Summary
Sporadic Medullary Thyroid Carcinoma (sMTC) is a rare but aggressive thyroid tumor. RET and RAS genes are present in about 50%–80% of cases, but most of the remaining cases are still orphan of a genetic driver. We studied the largest series of sMTC by deep sequencing to define the mutational landscape. With this methodology we greatly reduced the number of RET- or RAS-negative cases and we confirmed the central role of RET and RAS mutations. Moreover, we highlighted the bad prognostic role of RET mutations in sMTC and consolidated the favorable prognostic role of RAS mutations. For the first time, we showed that the variant allele frequency represents an additional prognostic marker inside the group of RET-mutated sMTC.
Subject Areas: Biological Sciences, Cancer, Genomics
Graphical Abstract
Highlights
-
•
We studied by NGS the largest cohort of sporadic MTC that has been studied so far
-
•
RET and RAS mutations have been confirmed as the major drivers in sporadic MTC
-
•
Allele frequency can be considered a new marker of bad prognosis in RET-mutated cases
-
•
Survival rate is significantly shorter in RET-mutated than in RAS-mutated cases
Biological Sciences; Cancer; Genomics
Introduction
Medullary Thyroid Carcinoma (MTC) originates from neural crest-derived parafollicular C-cells and can occur in hereditary (25%) or sporadic forms (75%) (Kouvaraki et al., 2005). Germline-activating RET mutations are found in 95%–98% of hereditary MTC, whereas somatic RET mutations are present in 25%–40% of sporadic MTC (sMTC) (Cerrato et al., 2009, Drosten and Putzer, 2006, Kouvaraki et al., 2005, Romei et al., 2011). Several types of somatic RET mutations have been reported in sMTC, with the most common mutation occurring in codon M918 within exon 16, which is present in up to 90% of RET-positive cases, followed by mutations in codon C634 within exon 11 (Elisei et al., 2014, Eng et al., 1994, Romei et al., 2016).
The presence of RET somatic mutations in sMTC has been shown to have a negative prognostic value (Elisei et al., 2008). In addition to point mutations, aneuploidy of chromosome 10 and RET gene amplification have been described in MTC cases, prevalently in cases with a somatic RET mutation (Ciampi et al., 2012).
Recently, activating point mutations in RAS genes (H-, K-, and NRAS) has been described in RET-negative sMTC, with a variable percentage depending on the different series and screening techniques employed (Agrawal et al., 2013, Boichard et al., 2012, Ciampi et al., 2013, Moura et al., 2015, Moura et al., 2011). RAS gene point mutations in MTC mainly occur in H- and KRAS, and they are usually mutually exclusive with RET mutations. In our previous study, we found that patients harboring RAS mutations showed a better prognosis than those harboring RET mutations or presenting no mutations (Ciampi et al., 2013).
Despite the presence of RET and RAS somatic mutations, 20–50% sMTC are still orphans of a genetic driver. Assessing the mutational status, especially for the RET gene, is crucial for targeted therapies with tyrosine kinase inhibitors currently employed, such as vandetanib and cabozantinib (Elisei et al., 2013, Wells et al., 2010), and the discovery of new oncogene alterations remains crucial to individuate novel targets for this type of therapy.
The recent advent of next-generation sequencing (NGS) techniques has dramatically changed our understanding of cancer genomics with the discovery of novel genetic alterations responsible for the pathogenesis of several cancer types (Berger and Mardis, 2018).
In these recent years, NGS has been applied in endocrine research as well (Persani et al., 2018), and several reports have been published for MTC, in some cases using a whole-exome approach (Agrawal et al., 2013, Chang et al., 2018) but mainly targeted sequencing (Heilmann et al., 2016, Ji et al., 2015, Simbolo et al., 2014, Wei et al., 2016). According to the results reported in these studies, despite the presence of some rare events present in a few cases, the common occurrence of mutually exclusive RET and RAS mutations has been confirmed to be the main pathogenic signature of sMTC. The few novel alterations found in these studies represent more likely a “private” mutation of that specific tumor than significantly recurrent genetic alterations. The major limits of these previous studies are the relative low number of cases analyzed and few data about the correlation between the mutations and clinical and pathological features of the tumors.
We aimed to analyze a large series of sMTC by NGS targeted sequencing using a thyroid-specific gene mutation panel to delineate their mutational landscape and correlate the molecular data with the pathological characteristics of the tumors and with both the clinical features and outcome of patients affected with sMTC.
Results
Sequencing Metrics and Overview of Gene Alterations Detected by NGS Targeted Sequencing
Of 209 cases studied, 28 were excluded as not informative due to technical reasons or insufficient quality of data obtained. Informative sequencing data were then obtained for 181/209 (86.6%) sMTC. The mean value of the variant vertical coverage obtained was 2,038X (median, 2,049.5; range, 117.7–5,713), and the mean number of reads for the sample was 385,564.1 (median, 396,496.5; range, 21,198–1,744,507).
In total, 166 genetic alterations were detected in 148 sMTC cases (Table S1). In particular, we found 152 single-nucleotide variations and 14 indels: 107/166 (64.5%) were found in the RET protooncogene, 48/166 (28.9%) in the three RAS genes (HRAS, KRAS, NRAS), 5/166 (3%) in the MET gene, 2/166 (1.2%) in the TP53 gene, 1/166 (0.6%) in the TSH receptor (TSHR) gene, 1/166 (0.6%) in the EIF1AX gene, 1/166 (0.6%) in the CHK2 gene, and 1/166 (0.6%) in thePPM1D gene. One hundred fifty-four gene alterations were validated by Sanger direct sequencing and confirmed to be somatic, five were found to be germline, one was confirmed in tissue DNA, but blood was not available for germline validation, and six were not validated by Sanger direct sequencing due to low Variation Allele Frequency (VAF) values or for other technical reasons.
All the mutations that were previously detected by Sanger sequencing for somatic RET and RAS mutations were confirmed by NGS while in eight cases we observed a discrepancy with a previous negative result by Sanger and a positive one by NGS. This apparent discrepancy was due either to the low VAF that was under the detection limit of Sanger (<20%) or bad Sanger sequencing quality.
Analysis of Genetic Alterations Occurring in sMTC Cases
General Distribution of Mutations
As shown in Table S1, the number of cases harboring one or more genetic alterations was 148/181 (81.7%), whereas the remaining 33/181 (18.3%) did not carry any alteration targeted in our panel. In particular, 132/148 (89.2%) mutated cases harbored one single mutation, whereas 11/148 (7.4%) showed a heterogeneous pattern due to the presence of a somatic driver mutation coupled with one or more other somatic mutations and 5/148 (3.4%) harbored one somatic driver mutation coupled with a second germline mutation (Table 1).
Table 1.
