mac-1 binding to ICAM-1 would allow LFA-1 mediated neutrophil migration upstream.
Here, we show that blocking Mac-1 via specific antibodies allowed both HL-60 cells and human neutrophils to migrate upstream on ICAM-1, ICAM-1 + VCAM-1, and HUVEC surfaces. We also determined that primary human neutrophils and the HL-60 cell line are unable to migrate upstream when using isotype, negative control antibodies. Elucidating the ability of this important cell type to crawl upstream suggests a fundamental biophysical mode of motility that is shared by many immune cells, worthy of continued investigation.
Materials and Methods
Cell culture
All primary and cell lines were cultured as described previously (8, 28, 29). Briefly, HL-60 cells (ATCC, Manassas, VA) were cultured as described previously in Iscove’s modified Eagle’s Medium (IMDM) containing 20% fetal bovine serum. HL-60 cells were differentiated into neutrophil-like cells in 1.25% DMSO (Sigma-Aldrich, St. Louis, MO) for 5 days before experimentation (30). HUVECs were maintained in EBM-2 growth media with BulletKit supplement (Lonza, Wakersville, MD). For experimentation, HUVECs were seeded in a 35 × 10 mm TC-treated dish (BD Falcon, Bedford, MA) and grown to confluence. HUVECs were then stimulated with IL-1β (BioLegend, San Diego, CA) for 4 h before experimentation.
Isolation of primary neutrophils from whole blood
The primary neutrophils were isolated from human donors and collected in sodium citrate as an anticoagulant. Donors were selected from both male and female populations, and every effort was made to obtain neutrophils from diverse ethnic populations. After lysis of the red-blood cells in a hypotonic lysis solution, the remaining blood cells were layered onto a gradient of Histopaque 1077 and 1119 (Sigma-Aldrich). The cells were spun at 700 × g for 30 min with no brake and the neutrophils collected from the interface of the Histopaque solutions (31). The collected cells were then washed and seeded in Hanks’ Buffered Saline Solution. All neutrophils were used for experimentation within 2–4 h of isolation. To assure no neutrophil activation occurred during the separation procedure, we kept the cells in Hanks’ Buffered Saline Solution without Ca2+/Mg2+ because the ions have been shown to prime cells while also resuspending the neutrophils slowly and using mild centrifugation settings (32).
Preparation of adhesion-molecule-coated surfaces
Surfaces were prepared as described before (23, 33, 34). Briefly, either TC-treated 24-well plates (BD Falcon) for static experiments or 1 inch × 3 inch plastic microscope slides (Thermo Fisher Scientific, Waltham, MA) for shear flow experiments were coated with 2 μg/mL Protein A/G (Biovision, Milpitas, CA) for overnight at 4°C. Upon washing three times with 1× phosphate-buffered saline, surfaces were treated with 0.2% Pluronic F127 (Sigma-Aldrich) for 1 h to block nonspecific binding to the surface. After three additional phosphate-buffered saline washes, surfaces were then coated with the desired total concentration (2.5 μg/mL, which is ∼122 sites/μm2) (35, 36) of recombinant human ICAM-1 Fc, VCAM-1 Fc (R&D Systems, Minneapolis, MN), or a mixture of the two for 1 h.
Static migration assays
After the preparation of CAM-coated 24-well plates, 500 μL of IMDM media + 20% fetal bovine serum was added to each experimental well. HL-60 cells were then seeded at a concentration of 5000 cells/cm2 and allowed to settle and attach to the surface for 30 min. The plate was then mounted on the microscope in a 5% CO2 and 37°C environment for 15 min to allow for equilibration. Images were captured every minute for 30 min on a motorized stage and observed using a Nikon Eclipse TE300 phase contrast microscope (Nikon, Tokyo, Japan). Images were captured using a 10× objective.
Flow chamber assembly and assay for recombinant surfaces
Experiments were conducted as described previously (3, 34). Briefly, a parallel-plate flow chamber (GlycoTech, Gaithersburg, MD) was used with a prepared plastic slide functionalized with either ICAM-1 Fc, VCAM-1 Fc, or equal mixtures of both. The channel template was cut from 0.01-inch-thick Duralastic sheeting (Allied Biomedical, Ventura, CA). For each flow experiment, the template was placed over the prepared slide. The template and slide were placed in the bottom well of the flow chamber, and the top was secured with screws. The chamber was assembled under water to minimize the introduction of air. It was then mounted on the microscope in a 5% CO2 and 37°C environment for 10 min to allow for equilibration. Before introduction of cells, the chamber was flushed with running media consisting of IMDM. A volume of 2 mL containing 1 × 106 cells in running media was introduced into the chamber, and cells were allowed to attach for 15 min. Fluid flow was initiated using a syringe pump (11 Plus; Harvard Apparatus, Holliston, MA), and volumetric flow rates were adjusted accordingly to correspond to desired shear rates. Shear rate was calculated using τw = (6 μQ)/(h2w), where μ is the fluid viscosity, Q is the volumetric flow rate, h is the channel height, and w is the channel width. For this chamber, h = 0.023 cm, w = 0.1 cm. Images were captured every minute on a motorized stage and observed using a Nikon Eclipse TE300 phase contrast microscope. Images were captured using a 10× objective every minute for 30 min. Migrating cells had a polarized morphology consisting of a lamellipod at the front and a uropod at the rear and were phase dark. Spherical and nonadherent cells were either washed away upon application of flow or demonstrated no motility and were not included in analysis.
Shear flow assays on stimulated HUVEC surfaces
Experiments were conducted under similar conditions as previous studies (23, 28, 37). In brief, HUVECs (Lonza) were seeded and grown to confluence in EGM-2 media with bullet supplements (Lonza) on 35 × 10 mm tissue-culture-treated polystyrene plates (BD Falcon) and gown to confluence at 37°C and 5% CO2. Before experimentation, HUVECs were supplied with fresh EGM-2 media supplemented with 10 ng/mL recombinant IL-1β (Biolegend) and allowed to incubate for a minimum of 4 h to ensure robust expression of E-selectin, ICAM-1, and VCAM-1 on the surface (38). To perfuse the HL-60 cells and PMNs across the HUVEC monolayer, we used a circular parallel-plate flow chamber (Glycotech) under vacuum, fitted with a rubber gasket to create a rectangular flow path (h = 127 μm, w = 0.25 cm). The chamber was assembled under water to minimize the introduction of air. It was then mounted on the microscope in a 5% CO2 and 37°C environment for 10 min to allow for equilibration. Before introduction of cells, the chamber was flushed with running media consisting of IMDM. A volume of 2 mL containing 1 × 106 cells in running media was introduced into the chamber, and cells were allowed to attach for 10 min. From this point on, the assay was performed under similar conditions to the previous experiments on recombinant surfaces as described above.
Antibody blocking
Functional blocking antibodies HL111 (anti-CD11a/αL-integrin), ICRF44 (anti-CD11b/αM-integrin), and 9F10 (anti-CD49d/α4-integrin) were obtained from Biolegend. 5 × 105 HL-60 cells or PMNs were blocked with a final concentration of 50 μg/mL blocking antibody in 25 μL running media and incubated for 30 min at 37°C and 5% CO2 as described previously (23, 37). Cells were then injected into flow chamber apparatus and allowed to adhere in the absence of flow for 30 min. Cells were exposed to flow for 10 min before quantification of speed and MI.
