Abstract
Human papillomavirus (HPV) infection is one of the most common sexually transmitted infections worldwide and is associated with precancerous lesions and cancers of the cervix, vulva, vagina, penis, anus, tonsils and base of the tongue. Several studies show an increased risk of HPV-associated cancers in solid organ transplant recipients (SOTR). The aims of this review are to investigate the evidence of efficacy for the HPV vaccination in transplant recipients and to discuss the known national guidelines. A systematic literature search has been conducted to identify studies where SOTR received the HPV vaccination to evaluate the efficacy of the HPV vaccine on this population. The primary outcome was antibody response against the HPV genotypes included in the vaccines and the secondary outcome was national guidelines recommending HPV vaccination of SOTR. Three cohort studies evaluated immunogenicity. Two studies found suboptimal effect of the HPV vaccine, while an early terminated study detected 100% seropositivity. We have identified four national guidelines in the following countries; United States of America, Canada, Australia and Ireland, along with a recommendation from the World Health Organization (WHO).
The results from the three studies were inconclusive due to the small sample sizes and the diverging results. Recommendations of HPV vaccination of SOTR is based on the knowledge about safety and efficiency in the general population and the safety of other inactivated (not live) vaccines in SOTR. Theoretically, the nonavalent vaccine should be recommended as the first choice in SOTR without age- or sex restrictions.
Abbreviations: HPV, human papilloma virus; SOTR, solid organ transplant recipients
Keywords: Human papillomavirus vaccine, HPV vaccine, Solid organ transplant recipient, Immunocompromised, Immunogenicity, Guideline
Introduction
Human papillomavirus (HPV) infection is one of the most common sexually transmitted infections worldwide. Typically, the virus types are classified into low risk and high risk. Low-risk viruses are related to warts, while high risk viruses are associated with precancerous lesions and cancers of the cervix, vulva, vagina, penis, anus, tonsils and the base of the tongue. The most common HPV-related cancer is cervical cancer [1]. Each year approximately 528,000 new cases of cervical cancers are diagnosed, causing 266,000 deaths globally. This makes cervical cancer the fourth most common cancer among women worldwide [2]
Today, the number of solid organ transplant recipients (SOTRs) and other immunocompromised patients is continuously increasing. Due to medical improvement in screening, diagnostics and treatment options, these patients have a continuously increasing life expectancy, and their quality of life is improved accordingly [3,4]. However, because SOTRs tend to live longer, they will also be exposed to immunosuppressants for a longer time, making the transplant recipients more susceptible to infections, such as HPV.
Several studies, including a large meta-analysis (n = 31,977) [5] and an extensive registry study (n = 187,649) [6], show increased risk of HPV-associated cancers (cervical, vaginal, vulvar, penile, anal and oropharyngeal) in SOTRs. Compared to the general population, Grulich et al.’s meta-analysis [5] found higher standardised incidence ratios (SIR) for cancers of the cervix (SIR 2.13), vulva and vagina (SIR 22.76), oral cavity and pharynx (SIR 3.23), penis (SIR 15.79) and anus (SIR 4.85) in SOTRs.
Currently, there are three prophylactic HPV vaccines available: the bivalent (HPV 16 and 18), the quadrivalent (HPV 6, 11, 16 and 18) and the nonavalent (HPV 6, 11, 16, 18, 31, 33, 45, 52 and 58) vaccines [7]. These HPV vaccines have been shown to be highly efficient in healthy individuals, preventing HPV-related lesions [[8], [9], [10], [11]]. Since 2006, publications [12,13] have requested trials on the efficacy of the HPV vaccines in immunocompromised patients.
The aims of this review are to investigate the evidence for the efficacy of HPV vaccination in SOTRs and to discuss the known national guidelines.
Material and methods
This systematic review was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [14]. Additionally, a PICO (population, intervention, comparison and outcome) strategy was adapted for the literature search. Our population consisted of patients who had undergone solid organ transplantation (kidney, liver, heart and lung), because we considered SOTRs to be good representatives for immune compromised individuals. The intervention was different types of HPV vaccines. Our primary outcomes were initially dysplasia and HPV-associated cancers. The secondary outcome was guidelines recommending HPV vaccination in SOTRs.
We conducted a literature search in the following databases: PubMed, Embase and Cochrane Library. The searching period lasted from the 10th of October 2017 to the 30th of November 2017. The PubMed and Cochrane Library search query is seen in the “Supplementary Table 1″. The same search strategy including equivalent search terms was performed in Embase. The search consisted of two searching blocks and a combination of “MeSH terms”/“Emtree” and “All fields”/ “Key words”/ “All text”. Search block 1 contained our population; “Organ transplant” and the specific organs with their synonyms. Search block 2 included our intervention, “Papillomavirus vaccines”, with synonyms. The two searching blocks were then combined, and no filters were used. Additional studies were identified using reference lists to find other relevant articles that might have been overlooked in our database search.