List of Cases Presenting Multiple Somatic Alterations and Somatic Coupled with a Germ-Line Alteration (in bold). Variant Allele Frequency (VAF) Values Are Reported in Brackets
| No | Alteration 1 | Alteration 2 | Alteration 3 | Alteration 4 |
|---|---|---|---|---|
| 41 | RET M918T (s) [47.3%] | RET D925A (s) [46.7%] | ||
| 242 | RET M918T (s) [32.2%] | RET R297H (s) [11.8%] | ||
| 196 | RET M918T (s) [12.3%] | RET R833C (s) [25.0%] | RET S891A (s) [20.3%] | MET T1010I (n.v.) [18.6%] |
| 140 | RET M918T (s) [39.0%] | KRAS K182E (n.v.) [20.0%] | ||
| 176 | RET C634W (s) [17.3%] | NRAS A18V (s) [7.5%] | ||
| 253 | RET C634W (s) [11.6%] | MET T1010I (s) [50.1%] | ||
| 132 | RET D898_E901del (s) [30.9%] | RET S904P (s) [30.8%] | ||
| 169 | RET C620R (s) [41.5%] | MET T1010I (n.v.) [6.7%] | ||
| 3 | RET C618G (n.v.) [40.5%] | TP53 R283C (n.v.) [48.1%] | ||
| 251 | HRAS G13R (s) [23.3%] | MET T1010I (s) [8.3%] | ||
| 39 | HRAS Q61R (s) [34.4%] | RET M918T (s) [3.0%] | ||
| 88 | RET M918T (s) [19.7%] | KRAS A130V (g) [44.9%] | ||
| 91 | RET C634Y (s) [37.3%] | RET R215L (g) [49.9%] | ||
| 201 | RET D898_E901del (s) [26.6%] | PPM1D K469E (g) [37.5%] | ||
| 20 | HRAS Q61R (s) [41.7%] | MET T1010I (g) [51.4%] | ||
| 52 | TSHR I630L (s) [31.0] | TP53 R158C (g) [52.8%] |
(s), verified somatic; (n.v.), not detectable by direct sequencing.
Types of Mutations
Cases presenting RET somatic alterations as the driver were 101/181 (55.8%): in 88 cases as a single alteration and in 13 cases as multiple alterations. Cases presenting RAS mutations as the driver were 44/181 (24.3%): in 42 cases as a single mutation and 2 cases in association with either a somatic or germline MET T1010I mutation. Finally, 3/181 (1.6%) cases presented mutations in other genes (i.e., CHK2 W114*, EIF1AX G135A, and TSHR I630L) (Table S1). The remaining 33/181 (18.3%) were negative for all alterations targeted in our panel.
RET Mutations: Prevalence, Types, and Associations with Other Mutations
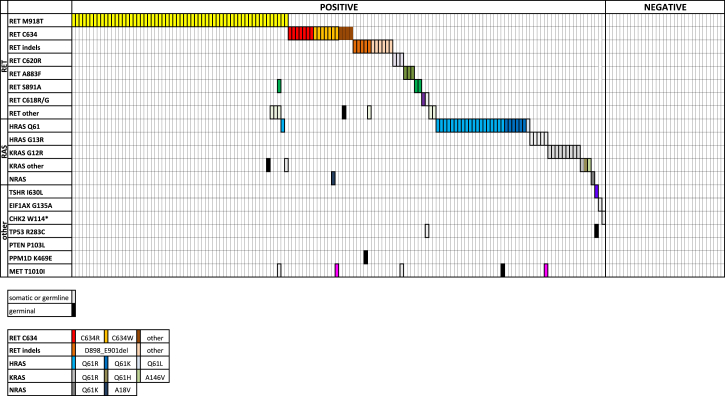
As shown in Figure 1, 60/148 (40.5%) mutated sMTC harbored the RET M918T mutation. In 54 cases, it was present as a single mutation; and in 6 cases, it was associated with other RET (n = 3) or RAS mutations (n = 3). The details of associated mutations are reported in Table 1. The RET gene C634 codon was mutated in 18/148 (12.2%) cases with different aminoacidic alterations (Figure 1). In 15 cases, it was present as a single mutation, whereas, in 3 cases, it was associated with other alterations. A RET indel was present in 14/148 (9.5%) cases (Table S1 and Figure 1): in 12 cases, it was present as a single mutation, while in 2 cases it was associated with other alterations. Additionally, 3/148 (2%) cases presented the C620R mutation, and one of them harbored a simultaneous MET T1010I mutation. Another 2/148 (1.3%) cases showed a C618R mutation, and one of them had a simultaneous TP53 R283C mutation. Finally, 2/148 (1.3%) cases presented the RET S891A mutation, and 1/148 (0.7%) cases showed the RET C630R and 1/148 (0.7%) cases showed the RET S1024F mutation. Among cases harboring RET multiple mutations, cases n. 41 and 132 presented the RET M918T + D925A and RET D898_E902del + S904P mutations, respectively. The analysis of the specific sequencing reads associated with these mutations showed that they were very close and on the same allele (i.e., in –cis), likely consequent to a single mutational event (data not shown). A complete detailed description of these mutations is summarized in Table S1 and Figure 1.
Figure 1.
Mutational Landscape of sMTC
Mutational profile of the 168 informative sMTC cases identified by NGS analysis. Each column corresponds to a single case. Genetic variations are listed on the left. The colored squares correspond to somatic mutations, whereas the black squares correspond to germline mutations, all validated by Sanger sequencing. Squares with a point-pattern represent mutations that were not validated by Sanger or not confirmed to be somatic or germline. See also Table S1.
RAS Mutations: Prevalence, Types, and Associations with Other Mutations
Alterations of the three RAS genes were present in 44/148 (29.7%) mutated cases. Of 148 sMTC cases, 31 (20.9%) were mutated in the HRAS gene and included 2 cases with a simultaneous somatic or germline MET T1010I mutation, respectively (Figure 1). Another 12/148 (8.2%) cases were positive in KRAS, and only 1/148 (0.7%) presented the NRAS Q61K mutation (Table S1 and Figure 1).
Other Unconventional Mutations: Prevalence, Types, and Associations
Only 3/148 (2%) sMTC cases harbored single mutations in other genes belonging to our panel, such as CHK2 W114*, EIF1AX G135A, and TSHR I630L. The last case was also associated with a germline TP53 R158C mutation. Although the TSHR I630L mutation has been validated as somatic, the other two mutations could not be, and consequently, we could not establish their potential driver role.
Rare and Uncommon Mutations In the Analyzed Genes
As shown in Table 2, among the above-reported mutations, we found a series of 18 uncommon and/or novel alterations. With the exception of the somatic or germline MET T1010I mutation, which was present in five separate cases already harboring either RET or RAS alterations (Table 3), all the others were single mutations in single cases. Considering their rarity and according to the in silico analysis (i.e., Clinic Var and MutTaster prediction tests) and public database of known gene alterations (i.e., dbSNP and COSMIC and HGMD), we hypothesized that they could be private mutations whose driver role in the pathogenesis of the sMTC is unclear (Table 3).