Measurement of cell trajectories, speed, and MI
Cell movement was tracked using the ImageJ plugin Manual Tracking as described previously (20, 23, 39). ImageJ and the plugin are both freely available through the National Institutes of Health website (https://imagej.nih.gov/ij/). The centroid of the cell was considered to represent the cell position. Time-lapse microscopy was used and images were taken every minute. The result was a series of (xi, yi) positions with time for each cell. The net displacement during the ith minute increment, Di, was calculated by the difference of the position at the beginning and end of the time step. The sum of total displacements (Di,accum) was used to calculate the cell speed over the entire experimental time course of 30 min. The migration index (MI) was defined as the ratio of the difference between the initial and final x-displacement/total displacement where MI = (xi,end − xi,initial)/(Di,accum). Values of the MI near −1 indicate that cells migrate in a straight trajectory against the direction of flow, whereas values near +1 indicate migration in a straight trajectory in the direction of flow. When the MI is near 0, there is no preferred direction in migration, indicating random motility. Only single cells actively migrating along the surface that remained in the field of view for the entire experiment were included in the analysis. Dividing cells and clusters of cells in which the cells interacted with one another’s path were not included in analysis, and neither were stationary cells or loosely adhered cells.
Statistics
Data are presented as mean ± standard error. Testing for differences between means was determined with either Student’s t-test or two-way ANOVA with post hoc comparisons in InStat 3 software (GraphPad, San Diego, CA). p < 0.05 was considered significant and is indicated in the figure legends.
Results
Untreated HL-60 cells and primary neutrophils are unable to migrate against the direction of shear flow on ICAM-1
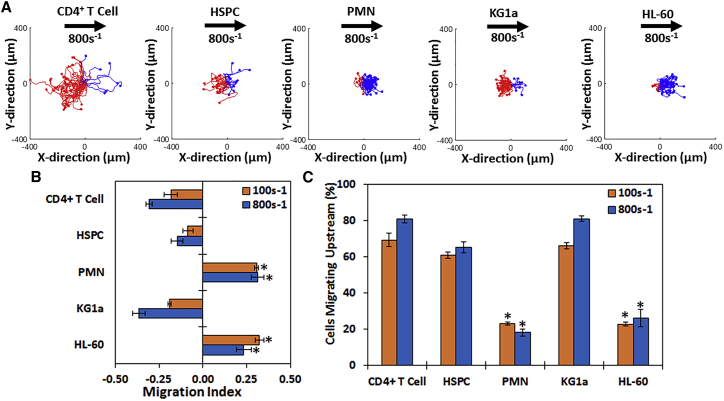
We first examined the ability of either HL-60 cells (an immortalized promyeloid cell line that can be differentiated into neutrophil-like cells) or primary human neutrophils to migrate upstream. The MI is defined as the ratio of the difference between the initial and final x-displacement/total displacement where MI = (xi,end − xi,initial)/(Di,accum), where positive values indicate downstream migration and negative values indicate upstream migration. Interestingly, the scattergrams (Fig. 1 A) and MI (Fig. 1 B) demonstrate that although the majority of primary CD4+ T cells, primary HSPCs, and the immortalized HSPC-like line KG1a cells migrate against the direction of flow on ICAM-1 surfaces (demonstrated by the abundance of red tracks and negative MI), differentiated HL-60 cells and primary neutrophils migrated preferentially downstream, as shown by the preponderance of blue tracks and a positive MI. Furthermore, more than half of the T cells, HSPCs, and KG1a cells migrated upstream (80.9, 65.2, and 81.1%, respectively), whereas a minority of HL-60 cells and neutrophils migrated upstream (23.2 and 18.1%, respectively) (Fig. 1 C).
Figure 1.
CD4+ T cells, HSPCs, and KG1a migrate upstream on ICAM-1 surfaces, whereas HL-60 cells and primary PMNs do not. (A) Cell traces of CD4+ T cells, bone marrow HSPCs, whole-blood-derived PMNs, KG1a cells, or differentiated HL-60 cells on 2.5 μg/mL ICAM-1 surfaces are shown at a shear rate of 800 s−1. Blue traces indicate cells that traveled downstream (with flow), and red traces indicate cells that traveled upstream (against flow). The direction of flow is from left to right, and the displacement has units of microns. In general, more of the cell traces indicate upstream motion (red tracks) in CD4+ T cells, HSPCs, and KG1a cells, whereas PMNs and HL-60 cells have more blue traces indicating downstream migration. (B) The directional migration of the five cell types under shear flow as expressed by the migration index (MI) is shown. A negative MI indicates migration against the flow (upstream), whereas a positive MI indicates migration with the flow (downstream). CD4+ T cells, HSPCs, and KG1a cells have a negative MI, whereas HL-60 cells and PMNs have a positive MI. (C) Percentage of migrating cells traveling upstream for each cell type at 100 and 800 s−1 shear rate on ICAM-1 is shown. HL-60 cells and PMNs have <50% of cells migrating upstream, whereas CD4+ T cells, HSPCs, and KG1a cells have >50% of cells migrating upstream. n = 4 independent experiments of at least 50 cells analyzed per experiment for each shear rate. ∗p < 0.05 with respect to CD4+ T cell experiments at similar conditions.
We characterized the expression of ICAM-1 binding molecules on HL-60 cells and primary neutrophils, compared it to the surface expression of molecules expressed on KG1a cells, and showed that HL-60 cells and primary neutrophils have a remarkably similar integrin profile. A common feature of cells in Fig. 1 that were unable to crawl upstream (HL-60 cells and primary neutrophils) was the expression of the integrin Mac-1 (Fig. S1). Because it is known that LFA-1 is responsible for upstream migration (17, 18, 19, 20, 21), we hypothesized that cells expressing Mac-1 are unable to migrate upstream because of competition between LFA-1 and Mac-1 for ICAM-1, suggesting that blocking Mac-1 in neutrophils and HL-60 cells may allow them to crawl upstream.
HL-60 cells are maximally motile at lower ICAM-1 concentrations than T cells and HSPCs
To determine the concentration in which HL-60 cells were maximally motile, we measured the motility of HL-60 cells on substrates coated with ICAM-1 in the absence of flow over a range of concentrations (1, 2.5, 5, and 10 μg/mL). In the absence of flow, the MI fell between −0.05 and 0.05 for all four ICAM-1 concentrations, indicating there was no preference in direction of migration in the absence of flow (Fig. S2 A). The persistence time mirrored the motility coefficient and was maximal at 2.5 μg/mL (Fig. S2 B), whereas the speed was inversely proportional to the concentration of ICAM-1 (Fig. S2 C). The two-dimensional area that HL-60 cells explored through migration was highest at 2.5 μg/mL; correspondingly, the random motility coefficient was highest at that surface density (Fig. S2 D).
