Summary
Analysis of kidney disease-causing genes and pathology resulting from systemic diseases highlight the importance of the kidney's filtering system, the renal corpuscles. To elucidate the developmental processes that establish the renal corpuscle, we performed single-nucleus droplet-based sequencing of the human fetal kidney. This enabled the identification of nephron, interstitial, and vascular cell types that together generate the renal corpuscles. Trajectory analysis identified transient developmental gene expression, predicting precursors or mature podocytes express FBLN2, BMP4, or NTN4, in conjunction with recruitment, differentiation, and modeling of vascular and mesangial cell types into a functional filter. In vitro studies provide evidence that these factors exhibit angiogenic or mesangial recruiting and inductive properties consistent with a key organizing role for podocyte precursors in kidney development. Together these studies define a spatiotemporal developmental program for the primary filtration unit of the human kidney and provide novel insights into cell interactions regulating co-assembly of constituent cell types.
Subject Areas: Biological Sciences, Cell Biology, Developmental Biology, Omics
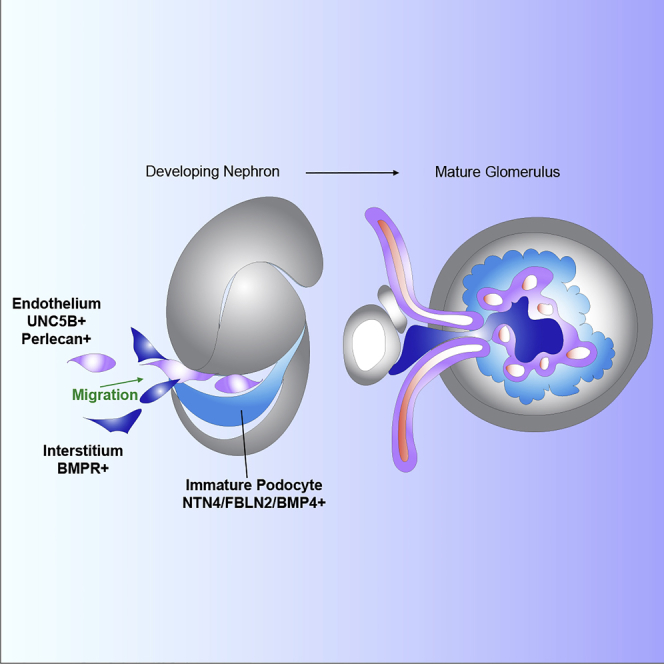
Graphical Abstract
Highlights
-
•
Single-nuclear RNA-seq analysis of human fetal kidney development
-
•
Co-ordinated programs of podocyte-driven glomerular development
-
•
Secreted podocyte factors act on endothelial and interstitial cells
Biological Sciences; Cell Biology; Developmental Biology; Omics
Introduction
The kidney is essential for metabolic waste excretion, the homeostatic balance of tissue fluids (water, salt, and pH), blood pressure and cell composition, and bone development and metabolism. Filtration is performed within the renal corpuscles (RCs) by a highly structured cellular device. This comprises a convoluted fenestrated glomerular endothelium supported by mesangial myofibroblasts that releases a plasma filtrate that enters the nephron between slit diaphragms generated by the foot processes of tightly adherent podocytes. Establishment of the glomerular filter is initiated by a stereotypic recruitment of pioneering endothelial cells to the developing podocytes followed secondarily by interstitial cells into the glomerular cleft of Comma and S-Shaped bodies (CSBs and SSBs) and sequential capillary formation (Figures 1A and 1B). Podocytes support development and maintenance of the glomerular vasculature via VEGFA (Eremina et al., 2003, Sison et al., 2010), whereas endothelial-derived PDGF signals promote mesangial development (Bjarnegård et al., 2004, Lindahl et al., 1998) before RC maturation (Figure 1C) (Levéen et al., 1994, Soriano, 1994). Single gene mutations resulting in end-stage renal disease cluster in genes showing podocyte-enriched expression, highlighting the central role of podocytes in normal kidney function (Brenner et al., 1996, Zhong et al., 2017). Advances in pluripotent stem cell-derived kidney organoid systems support parallel efforts to mechanistically dissect human kidney development to gain new insights into developmental programs relevant to treating or modeling disease states or generating functional systems (Takasato et al., 2016, Morizane et al., 2015, Wilson and Humphreys, 2019, Taguchi and Nishinakamura, 2015, Combes et al., 2019). A human-focused understanding of kidney development will complement other mammalian model systems to maximize effective application, predict new disease relationships, and identify novel developmental mechanisms (Combes et al., 2019, Lindström et al., 2018b, Lindström et al., 2018c, Lindström et al., 2018d, Menon et al., 2018, Wu et al., 2018a).