Table 2.
Details of Unconventional Alterations Found by NGS Targeted Sequencing
| Case | Gene Mutation | VAF (%) | Status | dbSNP ID | MAF | COSMIC ID α HGMD ID β | ClinVar Prediction γ MutTaster Prediction δ | Notes |
|---|---|---|---|---|---|---|---|---|
| 88 | KRAS c.389C > T; p.A130V | 44.9 | Germ line | rs730880473 | <0.01 | COSM4169153 α | Uncertain significance γ | Simultaneous RET M918T (s); reported as “neutral” (Wang et al., 2019) |
| 41 | RET c.2774A > C; p.D925A | 46.7 | Somatic | Novel | – | Novel | Disease causing δ | Occurring in -cis with RET M918T (s) |
| 242 | RET c.890G > A; p.R297H | 11.8 | Somatic | Novel | – | Novel | Polymorphism δ | Simultaneous RET M918T (s) |
| 196 | RET c.2497C > T; p.R833C | 25 | Somatic | rs377767422 | <0.01 | CM068590 β | Likely pathogenic γ | Simultaneous RET M918T (s) |
| 20,169,196251,253 | MET c.3029C > T; p.T1010I | Various | Somatic; germ line | rs56391007 | <0.01 | COSM707 α CM118113 β |
Conflicting results δ | |
| 176 | NRAS c.53C > T; p.A18V | 7.5 | Somatic | Novel | – | Novel | Disease causing δ | Simultaneous RET C634W (s) |
| 91 | RET c.644G > T; R215L | 49.9 | Germ line | rs748128929 | <0.01 | – | Polymorphism δ | Simultaneous RET C634Y (s) |
| 201 | PPM1D c.1405A > G; p.K469E | 37.5 | Germ line | rs61756416 | <0.01 | – | Disease causing δ | Simultaneous RET E898_E901del (s); reported as “benign” in breast and ovarian cancer (Ruark et al., 2013) |
| 132 | RET c.2710T > C; p.S904P | 30.8 | Somatic | Novel | – | Novel | Disease causing δ | Occurring in -cis with RET E898_E901del (s) |
| 128 |
RET c.1908_1909insTGCCG CACG; p.T636_V637delinsCRT |
35.4 | Somatic | rs377767437 | – | CI983210 β | Likely pathogenic γ | Described germ-line in MEN2A (Höppner et al., 1998) |
| 122 | RET c.1886_1891delTGTGCG; p.L629_D631delinsH | 38.4 | Somatic | NA | – | COSM27040 α | – | Likely driver |
| 302 |
RET c.1894_1902delGAGCT GTGC; p.E632_C634del |
42.9 | Somatic | Novel | – | Novel | – | Likely driver |
| 252 | RET c.3071C > T; p.S1024F | 17.6 | Somatic | Novel | – | Novel | Disease causing δ | Likely driver |
| 3 | TP53 c.847C > T; p.R283C | 48.1 | n.v. | rs149633775 | <0.01 | COSM10911 α/CM041458 β | Conflicting results δ | Simultaneous RET C618G (s) |
| 52 | TSHR c.1888A > C; p.I630L | 31 | Somatic | – | – | COSM26432 α/CM100952 β | Disease causing δ | Simultaneous TP53 R158C (g) |
| 52 | TP53 c.472C > T; p.R158C | 52.8 | Germinal | rs587780068 | <0.01 | COSM43848 α/CM121763 β | Pathogenic γ | Simultaneous TSHR I630L (s) |
| 196 | EIF1AX c.404G > C; G135A | 41.4 | n.v. | Novel | Novel | Disease causing δ | ||
| 198 | CHK2 c.341G > A; W114* | 10.1 | n.v. in blood | Novel | Novel | Disease causing δ |
n.v., not detectable by direct sequencing.
Table 3.
List of Cases Presenting the MET T1010I Mutation in Association with RET or RAS Somatic Mutations
| N. | Somatic Mutation | MET T1010I |
|---|---|---|
| 196 |
RET M918T (s) [12.3%] RET S891A (s) [20.3%] RET R833C (s) [25.0%] |
MET T1010I (n.v.) [18.6%] |
| 253 | RET C634W (s) [11.6%] | MET T1010I (s) [50.1%] |
| 169 | RET C620R (s) [41.5%] | MET T1010I (n.v.) [6.7%] |
| 110 | HRAS Q61R (s) [41.7%] | MET T1010I (g) [51.4%] |
| 251 | HRAS G13R (s) [23.2%] | MET T1010I (s) [8.3%] |
Variant allele frequency (VAF) value is reported in brackets.
(s), verified somatic; (g), verified germinal; (n.v.), not detectable by direct sequencing.
TERT Promoter C228 and C250 Mutational Status
The sequencing data for the TERT promoter were available for 148/181 (81.8%) cases. Neither C228T nor C250T mutations were found in any of the studied cases.
Whole-Exome Sequencing
The whole-exome sequencing (WES), despite the wide and deep analysis, did not reveal any other recurrent somatic mutation either in the four sMTC negative at the targeted sequencing or in those already known to be RET mutated.
Correlation of the Mutational Status of Primary sMTC with the Clinical and Pathological Features of the Patients
The 175/209 (83.7%) sMTC cases, whose primary tumor was analyzed, were divided into four categories depending on the mutational status (RET M918T, RET other, RAS mutations, and not RET/not RAS) and were correlated with the clinical and pathological features of the patients (Table 4). A statistically significant correlation was found between the presence of RET mutations, both together and when considering M918T alone, and the advanced stage of the disease (p = 0.0025), higher T category (p < 0.0001), and the presence of both lymph-node (N) (p = 0.0021) and distant metastases (M) (p = 0.0073).
Table 4.