Migration of both resting and activated HL-60 cells is upstream on ICAM-1 and downstream on VCAM-1 under shear flow after Mac-1 blocking
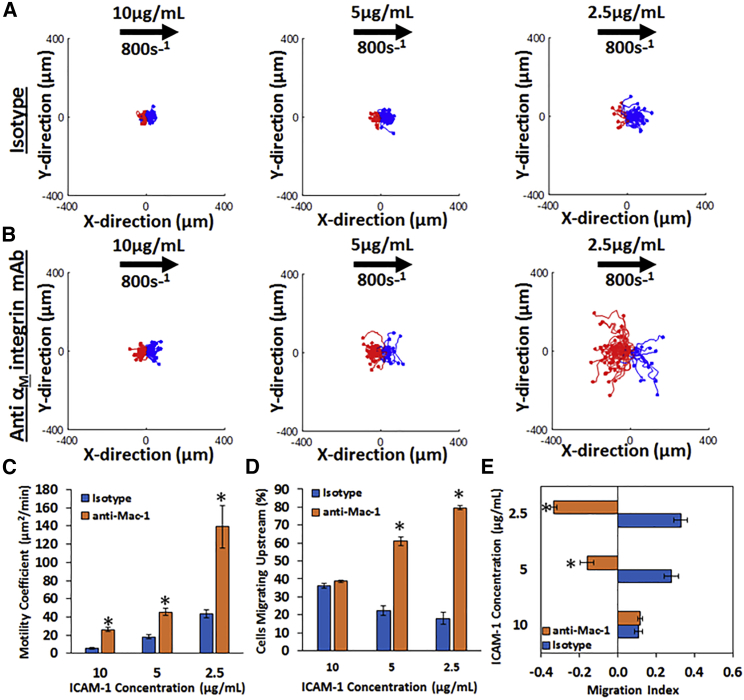
We then tested the motility of HL-60 at a shear rate of 800 s−1 at a range of ICAM-1 surface concentrations (2.5, 5, and 10 μg/mL) with and without preincubation of a Mac-1-blocking antibody (Fig. 2). As seen from the cell tracks on ICAM-1 surfaces, HL-60 cells treated with an isotype antibody have a preference for migration with the direction of flow, indicated by the preponderance of blue tracks (Fig. 2 A), with less than 50% of cells traveling upstream (Fig. 2 D), and a positive MI (Fig. 2 E). Also, HL-60 cells explore a greater area with decreasing concentration, as evidenced by the motility coefficient (Fig. 2 C), which increases with decreasing concentration.
Figure 2.
The migration of HL-60 cells under shear flow is dependent on ICAM-1 concentration and Mac-1 binding: cell traces of HL-60 cells on ICAM-1 at concentrations of 10μg/mL (first column), 5μg/mL (second column), and 2.5μg/mL (third column) under (A) isotype or (B) anti-αM-integrin blocking at a shear rate of 800 s−1 are shown. The traces depicted are the cumulative tracks of two independent experiments and have units of microns. Blue traces indicate cells that traveled downstream (with flow), and red traces indicate cells that traveled upstream (against flow). The direction of flow is from left to right in these traces and has units of microns. (C) The random motility coefficient (μm2/min), (D) the percentage of cells migrating upstream, and (E) the MI of HL-60 cells on varying ICAM-1 concentrations under isotype or anti-αM-integrin blocking at 800 s−1 shear rate are shown. A negative MI indicates migration against the flow (upstream), whereas a positive MI indicates migration with the flow (downstream). HL-60 cells become more motile at lower ICAM-1 concentrations and migrate upstream under Mac-1-blocking conditions. n = 4 independent experiments of at least 50 cells analyzed per experiment. ∗p < 0.05 with respect to isotype conditions.
Blocking Mac-1 with a monoclonal antibody allows HL-60 cells to migrate upstream on ICAM-1 surfaces, indicated by the greater number of red tracks (Fig. 2 B). Furthermore, more than 50% of cells are traveling upstream at ICAM-1 concentrations of 5 μg/mL and 2.5 μg/mL (Fig. 2 D). After blocking Mac-1, HL-60 cells display a negative MI (Fig. 2 E). HL-60 cells blocked with Mac-1 also explore a greater area and are more motile with decreasing concentration of ICAM-1 as compared to cells treated with a negative control isotype antibody. This is demonstrated by the random motility coefficient (Fig. 2 C), which increases with decreasing ICAM-1 concentration. In all, these data demonstrate that HL-60 cells are indeed able to migrate upstream on ICAM-1 surfaces, provided Mac-1 is blocked, and that the inverse dependence of motility on ICAM-1 concentration may account for previous reports in which neutrophils were unable to migrate upstream even when an attempt was made to block Mac-1 (17).
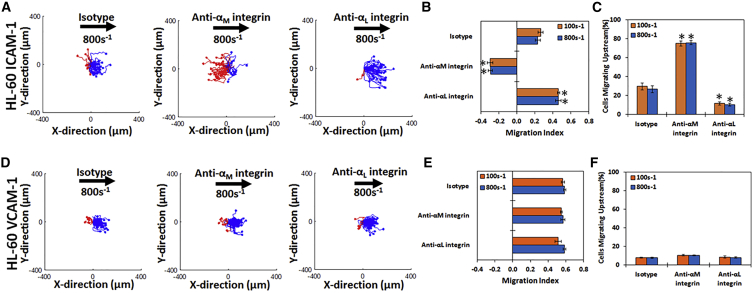
Next, we measured the motility of HL-60 cells on surfaces coated with either ICAM-1 or VCAM-1 at two shear rates (100 and 800 s−1) and with or without blocking antibodies against either the αL-integrin (LFA-1) or the αM-integrin (Mac-1) (Fig. 3). On surfaces containing only ICAM-1, HL-60 cells migrate with the direction of flow when treated with an isotype antibody (Fig. 3 A, left panel; Video S1), but the direction of migration is reversed to be against the direction of flow upon blocking the αM-integrin (Fig. 3 A, middle panel; Video S2). Under Mac-1 blocking conditions, HL-60 cells migrated upstream with a negative MI (Fig. 3 B) and >50% of cells travelled upstream (Fig. 3 C). Conversely, blocking LFA-1 showed the opposite effect by increasing the migration in the direction of flow (Fig. 3 A, right panel). Cells travel a shorter distance under either of the blocking conditions as compared to isotype (Fig. S3 A).
Figure 3.