Titles and abstracts, as well as full-text articles, were screened by two different reviewers using predefined criteria for inclusion and exclusion. Criteria necessary for inclusion were (1) publications in English or the Scandinavian languages, (2) literature from 2005 to date, (3) studies in humans, (4) SOTRs that had received HPV vaccination, (5) patients of both sexes and (6) no age restrictions. Conference abstracts and publications not available in full-text were excluded. If any discrepancies arose between the two reviewers, it was resolved through consensus.
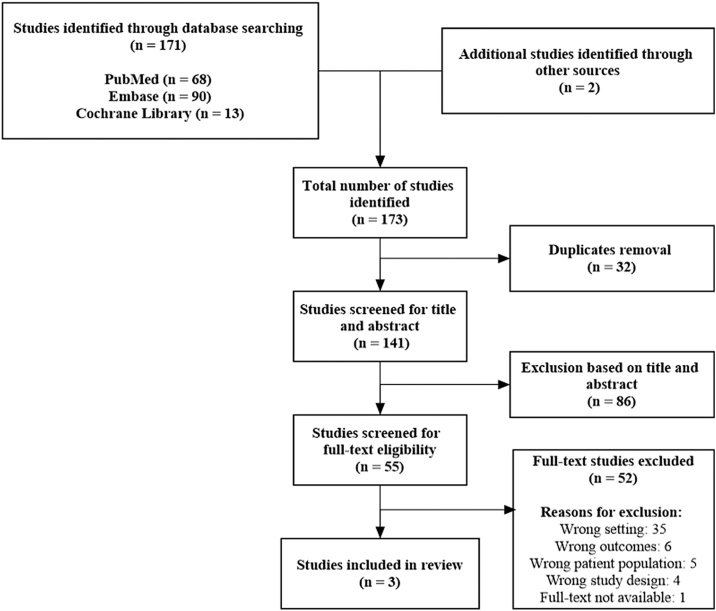
The literature search yielded a total of 173 studies for further evaluation. After removing 32 duplicates, 141 studies were screened for title and abstract. Of these, 86 studies were excluded and 55 were found relevant for full-text eligibility screening. After full-text article screening, we were not successful in identifying any articles with our primary outcomes. However, we were able to detect three studies [[15], [16], [17]] and one conference abstract (not accessible data and excluded) [18] investigating HPV antibody response. As this is a theoretical measure of effect for the HPV vaccine, we found it reasonable to change our outcome to immunogenicity. Figure 1 gives an overview of the screening strategy of our studies.
Fig. 1.
Flowchart of the study selection process.
A search in the clinical trials’ database also showed that there are some upcoming studies on both the quadrivalent and the nonavalent vaccines [19].
Guidelines and recommendations were identified through the reference lists of the 173 studies and additional relevant websites. This yielded four different national guidelines [[20], [21], [22], [23], [24]] and one recommendation [25]. Due to language restrictions, we were only able to address guidelines written in English or the Scandinavian languages.
Results
Out of 173 identified studies, three cohorts fulfilled our eligibility criteria [[15], [16], [17]]. The studies used a variation of methods for measuring antibody response: competitive Luminex immunoassay (cLIA), immunoglobulin G competitive Luminex immunoassay (IgG cLIA) and immunoglobulin G enzyme-linked immunosorbent assay (IgG ELISA). The three cohort studies [[15], [16], [17]] all used cLIA, which was, in fact, also used in the licensing study of the HPV vaccine [26]. We used the “Critical Appraisal Skills Programme (CASP)” checklist [27] as a supplement to our analysis of the three studies [[15], [16], [17]]. Study characteristics are shown in Table 1, and cLIA results with seropositivity threshold values are presented in Table 2.
Table 1.
Study characteristics.
| Nelson et al. (17): | Kumar et al. (16): | Gomez-Lobo et al. (15): | |
|---|---|---|---|
| Publication year: | 2016 | 2013 | 2014 |
| Nation: | USA | Canada | USA |
| Number of hospitals or centres: | Two | One | Two |
| Vaccine type: | Quadrivalent HPV vaccinea | Quadrivalent HPV vaccinea | Quadrivalent HPV vaccinea |
| Methods of measuring: | IgG cLIA and cLIA | IgG ELISA and cLIA | cLIA |
| Participants: | n = 23b | n = 47c | n = 17d |
| Sex (n): | Females (23) | Females (31) | Not specified |
| Males (16) | |||
| Age range in years: | 11- 21 | 18 - 35 | 11-19 |
| Type of organ transplant (n): | Kidney (23) | Kidney (30) | Kidney (14) |
| Liver (1) | Liver (3) | ||
| Lung (11) | |||
| Heart (3) | |||
| Heart-lung / multivisceral (2) |
cLIA; competitive Luminex Immunoassay, IgG cLIA; Immunoglobulin G competitive Luminex Immunoassay, IgG ELISA; Immunoglobulin G enzyme-linked immunosorbent assay.