Correlation between Mutational Status of RET and RAS Genes with Clinical and Pathological Features of the Primary sMTC Cases
| RET M918T | RET Other | RAS | Not RET/Not RAS | p Value | |
| Sex | 0.1378a | ||||
| Female | 51.2% (22/43) | 52.8% (19/36) | 72.5%(29/40) | 67.9% (19/28) | |
| Male | 48.8% (21/43) | 47.2% (17/36) | 27.5% (11/40) | 32.1% (9/28) | |
| Age at diagnosis (mean ± SD) (years) | 49.43 ± 13.93 | 55.67 ± 14.32 | 55.81 ± 15.44 | 58.84 ± 15.70 | 0.0779b |
| Primary/metastases | 0.1609a | ||||
| Primary | 74.1% (43/58) | 83.7% (36/43) | 90.9% (40/44) | 84.8% (28/33) | |
| Metastases | 25.9% (15/58) | 16.3% (7/43) | 9.1% (4/44) | 15.2% (5/33) | |
| Outcome | <0.0001a | ||||
| Disease-free | 26.3% (10/38) | 66.7% (20/30) | 61.8% (21/34) | 77.3% (17/22) | |
| Biochemical | 7.9% (3/38) | 6.7% (2/30) | 29.4% (10/34) | 9.1% (2/22) | |
| Metastatic/dead | 65.8% (25/38) | 26.6% (8/30) | 8.8% (3/34) | 13.6% (3/22) | |
| Stage | 0.0001a | ||||
| I | 18.9% (7/37) | 46.9% (15/32) | 62.5% (25/40) | 70.8% (17/24) | |
| III | 81.1% (30/37) | 53.1% (17/32) | 37.5% (15/40) | 29.2% (7/24) | |
| T Categories | <0.0001a | ||||
| T1+T2 | 37.1% (13/35) | 72.7% (24/33) | 90.0% (36/40) | 83.3% (20/24) | |
| T3+T4 | 62.9% (22/35) | 27.3% (9/33) | 10.0% (4/40) | 16.7% (4/24) | |
| Lymph-node metastasis (N) | 0.0021a | ||||
| N0 | 30.6% (11/36) | 54.5% (18/33) | 66.7% (26/39) | 75.0% (18/24) | |
| N1 | 69.4% (25/36) | 45.5% (15/33) | 33.3% (13/39) | 25.0% (6/24) | |
| Distant metastasis (M) | 0.0073a | ||||
| M0 | 77.8% (28/36) | 90.6% (29/32) | 97.5% (39/40) | 100.0% (24/24) | |
| M1 | 22.2% (8/36) | 9.4% (3/32) | 2.5% (1/40) | 0 (0/24) |
Unless stated, values are expressed in % (number/total number).
Chi-squared test.
One-way ANOVA test.
In contrast to RET-mutated cases, RAS-mutated sMTC cases were significantly associated with a better outcome (p = 0.001), a lower stage of disease (p = 0.0037), and lower T category (i.e., T1/T2) (p = 0.0015), but no correlation was observed between the presence of RAS mutation and other epidemiological and pathological features (Table 5).
Table 5.
Association of RAS-Mutated sMTC Cases and Clinical and Pathological Features
| RAS+ | RAS- | p Value | |
|---|---|---|---|
| Sex | 0.089a | ||
| Female | 72.5% (29/40) | 56.4% (62/110) | |
| Male | 27.5% (11/40) | 43.6% (48/110) | |
| Age at diagnosis (mean ± SD) | 55.81 ± 15.44 | 58.84 ± 15.70 | 0.571b |
| Outcome | 0.0003a | ||
| Disease-free | 61.8% (21/34) | 52.2% (47/90) | |
| Biochemical | 29.4% (10/34) | 7.8% (7/90) | |
| Metastatic/dead | 8.8% (3/34) | 40% (36/90) | |
| Stage | 0.0037a | ||
| I + II | 62.5% (25/40) | 41.5% (39/94) | |
| III + IV | 37.5% (15/40) | 58.5% (55/94) | |
| T Categories | 0.0015a | ||
| T1+T2 | 90.0% (36/40) | 62.4% (58/93) | |
| T3+T4 | 10.0% (4/40) | 37.6% (35/93) | |
| Lymph-node metastasis (N) | 0.0857a | ||
| N0 | 66.7% (26/39) | 49.5% (46/93) | |
| N1 | 33.3% (13/39) | 50.5% (47/93) | |
| Distant metastasis (M) | 0.107a | ||
| M0 | 97.5% (39/40) | 88.2% (82/93) | |
| M1 | 2.5% (1/40) | 11.8 (11/93) |
Unless stated, values are expressed in % (number/total number).
Chi-squared test.
Student Unpaired t test.
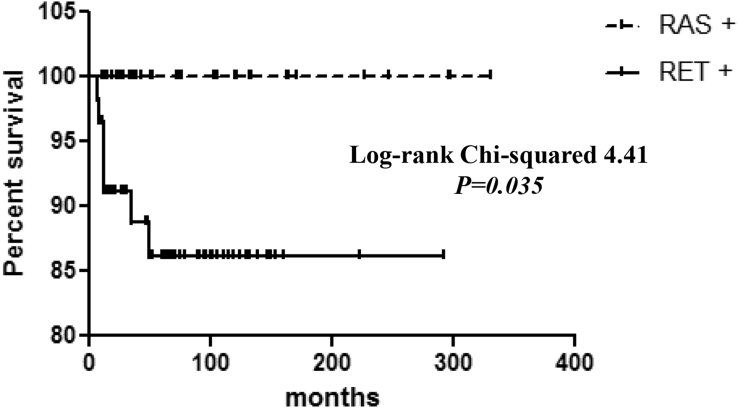
A strong correlation was also found between the presence of RET mutations and a worse patient outcome (p < 0.0001), and the survival of Kaplan-Meier curves confirmed that patients with sMTC harboring the RET mutation had a higher rate of cancer-related deaths than patients harboring RAS mutations (log rank = 4.41; p = 0.035) (Figure 2).
Figure 2.
Survival in RET- and RAS-Mutated sMTC Cases
Kaplan-Meier curves showing survival in patients with sMTC harboring RET mutations or RAS mutations. The difference in the curves was statistically significant (log rank = 4.41; p = 0.035) and demonstrated that RET-positive cases have a higher probability to die of the disease.
Correlation of the Variant Allele Frequency Value with the Tumor Size and Outcome of the Patients
The overall mean VAF of the mutations found was 35.1% (median, 30.2; range, 4.4–95.2). However, a big difference in VAF was observed among different cases with the lowest VAF observed in the rare and uncommon alterations (Table S1). According to the VAF, we could hypothesize the role of the mutations, especially in those cases with more than one alteration: the mutation with the greatest VAF would likely be the driver mutation (Li et al., 2017).
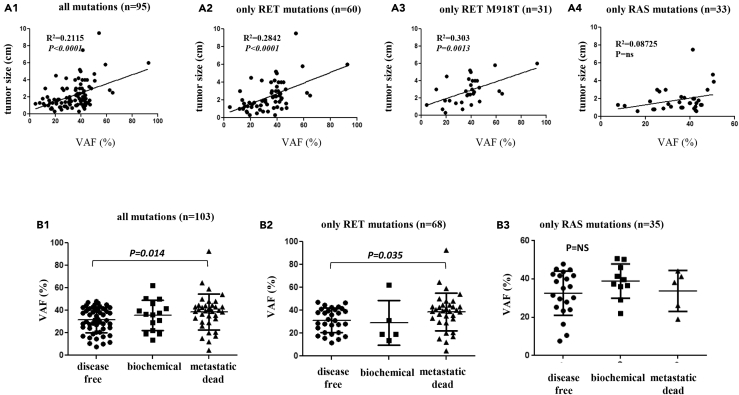
As shown in Figure 3, panel A1, when we compared in 95 patients with primary tumor the VAF value of the driver mutation, any type, with the sMTC tumor size (in centimeters), we observed that larger tumors harbored mutations with a higher VAF value (p < 0.0001). However, when we performed the same analysis in subgroups according to the type of mutation, the correlation was confirmed in the subgroups of RET-mutated cases, either when all RET (Figure 3, panel A2) or only RET M918T-mutated cases were considered (Figure 3, panel A3) (p < 0.0001 and p = 0.0013, respectively) but not in the subgroup harboring RAS mutations (Figure 3, panel A4).