HL-60 cells migrate upstream on ICAM-1 and downstream on VCAM-1 once Mac-1 is blocked: cell traces of HL-60 cells on (A) ICAM-1 and (D) VCAM-1 under isotype (first column), with anti-αM-integrin blocking (second column), or with anti-αL-integrin blocking (third column) at 800 s−1 shear rate at a concentration of 2.5 μg/mL. The traces depicted are the cumulative tracks of two independent experiments and have units of microns. Blue traces indicate downstream migration (with flow), and red traces indicate upstream migration (against flow). The direction of flow is from left to right in these traces and the traces have units of microns. The direction of HL-60 cell migration under shear flow as expressed by the MI under isotype, anti-αM-integrin, or anti-αL-integrin blocking at 100 and 800 s−1 shear rate on (B) ICAM-1 and (E) VCAM-1 is shown. A negative MI indicates migration against the flow (upstream), whereas a positive MI indicates migration with the flow (downstream). The percentage of migrating cells traveling upstream under isotype, anti-αL-integrin, or anti-αM-integrin blocking at 100 and 800 s−1 shear rate on (C) ICAM-1 or (F) VCAM-1 is shown. HL-60 cells migrate downstream on ICAM-1 and VCAM-1 under isotype conditions, whereas blocking the αM-integrin promotes upstream migration on ICAM-1 but not VCAM-1. Blocking the αL-integrin of removes all upstream migration in ICAM-1 and does not affect VCAM-1 migration. n = 4–5 independent experiments of at least 60 cells analyzed per experiment for each CAM. ∗p < 0.05 with respect to isotype conditions.
On VCAM-1-only surfaces, HL-60 cells migrate downstream in the presence of a negative control antibody (Fig. 3 D, left plot), if αM-integrin is blocked (Fig. 3 D, middle plot), or if αL-integrin is blocked (Fig. 3 D, right plot). There was no significant difference in the direction of migration under any blocking conditions, as shown by the MI (Fig. 3 E). Less than 10% of HL-60 cells migrated upstream on VCAM-1 under any conditions (Fig. 3 F). In all, these data demonstrate that blocking Mac-1 on HL-60 cells leads to upstream migration on ICAM-1, whereas VCAM-1 only supported downstream migration under all conditions tested. Cells travel similar distances under all conditions tested (Fig. S3 B).
To determine whether activation (which the neutrophils would experience during an inflammatory insult) changes the directional preferences of the HL-60 cells, these cells were stimulated with N-Formylmethionine-leucyl-phenylalanine (fmlp) before experimentation. As seen in Fig. S4, activation of the HL-60 with fmlp does not change the directional preference of the cells on either ICAM-1 or VCAM-1 (from Fig. 3) because cells will only migrate upstream on ICAM-1 if Mac-1 is blocked and HL-60 cells migrate downstream on VCAM-1 under all conditions. On the other hand, the HL-60 cells stimulated with fmlp are significantly more motile (Fig. S4 E) than their unstimulated counterparts (data not shown). In sum, although fmlp stimulation increases the motility of the HL-60 cells, the directional preferences are similar, as seen with unstimulated HL-60 cells.
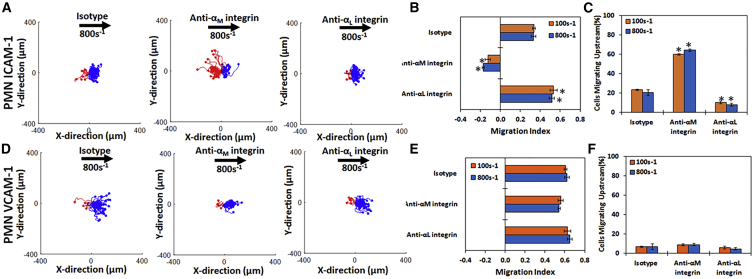
Primary neutrophils from whole blood behave similarly to HL-60 cells on ICAM-1 and VCAM-1 surfaces
To determine whether the results we found in our assays on cell lines were transferrable to primary cells, we tested whether primary neutrophils from whole blood display similar patterns of directional migration on ICAM-1 (Fig. 4, A–C) and VCAM-1 (Fig. 4, D–F). On ICAM-1 surfaces, primary PMNs migrate with the direction of flow when treated with an isotype antibody (Fig. 4 A, left panel; Video S3), and the direction of motility was reversed to against the direction of flow if the αM-integrin was blocked (Fig. 4 A, middle panel; Video S4). Blocking the αL-integrin led to migration that was more directionally downstream compared to migration when isotype antibodies were used (Fig. 4 A, right panel). Quantification of the direction of migration confirmed these qualitative results; blocking with either αL or isotype antibody led to positive MIs, whereas blocking Mac-1 led to a negative MI (Fig. 4 B). Measuring the fraction of cells migrating upstream yielded similar results regarding the directionality of migration (Fig. 4 C), and PMNs traveled similar distances under all conditions tested (Fig. S3 A).
Figure 4.
Primary PMNs display similar migration profiles to HL-60 cells on ICAM-1 and VCAM-1: cell traces of neutrophils from whole blood on (A) ICAM-1 and (D) VCAM-1 under isotype (first column), with anti-αM-integrin blocking (second column), or with anti-αL-integrin blocking (third column) at 800 s−1 shear rate at a concentration of 2.5 μg/mL. The traces depicted are the cumulative tracks of two independent experiments and have units of microns. Blue traces indicate cells that traveled downstream (with flow), and red traces indicate cells that traveled upstream (against flow). The direction of flow is from left to right in these traces and has units of microns. The direction of PMN migration under shear flow as expressed by the MI under isotype, anti-αM-integrin, or anti-αL-integrin blocking at 100 and 800 s−1 shear rate on (B) ICAM-1 and (E) VCAM-1 is shown. A negative MI indicates migration against the flow (upstream), whereas a positive MI indicates migration with the flow (downstream). Percentage of migrating cells traveling upstream under isotype or anti-αL-integrin blocking at 100 and 800 s−1 shear rate on (C) ICAM-1 or (F) VCAM-1 is shown. PMNs behave exactly like HL-60 cells by migrating downstream on ICAM-1 and VCAM-1 under isotype conditions, whereas blocking the αM-integrin promotes upstream migration on ICAM-1 but not on VCAM-1. Blocking the αL-integrin of removes all upstream migration on ICAM-1 and does not affect VCAM-1 migration. n = 4–5 independent experiments of at least 50 cells analyzed per experiment for each CAM. ∗p < 0.05 with respect to isotype conditions.
On VCAM-1 surfaces, PMNs migrate downstream (Fig. 4 D, left plot), and this is unchanged upon blocking either the αM- (Fig. 4 D, middle plot) or the αL-integrin (Fig. 4 D, right plot). Under all conditions, the MI remained negative (Fig. 4 E), less than 10% of cells migrated upstream (Fig. 4 F), and PMNs traveled similar distances (Fig. S3 B).
In all, these data demonstrate that like HL-60 cells, blocking Mac-1 on the surface of primary neutrophils supports upstream migration on ICAM-1 but downstream migration on VCAM-1.