The quadrivalent HPV vaccine was given at month 0, month 2 and month 6.
Nelson et al.(17) enrolled 67 patients, 57 patients completed the three-dose vaccine series and had at least one blood draw after vaccination. Of these 57 patients; 25 had chronic kidney disease, nine were dialysis patients and 23 were kidney transplant recipients.
Kumar et al.(16) enrolled 50 patients, two patients did not receive any doses of the vaccine and one was found to have a prior history of low-grade squamous lesion. These three patients were excluded, while n = 47 continued in the study.
Gomez-Lobo et al.(15) enrolled 25 liver and kidney transplant recipients, of these 17 patients fulfilled criteria to start vaccination.
Table 2.
Geometric mean titres (GMTs) for competitive Luminex Immunoassay (cLIA) in milliMerck units per millilitre (mMu/mL) obtained at seven months (four weeks/one month after the third vaccine dose).
| Age range in years: | Number of patients: | HPV-6: | HPV-11: | HPV-16: | HPV-18: | |
|---|---|---|---|---|---|---|
| Seropositivity threshold values [31]: | 20 | 16 | 20 | 24 | ||
| Nelson et al. (17)a: | 11 – 15 | n = 8b | 154 | 222 | 409 | 61 |
| 16 – 21 | n = 13b | 52 | 72 | 137 | 36 | |
| Kumar et al. (16): | 18 – 35 | n = 32c | 14.7d | 32.6d | 36.4d | 11.3d |
| Gomez-Lobo et al. (15): | 12 – 18 | n = 7 (Kidney) | 1056 | 1303 | 6872 | 1619 |
| n = 1 (Liver) | 158 | 1882 | 824 | 1616 | ||
| Healthy population (26): | 9 – 15 | 929.2 (n = 917) | 1304.6 (n = 917) | 4918.5 (n = 915) | 1042.6 (n = 922) | |
| 16 – 26 | 545.0 (n = 3329) | 748.9 (n = 3353) | 2409.2 (n = 3249) | 475.2 (n = 3566) | ||
| 27 – 34 | 435.6 (n = 439) | 577.9 (n = 439) | 2348.5 (n = 435) | 385.8 (n = 501) |
Obtained 1–12 months after completion of the vaccination series.
Only 21/23 kidney transplant recipients with Luminex measures at seven months.
Only 32/46 transplant recipients with Luminex measures at seven months.
Kumar et al.(16) measured GMTs in milliMerck units per litre (mMu/L).
Nelson et al. [17] studied the antibody response to HPV vaccination in patients with chronic kidney disease; dialysis patients and kidney transplant recipients (KTRs). This cohort included 57 young females for analysis; among these, 23 were KTRs (Table 1). The first blood samples were drawn before vaccine dose 1 (baseline), the second blood samples were drawn at 1–12 months after vaccine dose 2, and the third blood samples were drawn at 12–35 months after vaccine dose 3 to measure antibody levels against HPV genotypes 6,11,16 and 18. Patients had to complete the three-dose vaccine series and have at least one blood sample drawn after vaccination to be included in the analysis. Their methods of measuring HPV antibodies were IgG cLIA and cLIA.
Percentages of seropositivity were calculated after measurement by IgG cLIA, yielding the following results in the second blood samples (n = 22): HPV 6: 63.6%, HPV 11: 63.7%, HPV 16: 100% and HPV 18: 72.7%. Seropositivity in the third blood samples (n = 8) were HPV 6: 62.5%, HPV 11: 50%, HPV 16: 75% and HPV 18: 50%.
Geometric mean titre (GMT) values in milliMerck units per millilitre (mMu/mL) by cLIA were obtained for the KTRs and sorted according to age. The second blood samples were obtained from eight patients aged 9 to 15 years and the other age group of 16 to 21 years yielded 13 patient results (Table 2). The third blood samples were obtained from five patients in the youngest age group; HPV 6: 79 mMu/mL, HPV 11: 107 mMu/mL, HPV 16: 156 mMu/mL and HPV 18: 38 mMu/mL. In the older age group, three patient results were obtained: HPV 6: 56 mMu/mL, HPV 11: 46 mMu/mL, HPV 16: 133 mMu/mL and HPV 18: 25 mMu/mL.