Figure 3.
Correlation of the Variant Allele Frequency with Tumor Size and Outcome of Patients with sMTC
(A) Correlation between the tumor size (cm) and VAF of the driver mutation in sMTC. The comparison considered all mutations (A1), only RET mutations (A2), only the RET M918T mutation (A3), and only RAS mutations (A4). In all cases, except RAS-mutated cases, a statistically significant difference was observed (A1: p < 0.0001; A2: p < 0.0001; A3: p = 0.0013; A4: p = ns).
(B) Correlation between the VAF value (%) of the driver mutations and outcome of patients when considering all mutations (B1), only RET mutations (B2), and only RAS mutations (B3). The differences between the outcome categories were significant between disease-free and metastatic patients in the former two cases (B1: p = 0.003; B2: p = 0.0047; ANOVA), whereas no difference was observed considering only RAS mutations (B3: p = ns). Data are represented as mean ± SEM.
Analyzing 103 patients, a higher VAF value of the driver mutation was also correlated with a worse outcome of the patients, as demonstrated by a significantly higher VAF value in patients with metastatic disease with respect to disease-free patients, both when considering all cases with any type of mutation (p = 0.003) (Figure 3, panel B1) and when analyzing the subgroup with only RET mutations (p = 0.047) (Figure 3, panel B2). By contrast, this correlation was not found in the subgroup with only RAS-positive cases (Figure 3, panel B3).
Discussion
In recent years, the introduction of NGS techniques has revolutionized research and the diagnosis of many diseases, including endocrine diseases (Persani et al., 2018). In particular, cancer research was hugely improved by this high-throughput techniques, being able to sequence large genome and transcript portions and increasing the probability of discovering novel mutations, especially in less frequently studied genes (Berger and Mardis, 2018, Kamps et al., 2017).
Several studies have been performed in MTC, both employing WES (Agrawal et al., 2013, Chang et al., 2018) and targeted sequencing with specific panels of gene mutations (Heilmann et al., 2016, Ji et al., 2015, Simbolo et al., 2014, Wei et al., 2016). In summary, these studies confirmed the role of RET and RAS somatic mutations as main drivers in the pathogenesis of sMTC; on the other hand, very few alternative genetic alterations have been discovered, still leaving a rather large portion of cases negative for common somatic gene alterations.
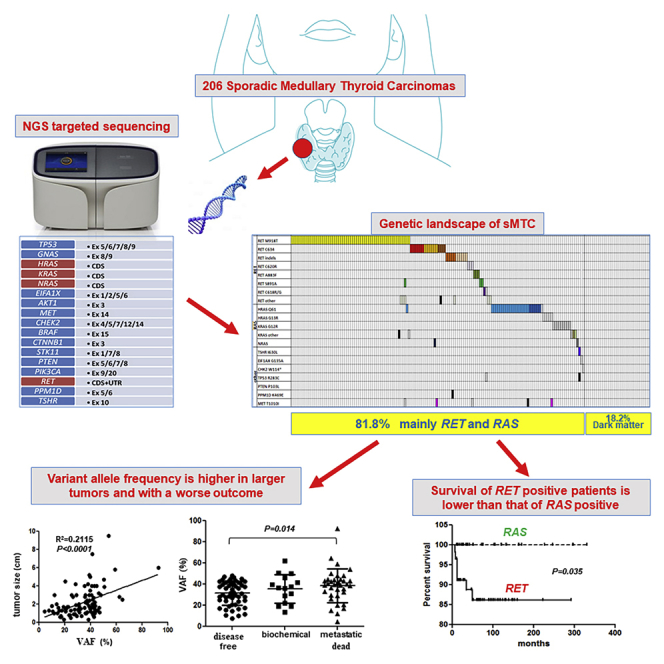
In the present study, we characterized 181 sMTCs by NGS targeted sequencing, and this series represents, to our knowledge, the largest analyzed so far. For this purpose, we designed a custom gene mutational panel that includes all amplicons covering the entire coding region of the RET, HRAS, KRAS, and NRAS genes that are known to be involved in C-cell tumorigenesis (Ciampi et al., 2013) and all the known gene alterations involved in follicular thyroid cancer tumorigenesis (Nikiforov et al., 2014, Nikiforova et al., 2013).
Our data showed that 55.8% of cases harbored RET genetic alterations, confirming that RET, particularly the M918T mutation, is the main driver oncogene in sMTC. The second main driver oncogene has been confirmed to be RAS, particularly HRAS and KRAS genes, which were altered in 24.3% of sMTC cases. Only a small subgroup, representing only 1.6% of cases, showed other types of uncommon mutations whose driver role remains unclear. According to these results, the prevalence of complete negative cases in our series was narrowed to 18.3% of cases (Figure 1), which increased up to 19.9% if we include the subgroup with the uncommon mutations. In agreement with the results of other two studies (Agrawal et al., 2013, Chang et al., 2018), also in our hands the WES was unable to identify other recurrence mutations that could further reduce this subgroup of negative cases. It is conceivable that for these cases other types of genetic and/or epigenetic alterations can play a role.
Compared with previous data obtained analyzing sMTC by Sanger direct sequencing (Ciampi et al., 2013, Elisei et al., 2008), we obtained an overall higher number of mutated cases, especially for RAS positive cases and, at the same time, we reduced the negative or “mutational orphan” sMTC cases. These results strongly support the use, whenever possible, of deep-sequencing techniques whose sensitivity is significantly higher than that of Sanger sequencing; this is a very important aspect to address, especially in metastatic patients who could benefit from targeted therapy whose choice largely depends on the knowledge of mutational status (Viola et al., 2016).
In our series, most cases harbored single mutually exclusive alterations (89.2%), whereas only a small portion of cases (7.4%) harbored multiple somatic mutations (Table 1), demonstrating that, genetically, sMTC is a rather stable tumor similar to what has been shown for papillary thyroid carcinoma (Cancer Genome Atlas Research Network, 2014). Moreover, according to the VAF values in the mutations, 9/11 cases harboring multiple somatic mutations showed a higher VAF of the RET mutation than that of the additional mutations, thus suggesting the driver role of RET also in these heterogeneous cases. It is noteworthy that, in two of four cases with two simultaneous RET mutations (Table 1, cases 41 and 132), the VAF was the same. This finding, combined with the observation that the mutations were closely mapping in –cis within the same sequencing read, indicates that they might be the result of a single simultaneous mutational event in a single allele of the same cell and not truly heterogeneous mutations, thus reducing the percentage of “true” heterogeneous cases to 6.5% of sMTC.