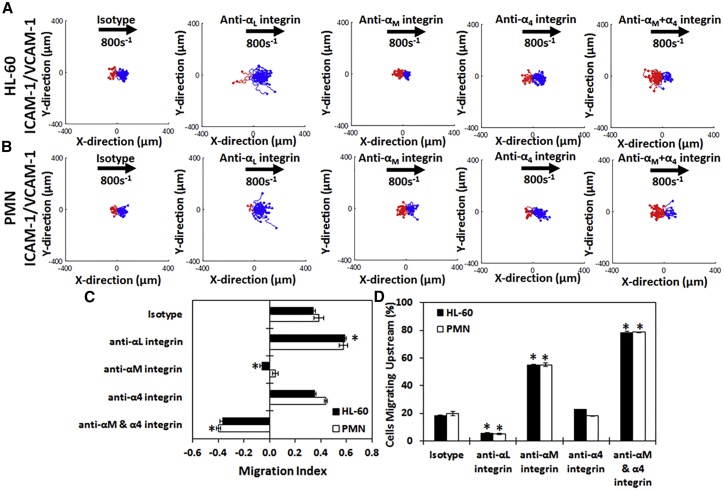
LFA-1 controls upstream motion while Mac-1 and VLA-4 control downstream migration on mixed surfaces
To see how the migration profiles were altered when more than one CAM was present (as the cell would encounter on the endothelial surface), we plated either HL-60 cells or primary PMNs on 50/50 mixtures by mass of ICAM-1 + VCAM-1 (I + V). As seen in the cell tracks (Fig. 5, A and B), both HL-60 cells and primary PMNs migrate with the direction of flow when an isotype antibody is present, as confirmed by the MI (Fig. 5 C) and the fraction of cells migrating upstream (Fig. 5 D). Next, we demonstrated that blocking Mac-1 allows HL-60 cells and PMNs to switch their direction of migration from downstream to upstream on I + V surfaces, as illustrated by the abundance of red traces after blocking (Fig. 5, A and B), the corresponding decrease in MI (Fig. 5 C), and the number of cells migrating upstream (Fig. 5 D). We then demonstrated blocking Mac-1 in combination with the α4-integrin (VLA-4) further enhances the migration of HL-60 cells and PMNs upstream on I + V surfaces, signified by the increase in red traces on the scattergrams (Fig. 5, A and B), a further decrease in MI (Fig. 5 C), and an increase in the fraction of cells migrating upstream (Fig. 5 D). Finally, we demonstrated that blocking the αL-integrin (LFA-1) eliminates upstream migration of HL-60 cells and PMNs on I + V surfaces, illustrated by the lack of red traces (Fig. 5, A and B) and the subsequent increase in MI (Fig. 5 C). The number of cells migrating upstream was also reduced (Fig. 5 D), and both PMNs and HL-60 cells migrated significantly further distances than any other subtype (Fig. S3 C). Blocking VLA-4 alone did not significantly affect the migration of HL-60 cells and PMNs on I + V surfaces as compared to isotype in either the scattergrams (Fig. 5, A and B, fourth panel), MI (Fig. 5 C), or cells migrating upstream (Fig. 5 D).
Figure 5.
HL-60 cells and PMN migrate upstream on mixed CAM surfaces once Mac-1 is blocked. Cell traces of (A) HL-60 cells and (B) neutrophils isolated from whole blood on surfaces containing an equal mixture of ICAM-1 and VCAM-1 (I + V) under isotype (first column), anti-αL-integrin blocking (second column), anti-αM-integrin blocking (third column), anti-α4-integrin blocking (fourth column), or with anti-αM-integrin and anti-α4-integrin blocking (fifth column) at 800 s−1 shear rate are shown. The traces depicted are the cumulative tracks of two independent experiments and have units of microns. Blue traces indicate cells that traveled downstream (with flow), and red traces indicate cells that traveled upstream (against flow). The direction of flow is from left to right, and traces have units of microns. (C) The direction of migration under shear flow as expressed by the MI or (D) percentage of migrating cells traveling upstream under isotype, anti-αL-integrin blocking, anti-αM-integrin blocking, anti-α4-integrin blocking, or anti-αM- and anti-α4-integrin blocking at 800 s−1 shear rate on I + V surfaces. Both HL-60 cells and PMNs migrate downstream on I + V surfaces until the αM-integrin is blocked, when they then migrate upstream. Blocking the αL-integrin removes all upstream migration, whereas blocking the α4-integrin with the αM-integrin enhances it. n = 4 independent experiments of at least 60 cells analyzed per experiment. ∗p < 0.05 with respect to isotype conditions.
Together, these data show that competition between Mac-1 and LFA-1 prevents upstream migration in HL-60 cells and primary neutrophils, and these cells can indeed migrate against the direction of flow on surfaces of mixed composition once Mac-1 is blocked. Furthermore, removing the VLA-4/VCAM-1 interactions in these cells in combination with the Mac-1/ICAM-1 interactions further enhances the ability of HL-60 cells and primary neutrophils to migrate upstream.
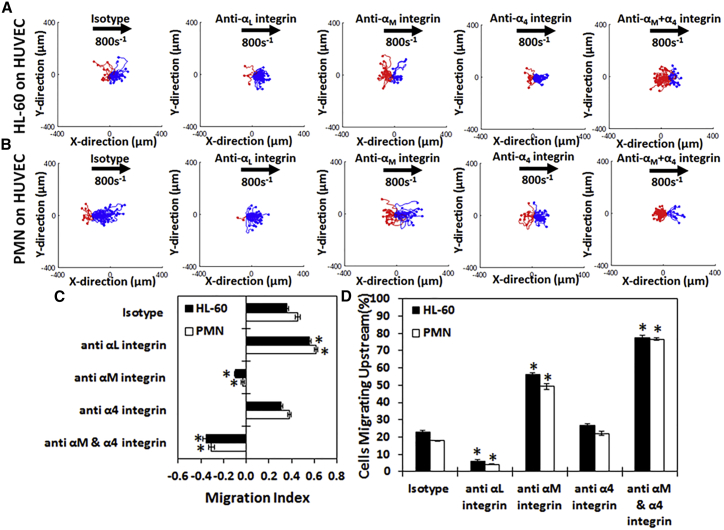
HL-60 cells and primary PMN can be induced to migrate upstream on HUVEC monolayers
Because both HL-60 cells and PMNs were able to migrate upstream on recombinant CAM surfaces, we tested whether HL-60 cells and primary PMNs exhibited upstream motility on HUVEC monolayers (Fig. 6). On unstimulated HUVEC monolayers, only 5% of HL-60 cells and PMNs attached to the HUVEC surface as compared to IL-1β-stimulated HUVECs (Fig. S5), and of those that attached, none actively migrated. On IL-1β-stimulated HUVECs, HL-60 cells (Fig. 6 A, first panel) migrate with the direction of flow, and all upstream motion could be completely removed upon blocking the αL-integrin (Fig. 6 A, second panel). However, the direction of migration becomes upstream if the αM-integrin is blocked (Fig. 6 A, third panel; Video S5), and this upstream migration is enhanced when both the αM- and the α4-integrins are blocked (Fig. 6 A, fourth panel). This change in directional migration is quantified with the MI (Fig. 6 C) and the percentage of cells migrating upstream (Fig. 6 D).
Figure 6.