The Mann–Whitney test was used to compare the KTRs’ GMTs to the healthy population’s GMTs. The healthy population data were extracted from the Gardasil (quadrivalent HPV vaccine) licensing study [26]. The differences in GMTs in the second blood samples were p = 0.02, while in the third blood samples they were p = 0.06 (9–15 years) and p = 0.15 (16–26 years).
Nelson et al. [17] concluded that there was a less robust reaction to the HPV vaccine in KTRs than in patients with chronic kidney disease and on dialysis based on the seropositivity percentages and GMTs. The study also suggested that female KTRs may benefit from an alternative HPV vaccine regimen to optimise their protection and requested the need for larger trials and efficacy studies.
Kumar et al. [16] analysed the immunogenicity of the quadrivalent HPV vaccine in SOTRs: kidney, liver, lung, heart and heart-lung/multivisceral transplants. The study contained 47 SOTRs (Table 1); 38 of these received all three vaccine doses and completed the blood draws before vaccination and the blood draws seven months after the first vaccine dose (i.e. the per-protocol population). Blood was drawn before vaccine dose 1 (baseline), prior to each vaccination, at seven months (four weeks after completed vaccine series) and one year post-vaccination. The immunogenicity was measured using HPV4-plex IgG ELISA and cLIA.
For the analysis using IgG ELISA, the vaccine response was evaluable for 38 patients. Seropositivity at seven months was as followed: HPV 6: 63.2%, HPV 11: 68.4%, HPV 16: 63.2% and HPV 18: 52.6%. A response to all four HPV genotypes was seen in 47.4% (18 of 38 patients).
GMT values in milliMerck units per litre (mMu/L) using cLIA were available for 32 patients at month 7 (Table 2). Percentage of cLIA for patients receiving all three vaccine doses at seven months; HPV 6: 23.1%, HPV 11: 66.7%, HPV 16: 51.9% and HPV 18: 14.8%. Only 7.4% responded to all four HPV genotypes, while 74.1% responded to at least one HPV genotype at seven months.
Kumar et al. [16] found suboptimal immunogenicity in SOTRs. Seropositivity was distinctively lower compared with two large randomised clinical trials of healthy individuals, where seropositivity ranged from 97% to 99% [28,29]. Some individuals (n = 27) had both assays (IgG ELISA and cLIA) performed at seven months. They found the results to be significantly correlated, although IgG ELISA had higher seropositivity than cLIA. Already six months after completed vaccination, the antibody titres had declined for all HPV genotypes. However, the seropositive proportion did not change, which is comparable to results found in immunocompetent persons [30].
Gomez-Lobo et al. [15] studied adolescent liver transplant recipients and KTRs. A total of 17 patients fulfilled the criteria to start HPV vaccination (Table 2). Blood samples were analysed at day 1 (baseline), as well as after seven months (one month after completed vaccine series). Immunogenicity was evaluated using cLIA. An emergency interim analysis was done due to concerns regarding acute rejection (AR) in KTRs. The study was terminated early, and only nine patients completed the three-dose vaccine series.
All eight transplant recipients (seven kidney and one liver) that completed both vaccine series and the seventh month blood draws were seronegative at baseline. GMTs at seven months for the KTRs are shown in Table 2.
Gomez-Lobo et al. [15] found an unexpectedly high antibody response of 100% to all four HPV genotypes in their eight patients, which is similar to GMTs measured in healthy individuals (Table 2) [26]. The study concluded that the reaction to the HPV vaccine was robust and that it elicited better immunogenicity than other similar vaccines given to SOTRs.
Several studies referred to guidelines and recommendations for HPV vaccination in SOTRs.
We identified four national guidelines in the following countries: the United States of America [20,21], Canada [22], Australia [23] and Ireland [24], along with a recommendation from the WHO [25]. These are presented in Table 3.
Table 3.