In some cases, the VAF observed in additional mutations was rather low and, in several cases, they represented “novel” mutations never described before and with an uncertain pathogenic role (Table 2). The meaning of these mutations is unknown, but being aware of their presence can be relevant for the follow-up of the patients because, if selected, these mutations could be responsible for acquired resistance during therapy with kinase inhibitors (Camidge et al., 2014, Chen and Fu, 2011), and their presence should not be overlooked.
We also found five cases in which a germinal mutation of key genes in thyroid tumorigenesis was also present and whose real pathogenic role is unclear (Table 1). These five alterations have been already reported in the dbSNP database, although VAF <0.01 excludes them as common SNPs (Table 2). The rarity of these alterations in the normal population suggests some pathogenic role for the tumor (Li et al., 2017), although in silico predictive scores describe only some of them as pathogenic. In particular, two of them (i.e, KRAS A130V and PPM1D K469E) have been described as non-pathogenic/neutral alterations (Ruark et al., 2013, Wang et al., 2019). Even less is known about the new somatic indel mutation (i.e., RET c.1894_1902delGAGCTGTGC; p.E632_C634del) that has never been described previously (Table 2).
Among cases presenting multiple mutations we did not find any difference in terms of number and type of mutations when comparing cases in which we analyzed the primary tumor tissues with in which we analyzed the metastatic lesions. The most recurrent additional mutation (5/181, 2.8%) observed in our series was the MET T1010I point mutation that was present at either the somatic or the germinal level (Table 3), as demonstrated also by the VAF (i.e., approximately 50% when germinal and less than 20% when somatic). In our series, MET T1010I was always associated to a driver mutation either in RET or RAS gene. The T1010I mutation is located in exon 14 of the MET gene encoding for the juxtamembrane portion of the tyrosine kinase (Comoglio et al., 2018) and exon 14 alterations, which are described in different tumor types, might be crucial for activation of the MET oncogene (Vigna et al., 1999). Germinal and/or somatic MET T1010I mutations have been described in several tumor types (Comoglio et al., 2018), including thyroid tumors (Wasenius et al., 2005). In this last study, 104 thyroid tumors were analyzed and somatic or germline MET T1010I mutations were found in 7% of samples: particularly, 1/12 (8.3%) sMTC harbored a germline MET T1010I. We also found either somatic (4/5) or germline (1/5) METT1010I-mutated cases, but all of them were characterized by the presence of another mutation likely driving the tumoral transformation. In general, the role of this mutation in cancer is not fully understood, and conflicting results exist, especially in other human tumors. As suggested for other human cancers (Neklason et al., 2011), a predisposing role of MET T1010I, especially when present at the germline level, might be hypothesized also for sMTC.
Other than describing the oncogene mutations involved in the pathogenesis of sMTC, we also compared the mutational status of these tumors with the clinical and pathological features of patients (Table 4). The presence of RET mutations and, in particular, the M918T was confirmed to be significantly associated with a worse outcome, a higher tumoral staging, a higher T category, and the presence of lymph-node and distant metastases. Moreover, when we compared the survival Kaplan-Meier curves of patients with sMTC with RET or RAS somatic mutations, a significantly lower percentage of surviving patients in the RET-positive cases was found. These results confirmed in a much larger series our previously reported data (Ciampi et al., 2013, Elisei et al., 2008) and those recently collected in a meta-analysis summarizing the clinical significance of the mutational status in MTC (Vuong et al., 2018).
Simultaneously, we observed that RAS-positive cases were significantly associated with a better outcome, a lower tumor staging, and a lower rate of T categories than RET-positive cases, and, also when compared with the RAS negative cases, independent from the presence of RET mutation (Table 5). With these results, we confirmed that sMTC with RAS mutations have, in general, a less aggressive phenotype and a better prognosis as we previously observed (Ciampi et al., 2013) and herein, as definitively demonstrated by the virtually 100% rate of survival of patients harboring RAS mutations (Figure 2).
Compared with previous studies, using the NGS approach, we could also evaluate the VAF value, adding a parameter that it is not possible to evaluate using Sanger direct sequencing. The mutated allele abundance has been demonstrated to be a prognostic factor itself in tumors, including PTC, in which a higher BRAF V600E VAF predicts a poorer outcome (Guerra et al., 2012). Our results demonstrated that, also in this series, larger tumors not only are more frequently RET mutated but also have a higher VAF that corresponds to a higher percentage of mutated cells. This observation suggests that the presence of a RET-mutated allele, particularly M918T, confers a growth advantage and results in larger clonal tumors. These findings are also in line with our previous observation of a lower rate of RET M918T mutation in micro-MTC that, when mutated, likely harbored a much lower VAF of the RET mutation not detectable with the traditional Sanger sequencing (Romei et al., 2012). Moreover, we demonstrated that a higher VAF correlated with a poorer prognosis, both when considering all mutations and when considering only RET mutations. According to these findings, VAF may be included in the list of bad prognostic markers in the subgroup of RET-mutated sMTC cases.
By contrast, RAS-mutated tumors appear to be clonal also in smaller tumors and no difference in the outcome of patients was observed compared with the RAS-mutated allele abundance. Based on this observation, we can hypothesize that two different sMTC types, in terms of both development and aggressiveness, might exist (i.e., RET-like and RAS-like) and further studies are needed to confirm this hypothesis.
Finally, we studied most of the sMTC for the presence of mutations in the TERT gene promoter hotspots (C228, C250), and, conversely, regarding what happens to other thyroid neoplasms (Alzahrani et al., 2016), TERT promoter mutations in C228 and C250, at least in our study, do not play any role in the pathogenesis and/or progression of sMTC.
In conclusion, in the present study, that included the largest series of sMTC studied so far by NGS, we confirmed that RET and RAS gene alterations are the main actors in driving the development of this rare human tumor. Moreover, we reinforced the concept that RET-mutated cases have a more aggressive phenotype and a poorer prognosis, particularly when the VAF is higher, so that this parameter can be considered a new marker of a worse prognosis in RET-mutated sMTC. At the same time, we demonstrated that RAS-mutated cases have a better outcome than RET-mutated cases. Finally, a lower-than-expected percentage of sMTC cases are still orphans of a recognized genetic driver, and further studies for alternative mechanisms of tumor transformation need to be performed.
Limitations of the Study
The main limitation of this study is that we used a custom targeting sequencing panel including a large series of genes involved in thyroid cancer pathogenesis that failed in providing evidence of strong alternative mechanisms of pathogenesis besides RET and RAS mutations. Although in some cases WES analysis was also performed, the possibility that other alterations may be involved cannot be completely ruled out with this study.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Data and Code Availability
For the following novel mutation a submission to COSMIC DATABASE has been requested and an identifier COSP47106 has been generated by COSMIC as a proof of successful data submission.