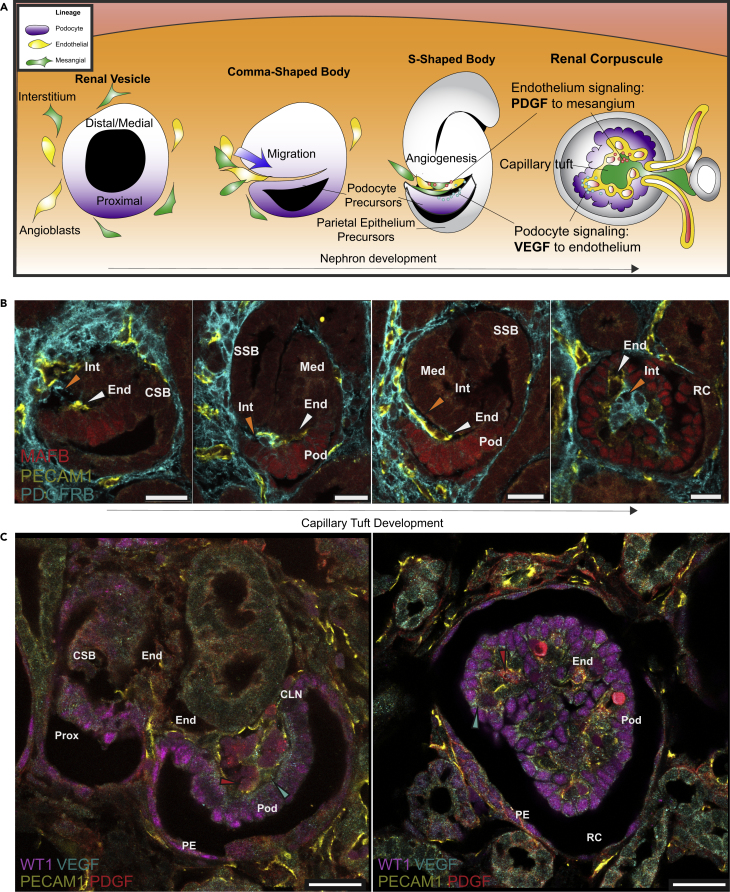
Figure 1.
Schematic Model of RC Development
(A) Differentiating NPCs epithelialize to form a renal vesicle with distal/proximal polarity. Nephron morphogenesis progresses through CSB and SSB stages concomitant with the recruitment and invasion of mesenchymal endothelial and interstitial cells to the glomerular cleft, which is lined by developing podocytes. Podocyte-derived VEGFA signaling to glomerular endothelial cells and PDGF secreted by endothelial cells acting on adjacent mesangial precursors are critical for the development, maintenance, and function of glomerular filtration.
(B) Immunostaining showing incremental stages of glomerular capillary tuft development starting with invasion of PECAM1+ endothelial cells (yellow) followed by PDGFRB + interstitial cells (cyan) into the glomerular cleft (left to right panels).
(C) Immunohistochemical staining of distinct stages of RC development labeling WT1+ PE, WT1+ podocytes colocalized with VEGFA (cyan arrowhead), and PECAM1+ endothelium colocalized with PDGF (red arrowhead).
(Scale bars, 25 μm). Prox, Proximal Nephron; Pod, Podocytes; PE, Parietal Epithelium; End, Endothelium; CSB, Comma-Shaped Body; CLN, Capillary Loop Nephron; RC, Renal Corpuscle.