Guidelines and recommendations for HPV vaccination.
| Professional society, Country: | Solid organ transplant recipients (SOTR): | Healthy individuals: |
|---|---|---|
| SST, Denmark (32): | No recommendation. | Females from 12 to 18 years of age. |
| WHO (25): | All immunocompromised, including transplant recipients (regardless of whether they are receiving antiretroviral therapy). | Female from 9 to 14 years of age. If resources are available, the age range could be expanded up to 18 years and could also include males. |
| CDC, USA (20): | All immunocompromised both females and males, from 9 to 26 years of age. | Females from 9 to 26 years of age. Males from 9 – 21 years of age. |
| IDSA, USA (21, 33): | All SOTR from 11 to 26 years of age(21). | Females from 9 to 26 years of age(33). |
| PHAC, Canada (22, 34): | Before or after transplantation in females and males from 9 to 26 years of age(22). | Both females and males from 9 to 26 years of age(34). |
| AGDH, Australia (23, 35): | Before or after transplantation in both females and males from 9 years of age and above(23). | Both females and males from 9 to 18 years of age(35). |
| HSE, Ireland (24, 36): | Before or after transplantation in patients from 10 years of age and above(24). | Females and males may be given the vaccine from 9 to 26 years of age(36). |
SST; Sundhedsstyrelsen (The Danish health authorities), WHO; World Health Organization, CDC; Center of disease control and prevention, IDSA; Infectious Diseases Society of America, PHAC; Public Health Agency of Canada, AGDH; Australian Government Department of Health, HSE; Health Service Executive.
Discussion
This review investigated whether there is any evidence for efficacy of HPV vaccination in SOTRs.
It is important to acknowledge that immunogenicity is only a theoretical measure of HPV vaccine efficacy. Interestingly, research has not been successful in determining the lower antibody thresholds associated with protection against HPV-associated lesions. This means that low GMTs do not necessarily result in limited protection against the development of these lesions. In fact, the efficacy of the vaccine can only be truly answered by using clinical outcomes (such as HPV-associated lesions) through long-term follow-up studies.
Based on the results of the three cohort studies [[15], [16], [17]], immunogenicity of the quadrivalent HPV vaccine seems to have had suboptimal effect in two of the studies [16,16,17], while an early-terminated study [15] found 100% seropositivity. However, the small sample sizes and the diverging results of the studies make it difficult to draw meaningful conclusions. (Table 2).
The HPV vaccine is found to be safe in healthy individuals and is associated with few adverse events [37]. Vaccination of SOTRs and other immunocompromised patients is challenging in terms of both efficacy and safety. Even though there are not many studies evaluating the safety specifically for the HPV vaccination in SOTRs, it is assumed to be safe since it is an inactivated (not live) vaccine [38]. This assumption was confirmed in two of the studies [16,17], which found no severe safety concerns regarding the HPV vaccination. Despite this, the third study [15] terminated early due to an unexpectedly high AR rate. Of 14 KTRs, six developed AR after receiving the HPV vaccine. Gomez-Lobo et al. [15] could not conclude whether the AR occurred regardless of the HPV vaccination or whether the HPV vaccine contributed to development of donor-specific antibodies and subsequently AR. Nevertheless, the two other studies and studies of similar vaccines could not find an association between vaccination and increased AR rate [16,17,39,40].
There are several factors that may influence the HPV vaccine response in SOTRs. Among these are time of vaccination, different immunosuppressants, type of organ transplant and HPV naivety. Kumar et al. [16] found that early vaccination after transplant’ high tacrolimus levels and having a lung transplant were all associated with reduced immunogenicity. Also, two studies [16,17] included patients who were not HPV naive prior to the vaccination. This may have influenced the results, as described in Kumar et al.’s [16] study, where these patients had a higher antibody response at seven months compared to HPV-naive patients.
Increased age is also associated with a weakened immune system, which could lead to a lowered antibody response. A randomised clinical trial by Dobson et al. [41] evaluated immunogenicity of the quadrivalent vaccine in two different age groups of healthy females. Two doses were given to girls aged 9–13 years, and three doses were given to women aged 16–26 years. Comparing the immunogenicity of both groups one month after the last vaccine dose, girls achieved 1.77–2.24-fold higher GMTs than women. This suggests that age itself contributes to a weakening of the immune system.
In a case report by Freiberger et al. [42], a 19-year-old female developed multiple HPV-associated lesions after bilateral lung transplantation. The young female received a two-dose vaccine series of the quadrivalent vaccine, but still developed lesions of the oesophagus, cervix and skin. Even if she had been fully vaccinated, the quadrivalent vaccine would probably not have protected her from these lesions, since her lesions were associated with HPV genotype 82. This illustrates that HPV genotypes other than those included in the vaccine may contribute to the development of HPV-associated lesions. At the same time, a weakened immune system does not necessarily react optimally to the vaccine. This highlights that vaccinations should be combined with screening (Pap smear) and that one does not exclude the importance of the other. In fact, a more intensive cervical screening programme in SOTRs is suggested by some publications [43,44], not only the first years post-transplantation, but for several years after.