RET c.2774A>C; p.D925A
RET c.890G>A; p.R297H
NRAS c.53C>T; p.A18V
RET c.2710T>C; p.S904P
RET c.1894_1902delGAGCTGTGC; p.E632_C634del
RET c.3071C>T; p.S1024F
EIF1AX c.404G>C; G135A
CHK2 c.341G>A; W114∗
Acknowledgments
This study has been supported by grants to R.E. from Associazione Italiana per la Ricerca sul Cancro (AIRC, Investigator grant 2018, project code 21790), Agenzia Italiana del Farmaco (AIFA, project code AIFA-2016-02365049), and Progetto di Ricerca di Ateneo (PRA_2018_27) from University of Pisa.
Author Contributions
R.C. and C.R. were responsible for the major part of the project, its design, and analysis of data. T.R. provided major technical support in collecting samples and nucleic acid extraction. A.P. provided clinical and pathological data from the patients and statistical assistance. A.T. performed the TERT promoter sequencing. V.C., D.V., and V.B. were responsible for recruiting the patients and collecting blood samples and informed consents. L.T., C.U., and F.B. provided FFPE tumor samples and were the pathologists responsible for all the histology classification of tumors. R.C. and R.E. wrote the manuscript. R.E. is the senior author and responsible for the supervision of the project.
Declaration of Interests
The authors declare that there are no conflicts of interest that could affect the impartiality of the reported research.
Published: October 25, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.09.030.
Supplemental Information
References
- Agrawal N., Jiao Y., Sausen M., Leary R., Bettegowda C., Roberts N.J., Bhan S., Ho A.S., Khan Z., Bishop J. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J. Clin. Endocrinol. Metab. 2013;98:E364–E369. doi: 10.1210/jc.2012-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani A.S., Alsaadi R., Murugan A.K., Sadiq B.B. TERT promoter mutations in thyroid cancer. Horm. Cancer. 2016;7:165–177. doi: 10.1007/s12672-016-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M.F., Mardis E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018;15:353–365. doi: 10.1038/s41571-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boichard A., Croux L., Al Ghuzlan A., Broutin S., Dupuy C., Leboulleux S., Schlumberger M., Bidart J.M., Lacroix L. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J. Clin. Endocrinol. Metab. 2012;97:E2031–E2035. doi: 10.1210/jc.2012-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge D.R., Pao W., Sequist L.V. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat. Rev. Clin. Oncol. 2014;11:473–481. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrato A., De Falco V., Santoro M. Molecular genetics of medullary thyroid carcinoma: the quest for novel therapeutic targets. J. Mol. Endocrinol. 2009;43:143–155. doi: 10.1677/JME-09-0024. [DOI] [PubMed] [Google Scholar]
- Chang Y.-S., Chang C.-C., Huang H.-Y., Lin C.-Y., Yeh K.-T., Chang J.-G. Detection of molecular alterations in Taiwanese patients with medullary thyroid cancer using whole-exome sequencing. Endocr. Pathol. 2018;29:324–331. doi: 10.1007/s12022-018-9543-6. [DOI] [PubMed] [Google Scholar]
- Chen Y., Fu L. Mechanisms of acquired resistance to tyrosine kinase inhibitors. Acta Pharm. Sin. B. 2011;1:197–207. doi: 10.1016/j.apsb.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi R., Mian C., Fugazzola L., Cosci B., Romei C., Barollo S., Cirello V., Bottici V., Marconcini G., Rosa P.M. Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid. 2013;23:50–57. doi: 10.1089/thy.2012.0207. [DOI] [PubMed] [Google Scholar]
- Ciampi R., Romei C., Cosci B., Vivaldi A., Bottici V., Renzini G., Ugolini C., Tacito A., Basolo F., Pinchera A., Elisei R. Chromosome 10 and RET gene copy number alterations in hereditary and sporadic Medullary Thyroid Carcinoma. Mol. Cell. Endocrinol. 2012;348:176–182. doi: 10.1016/j.mce.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Comoglio P.M., Trusolino L., Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat. Rev. Cancer. 2018;18:341–358. doi: 10.1038/s41568-018-0002-y. [DOI] [PubMed] [Google Scholar]
- Drosten M., Putzer B.M. Mechanisms of Disease: cancer targeting and the impact of oncogenic RET for medullary thyroid carcinoma therapy. Nat. Clin. Pr. Oncol. 2006;3:564–574. doi: 10.1038/ncponc0610. [DOI] [PubMed] [Google Scholar]
- Elisei R., Cosci B., Romei C., Bottici V., Renzini G., Molinaro E., Agate L., Vivaldi A., Faviana P., Basolo F. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J. Clin. Endocrinol. Metab. 2008;93:682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- Elisei R., Molinaro E., Agate L., Bottici V., Viola D., Biagini A., Matrone A., Tacito A., Ciampi R., Vivaldi A., Romei C. Ret oncogene and thyroid carcinoma. J. Genet. Syndr. Gene Ther. 2014;5:10. [Google Scholar]
- Elisei R., Schlumberger M.J., Müller S.P., Schöffski P., Brose M.S., Shah M.H., Licitra L., Jarzab B., Medvedev V., Kreissl M.C. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C., Smith D.P., Mulligan L.M., Nagai M.A., Healey C.S., Ponder M.A., Gardner E., Scheumann G.F., Jackson C.E., Tunnacliffe A. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum. Mol. Genet. 1994;3:237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- Guerra A., Fugazzola L., Marotta V., Cirillo M., Rossi S., Cirello V., Forno I., Moccia T., Budillon A., Vitale M. A high percentage of BRAFV600E alleles in papillary thyroid carcinoma predicts a poorer outcome. J. Clin. Endocrinol. Metab. 2012;97:2333–2340. doi: 10.1210/jc.2011-3106. [DOI] [PubMed] [Google Scholar]
- Heilmann A.M., Subbiah V., Wang K., Sun J.X., Elvin J.A., Chmielecki J., Sherman S.I., Murthy R., Busaidy N.L., Subbiah I. Comprehensive genomic profiling of clinically advanced medullary thyroid carcinoma. Oncology. 2016;90:339–346. doi: 10.1159/000445978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höppner W., Dralle H., Brabant G. Duplication of 9 base pairs in the critical cysteine rich domain of the ret proto-oncogene causes multiple endocrine neoplasia type 2A. Hum. Mutat. 1998;(Suppl 1):S128–S130. doi: 10.1002/humu.1380110143. [DOI] [PubMed] [Google Scholar]