Results
Single-Nucleus Interrogation of Human Fetal Kidney
To generate insights into RC formation, we applied snDrop-seq to transcriptionally profile nuclei isolated from human fetal kidneys (Figure 2A). Our group, and others, have captured single-nuclear transcriptomes from other challenging tissues including postmortem adult brain (Lake et al., 2016, Lake et al., 2018) resolving distinct cell types (Lake et al., 2017, Wu et al., 2019). The benefits of the nuclear approach are that cryopreserved tissue with potentially clinical value are compatible and epithelialized cells including those of sieved mature RCs are more accessible than by enzymatic cell dissociation methods required for whole cell. Podocytes are consistently under-represented (or missing) from prominent large kidney single cell RNA sequencing (RNA-seq) datasets (Adam et al., 2017, Park et al., 2018). Furthermore, our previous studies were unable to sufficiently access epithelialized cell types beyond SSB using an enzymatic cortical digestion method (Lindström et al., 2018a, Lindström et al., 2018b, Lindström et al., 2018d). To this end, we processed nephrogenic cortex and RCs from 13- to 16.5-week human fetal kidney samples that incorporated all stages of RC development to active filtering nephrons (Figures 2A and S1, Table S1). Nuclear RNA preferentially detects genes with high intron count compared with whole-cell RNA, leading to a systemic biased sensitivity toward genes that may escape detection in conventional single-cell approaches. Variable numbers of nuclei were obtained from each sample; whether the variation in number of nuclei reflects biological variability among the samples or technical variability in the procedures is not clear. Altogether, we generated data on 7,018 single nuclei from these combined samples sequenced to an average depth of 10,298 useful reads per nucleus (Table S1). This permitted detection of a median of 879 unique transcripts and 707 genes per nucleus, with genomic mapping rates showing the expected higher proportion of reads corresponding to intronic sequences (Table S1), as previously observed.
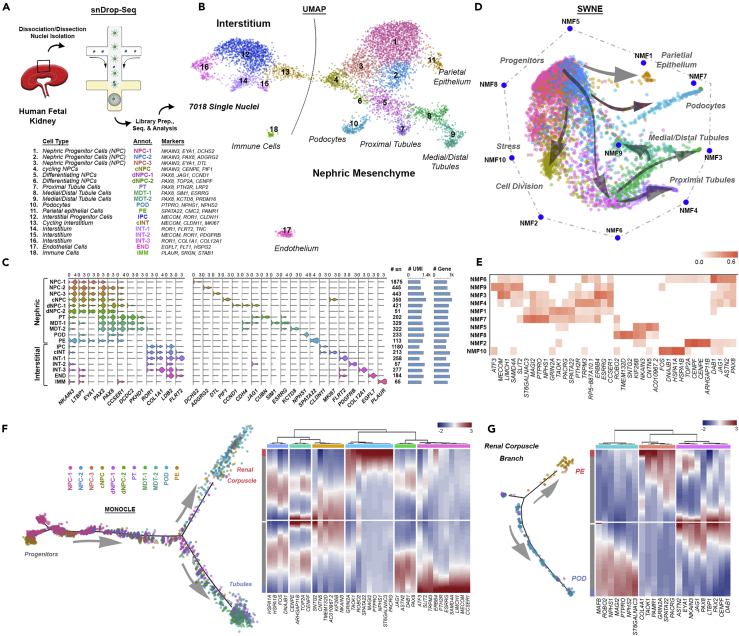
Figure 2.
Single-Nucleus RNA Sequencing of the Developing Human Kidney and Pseudotemporal Ordering Resolves Major Cell Types and Informs on Podocyte Developmental Programs
(A) An overview of the snDrop-seq pipeline on fetal kidney samples (Table S1) including dissociated kidney cortical cells (14 and 16 weeks) and sieved glomeruli (13 and 15 weeks).
(B) Combined expression data (four individuals over five experiments) visualized by UMAP dimensional reduction showing 18 distinct cellular populations encompassing the nephrogenic, interstitial, endothelial, and immune cell types. Cell type annotations and associated select marker genes are indicated for each cluster.
(C) Violin plots showing expression values for select broad or cell-type specific marker genes. For each cluster, the number of datasets (SN), average number of transcripts (UMI), and average number of genes detected are indicated.