In a global context, SOTRs are a quite small population, which may lead to ignoring the importance of their receiving the HPV vaccine. Absence of financial support from the vaccine industry may also be challenging due to lack of profit. SOTRs along with other patients suffering from, e.g., leukaemia, lymphoma, AIDS, psoriasis and inflammatory bowel disease are just some of many patients accounting for the large group known as immunocompromised patients. Immunocompromised patients are continuously increasing in number. In recent years, improved immunosuppressive treatments, screening methods, diagnostics and other medical achievements have enhanced the survival and life quality in these patients [3,4]. Despite this, new challenges arise. Increased survival makes immunosuppressed individuals more susceptible to HPV infection, and a prolonged life expectancy makes them more prone to develop HPV-associated lesions and malignancies.
Despite the increased risk of HPV-associated diseases in SOTRs, there are, to own knowledge, only a few countries that have recommendations regarding HPV vaccination of this population (Table 3). Nonetheless, these guidelines and recommendations [[20], [21], [22], [23], [24], [25]] are not tailored to this population, but are based on knowledge concerning the safety of receiving inactivated (not live) vaccines [38]. In fact, these guidelines and recommendations [[20], [21], [22], [23], [24], [25]] are quite similar to those that apply to healthy individuals [20,25,[33], [34], [35], [36]], as seen in Table 3.
Today, there are many countries with public health vaccination programmes that include the HPV vaccine. For instance, all 12-year-old girls in Denmark are offered the HPV vaccination [32]. Given that the national childhood vaccination programmes had high endorsement in both girls and boys, many of the future SOTRs would already have been protected against HPV-associated lesions before they even became immunosuppressed.Unfortunately, this is not the situation in Denmark, where the HPV vaccination rates are rather low and the vaccine is only given to adolescent girls [45]. This illustrates the need for developing guidelines for HPV vaccination of SOTRs.
Limitations to the included studies
First, they are all small studies [[15], [16], [17]], the largest being Kumar et al. [16] with only 38 patients for analysis. Second, in two of the studies [15,16], the inclusion of the patients was not sufficiently described. This makes it difficult to rule out systematic selection bias. Third, in the three studies [[15], [16], [17]], some participants were excluded without proper description of the reasons. Therefore, we found it problematic to follow the process of exclusion. Fourth, none of the cohorts [[15], [16], [17]] had a control group; rather, they compared their results to large studies of healthy individuals. This may be due to their small sample sizes and that it seems unethical to withhold the HPV vaccine. Fifth, Kumar et al. [16] used two different measuring methods. However, both methods were not applied to all the participants, making it a possible source of bias. Sixth, Kumar et al. [16] listed their results for cLIA in mMu/L instead of the usual unit (mMu/mL). Due to the study’s conclusion of suboptimal immunogenicity, it seems likely to be a typographical error. Seventh, completion of blood draws was a challenge for all the included cohorts [[15], [16], [17]]. Influencing factors were variable time of blood sampling, lacking baseline results and incompletion of the second and the third blood draws. Eighth, the early termination of Gomez-Lobo et al.’ study [15] makes their results rather inadequate.
Limitations to our systematic review
First, few studies with small sample sizes and diverging results made them inconclusive [[15], [16], [17]]. Second, the three cohorts [[15], [16], [17]] varied with regard to the age of the population; this may be a possible limitation when comparing the results. Third, KTRs were overrepresented compared to other transplant types in the studies [[15], [16], [17]]. Therefore, these results may not be representative for all SOTRs. Fourth, the three studies [[15], [16], [17]] used cLIA as a common measure method, but it was only the main method in Gomez-Lobo et al.15. In the two other studies [16,16,17], limited cLIA results were obtained, which made the comparability of the three studies challenging [[15], [16], [17]]. Fifth, we cannot exclude the risk of selection or publication bias, although an effort has been made to include all relevant articles.
Conclusions
To optimally evaluate the efficacy of the HPV vaccine in SOTRs, trials investigating development of HPV-associated lesions would be necessary. Since HPV-associated cancers develop over time, a study would be quite comprehensive. It also seems unethical to withhold the HPV vaccine, given that it is highly efficient in healthy individuals [[8], [9], [10], [11]] and that it is seemingly safe in SOTRs [38]. Based on these findings, there is convincing argumentation to recommend vaccination of SOTRs.
Theoretically, the nonavalent vaccine should be recommended as the first choice in SOTRs due to its broad coverage of HPV genotypes. The HPV vaccine should be administered without any age or sex restrictions, as long as no evidence for an age recommendation exists. We consider it preferable to vaccinate at a time when the immune system is still robust, e.g. pre-transplantation in SOTRs. There are still some unanswered questions. For instance, whether an alternative and more intensive vaccine regimen would be more efficient, and whether the HPV vaccination should be a part of the common treatment strategies for transplant recipients and candidates. Further studies are needed for addressing these questions.