- Ji J.H., Oh Y.L., Hong M., Yun J.W., Lee H.-W., Kim D., Ji Y., Kim D.-H., Park W.-Y., Shin H.-T. Identification of driving ALK fusion genes and genomic landscape of medullary thyroid cancer. PLoS Genet. 2015;11:e1005467. doi: 10.1371/journal.pgen.1005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps R., Brandão R.D., van den Bosch B.J., Paulussen A.D.C., Xanthoulea S., Blok M.J., Romano A. Next-generation sequencing in oncology: genetic diagnosis, risk prediction and cancer classification. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvaraki M.A., Shapiro S.E., Perrier N.D., Cote G.J., Gagel R.F., Hoff A.O., Sherman S.I., Lee J.E., Evans D.B. RET proto-oncogene: a review and update of genotype-phenotype correlations in hereditary medullary thyroid cancer and associated endocrine tumors. Thyroid. 2005;15:531–544. doi: 10.1089/thy.2005.15.531. [DOI] [PubMed] [Google Scholar]
- Li M.M., Datto M., Duncavage E.J., Kulkarni S., Lindeman N.I., Roy S., Tsimberidou A.M., Vnencak-Jones C.L., Wolff D.J., Younes A., Nikiforova M.N. Standards and guidelines for the interpretation and reporting of sequence variants in cancer. J. Mol. Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura M.M., Cavaco B.M., Leite V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr. Relat. Cancer. 2015;22:R235–R252. doi: 10.1530/ERC-15-0070. [DOI] [PubMed] [Google Scholar]
- Moura M.M., Cavaco B.M., Pinto A.E., Leite V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J. Clin. Endocrinol. Metab. 2011;96:E863–E868. doi: 10.1210/jc.2010-1921. [DOI] [PubMed] [Google Scholar]
- Neklason D.W., Done M.W., Sargent N.R., Schwartz A.G., Anton-Culver H., Griffin C.A., Ahnen D.J., Schildkraut J.M., Tomlinson G.E., Strong L.C. Activating mutation in MET oncogene in familial colorectal cancer. BMC Cancer. 2011;11:424. doi: 10.1186/1471-2407-11-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov Y.E., Carty S.E., Chiosea S.I., Coyne C., Duvvuri U., Ferris R.L., Gooding W.E., Hodak S.P., LeBeau S.O., Ohori N.P. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by thyroseq V2 next-generation sequencing assay. Cancer. 2014;120:3627–3634. doi: 10.1002/cncr.29038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforova M.N., Wald A.I., Roy S., Durso M.B., Nikiforov Y.E. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J. Clin. Endocrinol. Metab. 2013;98:1852–1860. doi: 10.1210/jc.2013-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persani L., de Filippis T., Colombo C., Gentilini D. GENETICS IN ENDOCRINOLOGY: genetic diagnosis of endocrine diseases by NGS: novel scenarios and unpredictable results and risks. Eur. J. Endocrinol. 2018;179:R111–R123. doi: 10.1530/EJE-18-0379. [DOI] [PubMed] [Google Scholar]
- Romei C., Ciampi R., Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat. Rev. Endocrinol. 2016;12:192–202. doi: 10.1038/nrendo.2016.11. [DOI] [PubMed] [Google Scholar]
- Romei C., Cosci B., Renzini G., Bottici V., Molinaro E., Agate L., Passannanti P., Viola D., Biagini A., Basolo F. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC) Clin. Endocrinol. 2011;74:241–247. doi: 10.1111/j.1365-2265.2010.03900.x. [DOI] [PubMed] [Google Scholar]
- Romei C., Ugolini C., Cosci B., Torregrossa L., Vivaldi A., Ciampi R., Tacito A., Basolo F., Materazzi G., Miccoli P. Low prevalence of the somatic M918T RET mutation in micro-medullary thyroid cancer. Thyroid. 2012;22:476–481. doi: 10.1089/thy.2011.0358. [DOI] [PubMed] [Google Scholar]
- Ruark E., Snape K., Humburg P., Loveday C., Bajrami I., Brough R., Rodrigues D.N., Renwick A., Seal S., Ramsay E. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493:406–410. doi: 10.1038/nature11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbolo M., Mian C., Barollo S., Fassan M., Mafficini A., Neves D., Scardoni M., Pennelli G., Rugge M., Pelizzo M.R. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows Arch. 2014;465:73–78. doi: 10.1007/s00428-014-1589-3. [DOI] [PubMed] [Google Scholar]
- Vigna E., Gramaglia D., Longati P., Bardelli A., Comoglio P.M. Loss of the exon encoding the juxtamembrane domain is essential for the oncogenic activation of TPR-MET. Oncogene. 1999;18:4275–4281. doi: 10.1038/sj.onc.1202791. [DOI] [PubMed] [Google Scholar]
- Viola D., Valerio L., Molinaro E., Agate L., Bottici V., Biagini A., Lorusso L., Cappagli V., Pieruzzi L., Giani C. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr. Relat. Cancer. 2016;23:R185–R205. doi: 10.1530/ERC-15-0555. [DOI] [PubMed] [Google Scholar]
- Vuong H.G., Odate T., Ngo H.T.T., Pham T.Q., Tran T.T.K., Mochizuki K., Nakazawa T., Katoh R., Kondo T. Clinical significance of RET and RAS mutations in sporadic medullary thyroid carcinoma: a meta-analysis. Endocr. Relat. Cancer. 2018;25:633–641. doi: 10.1530/ERC-18-0056. [DOI] [PubMed] [Google Scholar]
- Wang Q., Mehmood A., Wang H., Xu Q., Xiong Y., Wei D.Q. Computational screening and analysis of lung cancer related non-synonymous single nucleotide polymorphisms on the human kirsten rat sarcoma gene. Molecules. 2019;24 doi: 10.3390/molecules24101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasenius V.M., Hemmer S., Karjalainen-Lindsberg M.L., Nupponen N.N., Franssila K., Joensuu H. MET receptor tyrosine kinase sequence alterations in differentiated thyroid carcinoma. Am. J. Surg. Pathol. 2005;29:544–549. doi: 10.1097/01.pas.0000156103.37756.e2. [DOI] [PubMed] [Google Scholar]
- Wei S., LiVolsi V.A., Montone K.T., Morrissette J.J.D., Baloch Z.W. Detection of molecular alterations in medullary thyroid carcinoma using next-generation sequencing: an institutional experience. Endocr. Pathol. 2016;27:359–362. doi: 10.1007/s12022-016-9446-3. [DOI] [PubMed] [Google Scholar]
- Wells S.A., Jr., Gosnell J.E., Gagel R.F., Moley J., Pfister D., Sosa J.A., Skinner M., Krebs A., Vasselli J., Schlumberger M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J. Clin. Oncol. 2010;28:767–772. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For the following novel mutation a submission to COSMIC DATABASE has been requested and an identifier COSP47106 has been generated by COSMIC as a proof of successful data submission.
RET c.2774A>C; p.D925A
RET c.890G>A; p.R297H
NRAS c.53C>T; p.A18V
RET c.2710T>C; p.S904P
RET c.1894_1902delGAGCTGTGC; p.E632_C634del
RET c.3071C>T; p.S1024F
EIF1AX c.404G>C; G135A
CHK2 c.341G>A; W114∗