(D) SWNE visualization of nephrogenic lineage cell clusters and associated non-negative factorization (NMF) identified gene signatures indicate cluster relationships.
(E) Heatmap of top genes associated with each NMF state.
(F) Trajectory analysis using Monocle of nephrogenic lineages showing developmental progression into distinct RC (proximal) and tubule (medial/distal) lineages. Corresponding heatmap indicating discrete gene expression intensity from the progenitor state (gray center line value) maturing to either RC (red tip value) or medial/distal (blue tip value) branches.
(G) Trajectory analysis of the developing RC branch into either parietal or visceral (podocyte) epithelial cell types. Heatmap showing expression values of select marker genes progressing from differentiating NPCs (center, gray) to either parietal (red) or podocyte (blue) cell types.
See also Figures S1–S4.
To resolve the cell type composition, we analyzed transcriptional heterogeneity by grouping cells by gene-gene covariance (see Transparent Methods). This approach identified twelve nephrogenic, five interstitial, one immune, and one endothelial cluster (Figure 2A and Tables S2 and S3). Clusters showed expression profiles and subgroup aggregations visualized with Uniform Manifold Approximation and Projection (UMAP) (Becht et al., 2018), independent of technical batch effects (Figures 2B, 2C, and S1). Cluster identification was supported by GO-Term analysis, immunohistological (protein-targeted) and in situ hybridization (mRNA-targeted) analysis with select known markers of mammalian kidney development, including LTBP1 (Schwab et al., 2006, Fetting et al., 2014), CDH4 (Dahl et al., 2002, Rosenberg et al., 1997), COL4A1 (Chen et al., 2016, Chew and Lennon, 2018), disease-related genes ESRRG (Berry et al., 2011, Harewood et al., 2010), PKHD1 (Igarashi and Somlo, 2002, Wilson, 2004), and novel marker PAMR1 (Figure S2, Tables S4 and S5). To visualize and infer relationships between clusters we employed similarity weighted non-negative embedding (SWNE) analysis (Figure 2D) (Wu et al., 2018b). Nephron progenitor cells (NPCs) and mitotic NPCs (cNPC) clusters were related to two differentiated NPC (dNPC) clusters enriched from cortex (Figure S1). Differentiated tubular clusters comprised medial/distal and proximal tubular identities (Figure 2D). DNPCs transitioned to parietal epithelium (PE), and podocyte clusters enriched in RC samples (Figures 2B and S1). Interstitial clusters were composed of interstitial progenitor cells (IPCs), mitotic interstitium (cINT), and three populations containing two mesangial clusters enriched in RC samples (INT1-3) (Figures 2B and S1).
Molecular Dissection of Podocyte Development
Given the nucleating role of the podocyte in the development of a glomerular filter we hypothesized that transiently expressed genes during podocyte development could be important coordinating glomerular and mesangial cell programs. An unsupervised pseudotemporal analysis in Monocle was used to identify intermediates in the podocyte developmental pathway (Figures 2C–2E, S3, and S4) (Qiu et al., 2017). Monocle analysis predicted that NPCs transitioned to dNPCs that expressed PAX8, JAG1, and LHX1 (Park et al., 2007, Leimeister et al., 2003, Plachov et al., 1990) (Figures 2D–2G, Tables S6 and S7). Lhx1 plays a key early role in mouse podocyte programs and mutations in LHX1 associated with congenital anomalies of the kidney and urinary tract (CAKUT) syndrome (Kobayashi et al., 2005, Boualia et al., 2013, Lindström et al., 2018d). Additionally, JAG1 and PAX8 are two markers of early nephron that are involved in kidney development and disease (Boualia et al., 2013, Narlis et al., 2007, Plachov et al., 1990, Lindström et al., 2018c, Liu et al., 2013, Chen and Al-Awqati, 2005, Piscione et al., 2004). DNPCs bifurcated between medial/distal and proximal identities including podocytes (Figures 2F, S3, and S4, Table S6). Glomerulus-related GO Terms were associated with the proximal branch, whereas cytoskeletal processes were associated with the medial/distal branch (Tables S7–S11). Monocle analysis of proximal transcriptomes bifurcated podocyte and PE trajectories (Figures 2F, 2G, and S2E–S2E’). Global pseudotemporal analysis of this dataset identified eight temporally distinct gene sets (GS1–GS8) with distinct ontologies (Figures 3A and 3B, and Table S12). At one end, NPCs (GS1) expressed ROBO2 and ECEL (Lindström et al., 2018b), whereas at the other end, mature podocytes (GS8) expressed FOXC1, SYNPO, NPHS2 (Table S12), key genes in mouse and human podocyte function (Lindström et al., 2018a, Lindström et al., 2018b, Motojima et al., 2017, Roselli et al., 2004, Yanagida-Asanuma et al., 2007, Mundel et al., 1997, Komaki et al., 2013, Kume et al., 2000, Franceschini et al., 2006, Sharif and Barua, 2018). GS6–GS8 gene-associated phenotypes included defects in ureteric bud, renal system, and podocyte foot processes accompanied with GO Terms for regulation of development, cell adhesion, and cell movement (Figure 3B and Table S12).