HL-60 cells and PMNs display upstream migration on HUVEC once Mac-1 is blocked: cell traces of (A) HL-60 cells and (B) neutrophils isolated from whole blood on IL-1β-stimulated HUVECs under isotype (first column), anti-αL-integrin blocking (second column), anti-αM-integrin blocking (third column), anti-α4-integrin blocking (fourth column), or with anti-αM-integrin and anti-α4-integrin blocking (fifth column) at 100 s−1 shear rate. The traces depicted are the cumulative tracks of two independent experiments and have units of microns. Blue traces indicate cells that traveled downstream (with flow), and red traces indicate cells that traveled upstream (against flow). The direction of flow is from left to right, and traces have units of microns. (C) The direction of migration under shear flow as expressed by the MI or (D) percentage of migrating cells traveling upstream under isotype, anti-αL-integrin blocking, anti-αM-integrin blocking, anti-α4-integrin blocking, or anti-αM- and anti-α4-integrin blocking at 100 s−1 shear rate on HUVECs is shown. Both HL-60 cells and PMNs migrate downstream on HUVECs until the αM-integrin is blocked, when they then migrate upstream. Blocking the αL-integrin removes all upstream migration, whereas blocking the α4-integrin with the αM-integrin enhances it. n = 3–4 independent experiments of at least 45 cells analyzed per experiment. ∗p < 0.05 with respect to isotype conditions.
Primary neutrophils from whole blood (Fig. 6 B, first panel; Video S5) migrate slightly with the direction of flow on HUVECs. Like HL-60 cells, all upstream motion could be eliminated upon blocking the αL-integrin (Fig. 6 B, second panel). Likewise, PMNs migrated upstream when αM-integrin (Fig. 6 B, third panel) was blocked. Upstream migration was enhanced even further when both the αM- and α4-integrins were blocked in combination (Fig. 6 B, fourth panel). This preference is also seen in the MI (Fig. 6 C) and the percentage of cells migrating upstream (Fig. 6 D). Much like the recombinant surfaces, blocking VLA-4 alone did not significantly affect the migration of HL-60 cells and PMNs on HUVEC surfaces as compared to isotype as demonstrated in either the scattergrams (Fig. 6, A and B, fourth panel), MI (Fig. 6 C), or cells migrating upstream (Fig. 6 D). However, when blocking VLA-4 alone or both Mac-1 + VLA-4, the distance traveled significantly decreased as compared to isotype (Fig. S3 D).
Taken all together, the data indicate that HL-60 cells and primary whole-blood PMNs can migrate upstream after blocking Mac-1 under physiologically relevant conditions and on primary endothelial cells at shear rates seen in vivo.
Discussion
The migration of immune cells along the activated endothelium is an important step of the leukocyte adhesion cascade and the innate immune response (1). This step is critical as cells probe the endothelial surface for the best place to transmigrate through the endothelium to the sites of inflammation (40). Neutrophils, which employ the leukocyte adhesion cascade, are among the first cells to arrive to a site of infection and resolve the inflammatory event (41). Although the individual steps of the leukocyte adhesion cascade have been studied extensively (1, 2, 42, 43, 44), we have been intrigued by the recent discovery that several immune cell subsets and tumor cells are able to crawl efficiently against the direction of shear slow. Our laboratory and others have demonstrated that both murine and human T lymphocytes (17, 45), human HSPCs (23), and murine marginal zone B cells (21, 22) are able to efficiently crawl against the direction of shear flow on ICAM-1 surfaces and surfaces coated with mixtures of ICAM-1 and VCAM-1 but maintain a preference for downstream migration on VCAM-1 surfaces (20, 45). In all immune cells that show upstream migration, migration against the direction of shear flow was is dependent on LFA-1/ICAM-1 interactions (16, 17, 19, 20, 21, 23). A better understanding of the critical mechanisms of migration can allow for the engineering of neutrophils that are able to migrate more quickly though the endothelium and, in turn, resolve inflammation faster (46).
The initial study that described the upstream migration in T cells demonstrated that neutrophils, despite expressing LFA-1 and being able to bind ICAM-1, were unable to migrate upstream (17). In our hands, both the immortalized neutrophil-like HL-60 cell line and primary whole-blood neutrophils were unable to migrate upstream on ICAM-1 surfaces (Fig. 1). But migration on ICAM-1 surfaces in neutrophils is more complicated than in lymphoid cells because myeloid cells have an additional cell-surface receptor for ICAM-1, Mac-1. Although a previous study demonstrated that blocking Mac-1 on the neutrophil surface was not enough to elicit LFA-1-dependent upstream migration on ICAM-1 surfaces (17), we sought to assess whether there were certain conditions under which neutrophils could be induced to migrate against the direction of flow. By lowering the concentration of ICAM-1 on the surface fourfold, we significantly increased the overall motility and area explored by the HL-60 cells and neutrophils. The ability to tune motility by modulating adhesion is well-known and has been demonstrated in other cell types and cell-free systems (19, 35, 36, 47). Also, we demonstrated that at lower concentrations of ICAM-1, blocking Mac-1 on the surface of both HL-60 cells and primary neutrophils induced upstream migration (Fig. 2). We suspect that the inability to have seen upstream migration upon Mac-1 reported by Valignat and co-workers might have been due to the high ICAM-1 concentration used in their study (10 μg/mL), which limited both the overall motility of the neutrophils and their ability to migrate upstream (17).
Our work expands upon the previous observations of upstream migration and demonstrates that neutrophils can, in fact, migrate upstream under flow once Mac-1 is blocked. Although both HL-60 cells and neutrophil are unable to crawl upstream on any surface with isotype (negative control) antibodies, both cell types can be induced to migrate upstream on ICAM-1 surfaces once Mac-1 binding was blocked with monoclonal antibodies. For both cell types, blocking LFA-1 removed upstream migration on ICAM-1 surfaces, demonstrating the indispensable nature of LFA-1-ICAM-1 interactions for migration against the direction of shear flow. Furthermore, surfaces of mixed composition that contained equal amounts of ICAM-1 and VCAM-1 (I + V) were enlightening because HL-60 cells and neutrophils showed a net overall downstream migration on these surfaces, with only ∼15% of cells migrating upstream. However, once Mac-1 is blocked, both neutrophils and HL-60 cells reverse their direction of migration to against the flow, with greater than 50% of cells crawling upstream. This upstream migration can be further enhanced (∼75–80% of cells crawling upstream) by additively blocking VLA-4 in combination with Mac-1 to remove the VLA-4/VCAM-1 interactions. Removing the VLA-4/VCAM-1 interactions did not induce a strong phenotype on the ICAM-1 and VCAM-1 surface because the behavior of neutrophils and HL-60 cells was not significantly different than cells blocked with an isotype antibody (Fig. 5). These experiments recapitulate the complex chemistry neutrophils will see on the endothelium upon activation by TNF-α and IL-1β during inflammation (48). Indeed, similar results were seen on stimulated HUVEC monolayers because both HL-60 cells and primary neutrophils were unable to crawl upstream when treated with isotype-blocking antibodies but could crawl upstream once Mac-1 was blocked. Blocking VLA-4 and Mac-1 increased the ability and number of neutrophils crawling upstream on HUVECs, whereas blocking LFA-1 completely removed upstream migration. In sum, neutrophils migrate against the direction of flow via LFA-1/ICAM-1 interactions and with the direction of flow via VLA-4/VCAM-1 interactions in all contexts. As for Mac-1/ICAM-1 interactions, in our studies, they caused the HL-60 cells and neutrophils to migrate with the direction of flow because the removal of Mac-1 binding function is a prerequisite for upstream migration. Previous studies have demonstrated that murine neutrophils are able to migrate perpendicularly to the direction of shear flow, in a Mac-1- and VAV-1-dependent manner on murine plasma and mouse cremaster venules (49). Although we do not see a prominent mode of perpendicular migration in our studies, species, surface-specific differences, and differences in the activation state of the neutrophils in migration may account for this disparity. We suspect neutrophils can utilize these versatile modes of motility to gain entry into tissues under a variety of conditions.