Conflicts of interest
J.B. has received lecture fees and advisory board fees from Merck, Sanofi Pasteur MSD, and Glaxo Smith Kline. The remaining authors have stated explicitly that they have no conflicts of interest in connection with this article.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurox.2019.100015.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.WHO: World Health Organization; 2019. Human papillomavirus (HPV) and cervical cancer.http://www.who.int/mediacentre/factsheets/fs380/en/ [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;(136):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Grinyó J.M. Why is organ transplantation clinically important? Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain A., Reyes J., Kashyap R., Dodson S.F., Demetris A.J., Ruppert K. Long-term survival after liver transplantation in 4.000 consecutive patients at a single center. Ann Surg. 2000;232:490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grulich A.E., van Leeuwen M.T., Falster M.O., Vajdic C.M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 6.Madeleine M.M., Finch J.L., Lynch C.F., Goodman M.T., Engels E.A. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant. 2013;13:3202–3209. doi: 10.1111/ajt.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute NC.; 2017. Gardasil 9 vaccine protects against additional HPV types.https://www.cancer.gov/types/cervical/research/gardasil9-prevents-more-HPV-types [Google Scholar]
- 8.Lehtinen M., Paavonen J., Wheeler C.M., Jaisamrarn U., Garland S.M., Castellsagué X. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 9.Paavonen J., Naud P., Salmeron J., Chow S.N., Apter D., Kitchener H. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 10.Dillner J., Kjaer S.K., Wheeler C.M., Sigurdsson K., Iversen O.E., Hernandez-Avila M. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldur-Felskov B., Dehlendorff C., Junge J., Munk C., Kjaer S.K. Incidence of cervical lesions in Danish women before and after implementation of a national HPV vaccination program. Cancer Causes Control. 2014;25:915–922. doi: 10.1007/s10552-014-0392-4. [DOI] [PubMed] [Google Scholar]
- 12.Esposito S., Prada E., Lelii M. Immunization of children with secondary immunodeficiency. Hum Vaccin Immunother. 2015;11:2564–2570. doi: 10.1080/21645515.2015.1039208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palefsky J.M., Gillison M.L., Strickler H.D. HPV vaccines in immunocompromised women and men. Vaccine. 2006;24(SUPPL. 3):S140–S146. doi: 10.1016/j.vaccine.2006.05.120. [DOI] [PubMed] [Google Scholar]
- 14.2017. PRISMA (Preferred reporting items for systematic reviews and meta-analyses) checklist.http://prisma-statement.org/PRISMAStatement/Checklist.aspx [Google Scholar]
- 15.Gomez-Lobo V., Whyte T., Kaufman S., Torres C., Moudgil A. Immunogenicity of a prophylactic quadrivalent human papillomavirus L1 virus-like particle vaccine in male and female adolescent transplant recipients. Pediatr Transplant. 2014;18:310–315. doi: 10.1111/petr.12226. [DOI] [PubMed] [Google Scholar]
- 16.Kumar D., Unger E.R., Panicker G., Medvedev P., Wilson L., Humar A. Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am J Transplant. 2013;13:2411–2417. doi: 10.1111/ajt.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson D.R., Neu A.M., Abraham A., Amaral S., Batisky D., Fadrowski J.J. Immunogenicity of human papillomavirus recombinant vaccine in children with CKD. Clin J Am Soc Nephrol. 2016;11:776–784. doi: 10.2215/CJN.09690915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nailescu C., Slaven J., Saha C., Shew M. The response to human papillomavirus vaccination in pediatric patients before and after kidney transplantation. Pediatr Transplant. 2015;19:80. [Google Scholar]
- 19.U.S. National Library of Medicine; 2017. Search: human papilloma virus vaccine | transplant.https://clinicaltrials.gov/ct2/results?cond=transplant&term=human+papilloma+virus+vaccine&cntry1=&state1=&Search=Search [Google Scholar]
- 20.CDC: Centers for Disease Control and Prevention; 2017. Use of a 2-Dose schedule for human papillomavirus vaccination - updated recommendations of the advisory committee on immunization practices.https://www.cdc.gov/mmwr/volumes/65/wr/mm6549a5.htm [DOI] [PubMed] [Google Scholar]
- 21.Rubin L.G., Levin M.J., Ljungman P., Davies E.G., Avery R., Tomblyn M. IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2013;2014(58):3022. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- 22.Government of Canada; 2017. Canadian immunization guide: part 3 - vaccination of specific populations; p. 8.https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-8-immunization-immunocompromised-persons.html#p3c7t4 [Google Scholar]