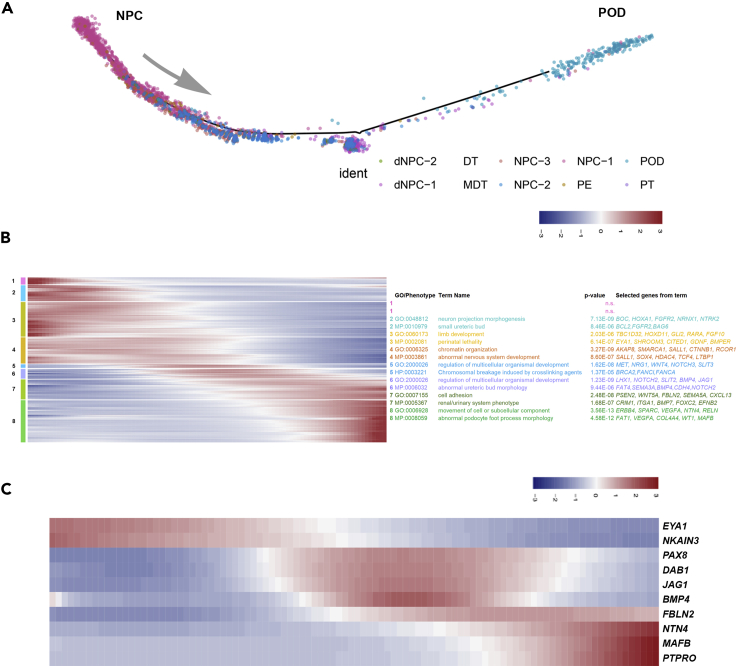
Figure 3.
Trajectory Analysis of Podocyte Lineage Cells Identifies Distinct Transient Gene Expression Signatures
(A) Unidirectional trajectory of undifferentiated NPCs and podocyte lineage cells (see Transparent Methods) identified in Figure 2G.
(B) Identification of temporally significant stages of gene expression and their associated top gene ontology (GO) and mouse/human phenotype terms (select genes from each term are indicated). Cells are ordered according to the trajectory shown in (A).
(C) Heatmap of gene expression values for select stage-specific and expressed factors during podocyte development for cells ordered as in (A).
See also Figure S5.