As to what the exact mechanism for upstream migration is and whether it is a passive or active process is still an open question. A previous study strongly suggested that the upstream migration of T cells on ICAM-1 is due to a passive steering mechanism of the uropod during shear flow (16). The “wind vane” hypothesis was borne from the lack of changes in upstream migration seen when intracellular calcium signaling and phosphotidyl-3-kinase were inhibited. The same group has further quantified this “wind vane” hypothesis by demonstrating that the direction of migration of the cells is principally a mechanism driven by where the cell contacts the endothelial surface. It was hypothesized that during LFA-1/ICAM-1 interactions, the cell contacts the surface at the leading edge and the uropod sticks up into the free stream, the wind vane is engaged, and the cell migrates upstream (50). On the other hand, with VLA-4/VCAM-1 interactions, the uropod is principally in contact with the surface, so the wind vane cannot engage and the cell swims downstream (50). It remains to be seen whether these modes of motility hold true in neutrophils, especially if blocking Mac-1 on ICAM-1 surfaces switches the point of contact with the surface from the uropod to the leading edge, activating the wind vane. This, as well as further investigation to understand the factors that control upstream migration from within the cell, would warrant further study outside the scope of this work.
The biological function of upstream migration is still unknown, but we have some clues as to its function from other types of cells displaying this mode of motility. Marginal zone B cells will crawl upstream on ICAM-1, against the blood flow that goes from the follicle to the red pulp in the spleen, when leaving the marginal zone to enter the follicle for further maturation (21, 22). Furthermore, human breast cancer cells are able to migrate against direction of interstitial shear flow in a three-dimensional collagen gel to escape into the leaky vasculature and metastasize further (51, 52). On the other hand, CD4+ T cells (20) and HSPCs (23) utilize upstream migration while migrating on the endothelium pre-transmigration, albeit for different reasons. The CD4+ T cells are trying to reach the lymph node to contact dendritic cells (53), and the HSPCs are trying to home to the bone marrow and reestablish hematopoiesis post-transplantation (54). Migration against the direction of shear flow may be a natural mechanism by which cells home back to their site of activation after rolling and arrest to better transmigrate through the endothelium (55). Further demonstrating this point, a recent study in our lab has shown that T cells are able to migrate upstream after completing the tethering and rolling portions of the leukocyte adhesion cascade, and furthermore, cells migrating upstream after attachment transmigrate significantly faster than those crawling downstream (19). Thus, upstream migration may be a universal mechanism of migration in which cells can backtrack to areas of the endothelium that are more amenable to transmigration.
Although small levels of upstream migration have previously been reported in mouse neutrophils migrating on activated murine cremaster venules (56), this work extends the fascinating phenomenon of upstream migration into human myeloid cells and neutrophils and critically suggests Mac-1 prevents neutrophils from migrating upstream. Because we demonstrate here upstream migration under biologically relevant shear flows, with the primary neutrophils, and in vitro on activated HUVEC monolayers, further study is warranted to determine whether 1) the upstream migration occurs in vivo naturally and 2) whether we can leverage Mac-1 blocking of neutrophils to get them to crawl upstream in vivo. If we understand the molecular origins of upstream motility, it might be possible promote it for clinical benefit such as for a more rapid resolution of inflammation.
Author Contributions
A.B. conceived the project, designed and performed experiments, and wrote the manuscript. N.R.A. designed and performed experiments and helped edit the manuscript. D.A.H. designed research, coordinated project activities, and helped write and edit the manuscript.
Acknowledgments
The authors would like to thank Jason Chen and Prof. Scott L. Diamond (University of Pennsylvania) for help with the phlebotomy of whole blood and isolation of primary human neutrophils. The authors would also like to thank Prof. Valerie M. Weaver (University of California-San Francisco) for the use of her facilities during the review experiments.
This work was supported by the National Institutes of Health, National Institute of General Medical Sciences grants NIH GM123019 and GM133060 to D.A.H.
Editor: Pablo Iglesias.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2019.08.044.
Supporting Material
References
- 1.Ley K., Laudanna C., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 3.Buffone A., Jr., Mondal N., Neelamegham S. Silencing α1,3-fucosyltransferases in human leukocytes reveals a role for FUT9 enzyme during E-selectin-mediated cell adhesion. J. Biol. Chem. 2013;288:1620–1633. doi: 10.1074/jbc.M112.400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malý P., Thall A., Lowe J.B. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins P.P., McEver R.P., Cummings R.D. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J. Biol. Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- 6.Hidalgo A., Peired A.J., Frenette P.S. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chase S.D., Magnani J.L., Simon S.I. E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease. Ann. Biomed. Eng. 2012;40:849–859. doi: 10.1007/s10439-011-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondal N., Stolfa G., Neelamegham S. Glycosphingolipids on human myeloid cells stabilize E-selectin-dependent rolling in the multistep leukocyte adhesion cascade. Arterioscler. Thromb. Vasc. Biol. 2016;36:718–727. doi: 10.1161/ATVBAHA.115.306748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimrichter L., Burdick M.M., Schnaar R.L. E-selectin receptors on human leukocytes. Blood. 2008;112:3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eash K.J., Greenbaum A.M., Link D.C. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimura T. Chemokine receptors and neutrophil trafficking. In: Harrison J.K., Lukacs N.W., editors. The Chemokine Receptors. Humana Press; 2007. pp. 71–86. [Google Scholar]
- 12.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 13.Luo D., McGettrick H.M., Nash G.B. The roles of integrins in function of human neutrophils after their migration through endothelium into interstitial matrix. PLoS One. 2015;10:e0118593. doi: 10.1371/journal.pone.0118593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heit B., Colarusso P., Kubes P. Fundamentally different roles for LFA-1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J. Cell Sci. 2005;118:5205–5220. doi: 10.1242/jcs.02632. [DOI] [PubMed] [Google Scholar]
- 15.Rowe R.G., Weiss S.J. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Valignat M.P., Nègre P., Theodoly O. Lymphocytes can self-steer passively with wind vane uropods. Nat. Commun. 2014;5:5213. doi: 10.1038/ncomms6213. [DOI] [PubMed] [Google Scholar]