- 23.Australian Government Department of Health; 2017. 3.3 Groups with special vaccination requirements. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10-home˜handbook10part4˜handbook10-4-6#4-6-4. [Google Scholar]
- 24.HSE: Health Service Executive; 2015. Immunisation of immunocompromised persons. [Google Scholar]
- 25.WHO: World Health Organization; 2017. Table 1: summary of WHO position papers - recommendations for routine immunization. [Google Scholar]
- 26.GmbH Merck. Whitehouse Station; New Jersey: 2006. Gardasil product insert 9682302. Merck GmbH. [Google Scholar]
- 27.CASP: Critical Appraisal Skills Programme; 2017. CASP cohort study checklistm.http://www.casp-uk.net/casp-tools-checklists [Google Scholar]
- 28.Munoz N., Manalastas R., Pitisuttithum P., Tresukosol D., Monsonego J., Ault K. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–1957. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 29.Giuliano A.R., Palefsky J.M., Goldstone S. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garland S.M., Hernandez-Avila M., Wheeler C.M., Perez G., Harper D.M., Leodolter S. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 31.Dias D., Van Doren J., Schlottmann S., Kelly S., Puchalski D., Ruiz W. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12:959–969. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dan Health Authority (SST: Sundhedsstyrelsen); 2017. Questions and answers on the HPV vaccine.https://www.sst.dk/en/disease-and-treatment/vaccination/hpv-vaccination/questions-and-answers-on-the-hpv-vaccine#1 [Google Scholar]
- 33.IDSA: Infectious Diseases Society of America; 2017. Statement on the human papillomavirus (HPV) vaccine.http://www.idsociety.org/HPV_Statement.aspx [Google Scholar]
- 34.Government of Canada; 2017. Canadian immunization guide: part 4 - active vaccines; p. 9.https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-9-human-papillomavirus-vaccine.html#a5 [Google Scholar]
- 35.Australian Government Department of Health; 2017. 4.6 Human papillomavirus. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10-home˜handbook10part4˜handbook10-4-6#4-6-4. [Google Scholar]
- 36.HSE: Health Service Executive; 2016. Chapter 10 Human papillomavirus. [Google Scholar]
- 37.CDC: Centers for Disease Control and Prevention; 2017. Human papillomavirus (HPV) vaccine safety.https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html [Google Scholar]
- 38.Danziger-Isakov L., Kumar D. Vaccination in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):311–317. doi: 10.1111/ajt.12122. [DOI] [PubMed] [Google Scholar]
- 39.Kimball P., Verbeke S., Flattery M., Rhodes C., Tolman D. Influenza vaccination does not promote cellular or humoral activation among heart transplant recipients. Transplantation. 2000;69:2449–2451. doi: 10.1097/00007890-200006150-00042. [DOI] [PubMed] [Google Scholar]
- 40.Lawal A., Basler C., Branch A., Gutierrez J., Schwartz M., Schiano T.D. Influenza vaccination in orthotopic liver transplant recipients: absence of post administration ALT elevation. Am J Transplant. 2004;4:1805–1809. doi: 10.1111/j.1600-6143.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 41.Dobson S.R., McNeil S., Dionne M., Dawar M., Ogilvie G., Krajden M. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793–1802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 42.Freiberger D., Lewis L., Helfand L. Human papillomavirus-related high-grade squamous intraepithelial lesions of the esophagus, skin, and cervix in an adolescent lung transplant recipient: a case report and literature review. Transpl Infect Dis. 2015;17:98–102. doi: 10.1111/tid.12322. [DOI] [PubMed] [Google Scholar]
- 43.Kasiske B.L., Vazquez M.A., Harmon W.E., Brown R.S., Danovitch G.M., Gaston R.S. Recommendations for the outpatient surveillance of renal transplant recipients. Am Soc Transplant J Am Soc Nephrol. 2000;11(Suppl 1):S1–S86. [PubMed] [Google Scholar]
- 44.Meeuwis K.A., van Rossum M.M., van de Kerkhof P.C., Hoitsma A.J., Massuger L.F., de Hullu J.A. Skin cancer and (pre)malignancies of the female genital tract in renal transplant recipients. Transpl Int. 2010;23:191–199. doi: 10.1111/j.1432-2277.2009.00975.x. [DOI] [PubMed] [Google Scholar]
- 45.SSI: Statens Serum Institut; 2017. Epi-nyt.https://www.ssi.dk/Aktuelt/Nyhedsbreve/EPI-NYT/2017/Uge%2025%20-%202017.aspx [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.