- 17.Valignat M.P., Theodoly O., Lellouch A.C. T lymphocytes orient against the direction of fluid flow during LFA-1-mediated migration. Biophys. J. 2013;104:322–331. doi: 10.1016/j.bpj.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abram C.L., Lowell C.A. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson N.R., Buffone A., Jr., Hammer D.A. T lymphocytes migrate upstream after completing the leukocyte adhesion cascade. Cell Adhes. Migr. 2019;13:163–168. doi: 10.1080/19336918.2019.1587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominguez G.A., Anderson N.R., Hammer D.A. The direction of migration of T-lymphocytes under flow depends upon which adhesion receptors are engaged. Integr. Biol. (Camb) 2015;7:345–355. doi: 10.1039/c4ib00201f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tedford K., Steiner M., Fischer K.-D. The opposing forces of shear flow and sphingosine-1-phosphate control marginal zone B cell shuttling. Nat. Commun. 2017;8:2261. doi: 10.1038/s41467-017-02482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tedford K., Tech L., Fischer K.D. Analysis of shear flow-induced migration of murine marginal zone B cells in vitro. J. Vis. Exp. 2018;141 doi: 10.3791/58759. e58759. [DOI] [PubMed] [Google Scholar]
- 23.Buffone A., Jr., Anderson N.R., Hammer D.A. Migration against the direction of flow is LFA-1-dependent in human hematopoietic stem and progenitor cells. J. Cell Sci. 2018;131:jcs205575. doi: 10.1242/jcs.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katagiri K., Kinashi T., Katagiri T. Differential regulation of leukocyte function-associated antigen-1/intercellular adhesion molecules-1-dependent adhesion and aggregation in HL-60 cells. Blood. 1996;87:4276–4285. [PubMed] [Google Scholar]
- 25.Ding Z.M., Babensee J.E., Ballantyne C.M. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- 26.Carrigan S.O., Weppler A.L., Stadnyk A.W. Neutrophil differentiated HL-60 cells model Mac-1 (CD11b/CD18)-independent neutrophil transepithelial migration. Immunology. 2005;115:108–117. doi: 10.1111/j.1365-2567.2005.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson D.C., Miller L.J., Springer T.A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J. Immunol. 1986;137:15–27. [PubMed] [Google Scholar]
- 28.Mondal N., Buffone A., Jr., Neelamegham S. ST3Gal-4 is the primary sialyltransferase regulating the synthesis of E-, P-, and L-selectin ligands on human myeloid leukocytes. Blood. 2015;125:687–696. doi: 10.1182/blood-2014-07-588590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolfa G., Mondal N., Neelamegham S. Using CRISPR-Cas9 to quantify the contributions of O-glycans, N-glycans and Glycosphingolipids to human leukocyte-endothelium adhesion. Sci. Rep. 2016;6:30392. doi: 10.1038/srep30392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Roozendaal K.E.P., Darling D., Farzaneh F. DMSO and retinoic acid induce HL-60 differentiation by different but converging pathways. Exp. Cell Res. 1990;190:137–140. doi: 10.1016/0014-4827(90)90155-4. [DOI] [PubMed] [Google Scholar]
- 31.English D., Andersen B.R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J. Immunol. Methods. 1974;5:249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- 32.Oh H., Siano B., Diamond S. Neutrophil isolation protocol. J. Vis. Exp. 2008;(17):745. doi: 10.3791/745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson N.R., Lee D., Hammer D.A. An experimentally determined state diagram for human CD4+ T lymphocyte CXCR4-stimulated adhesion under shear flow. Cell. Mol. Bioeng. 2018;11:91–98. doi: 10.1007/s12195-018-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buffone A., Jr., Nasirikenari M., Lau J.T.Y. Leukocyte-borne α(1,3)-fucose is a negative regulator of β2-integrin-dependent recruitment in lung inflammation. J. Leukoc. Biol. 2017;101:459–470. doi: 10.1189/jlb.3A0516-215RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eniola A.O., Krasik E.F., Hammer D.A. I-domain of lymphocyte function-associated antigen-1 mediates rolling of polystyrene particles on ICAM-1 under flow. Biophys. J. 2005;89:3577–3588. doi: 10.1529/biophysj.104.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eniola A.O., Willcox P.J., Hammer D.A. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys. J. 2003;85:2720–2731. doi: 10.1016/s0006-3495(03)74695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKay J.L., Hammer D.A. Stiff substrates enhance monocytic cell capture through E-selectin but not P-selectin. Integr. Biol. 2016;8:62–72. doi: 10.1039/c5ib00199d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dustin M.L., Springer T.A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J. Cell Biol. 1988;107:321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bendell A.C., Williamson E.K., Hammer D.A. The Arp2/3 complex binding protein HS1 is required for efficient dendritic cell random migration and force generation. Integr. Biol. (Camb) 2017;9:695–708. doi: 10.1039/c7ib00070g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller W.A. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013;50:7–22. doi: 10.1177/0300985812469883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selders G.S., Fetz A.E., Bowlin G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017;4:55–68. doi: 10.1093/rb/rbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chavakis E., Choi E.Y., Chavakis T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb. Haemost. 2009;102:191–197. doi: 10.1160/TH08-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nourshargh S., Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Schnoor M. Endothelial actin-binding proteins and actin dynamics in leukocyte transendothelial migration. J. Immunol. 2015;194:3535–3541. doi: 10.4049/jimmunol.1403250. [DOI] [PubMed] [Google Scholar]
- 45.Steiner O., Coisne C., Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J. Immunol. 2010;185:4846–4855. doi: 10.4049/jimmunol.0903732. [DOI] [PubMed] [Google Scholar]
- 46.de Oliveira S., Rosowski E.E., Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat. Rev. Immunol. 2016;16:378–391. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palecek S.P., Loftus J.C., Horwitz A.F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 48.Mackay F., Loetscher H., Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J. Exp. Med. 1993;177:1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phillipson M., Heit B., Kubes P. Vav1 is essential for mechanotactic crawling and migration of neutrophils out of the inflamed microvasculature. J. Immunol. 2009;182:6870–6878. doi: 10.4049/jimmunol.0803414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hornung A., Sbarrato T., Valignat M.-P. A bistable mechanism mediated by integrins controls mechanotaxis of leukocytes. bioRxiv. 2018 doi: 10.1016/j.bpj.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polacheck W.J., Charest J.L., Kamm R.D. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl. Acad. Sci. USA. 2011;108:11115–11120. doi: 10.1073/pnas.1103581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polacheck W.J., German A.E., Kamm R.D. Mechanotransduction of fluid stresses governs 3D cell migration. Proc. Natl. Acad. Sci. USA. 2014;111:2447–2452. doi: 10.1073/pnas.1316848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunter M.C., Teijeira A., Halin C. T cell trafficking through lymphatic vessels. Front. Immunol. 2016;7:613. doi: 10.3389/fimmu.2016.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahin A.O., Buitenhuis M. Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adhes. Migr. 2012;6:39–48. doi: 10.4161/cam.18975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beste M.T., Lee D., Hammer D.A. An integrated stochastic model of “inside-out” integrin activation and selective T-lymphocyte recruitment. Langmuir. 2012;28:2225–2237. doi: 10.1021/la203803e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumagin R., Prizant H., Sarelius I.H. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J. Immunol. 2010;185:7057–7066. doi: 10.4049/jimmunol.1001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.