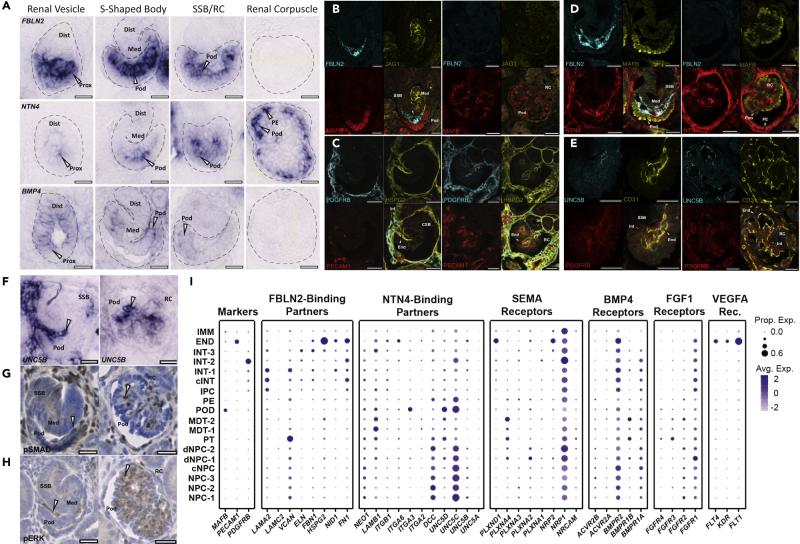
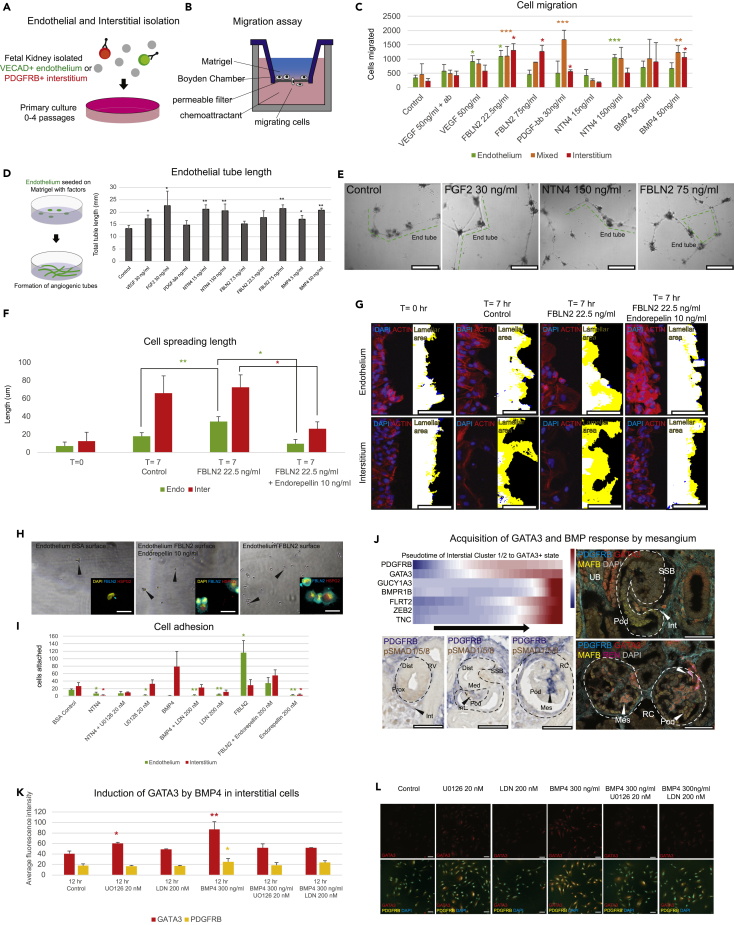
Examining these data for podocyte-derived, stage-specific developmental signals as potential organizers of the glomerular filter identified three expressed factors predicted to display partial temporal overlap: BMP4 (GS6; a member of the BMP subfamily of TGFβ signals) (Padgett et al., 1993), FBLN2 (GS7; a calcium-binding extracellular matrix protein of the fibulin family) (Zhang et al., 1994), and NTN4 (GS8; a Netrin family member) (Figure 3C) (Yin et al., 2000). In situ hybridization demonstrated each exhibited expression in podocytes (but also other cell types) surrounding the glomerular cleft in conjunction with angioblast recruitment, although their spatiotemporal profiles differed (Figure 4A). In particular, FBLN2 was detected at high levels in the earliest podocyte precursors, continued throughout RC development, but terminated in the functional RC (Figure 4A). FBLN2 was detected within the glomerular cleft and angioblasts produced PERLECAN/HSPG2, a potential interaction partner for FBLN2(Brown et al., 1997) (Figures 4B and 4C). UNC5B, a surface receptor for NTN4 known to be involved in diabetic nephropathy (Lu et al., 2004, Hoang et al., 2009, Lejmi et al., 2014, Ranganathan et al., 2015, Wang et al., 2008), was also detected on the angioblast (Figures 4D and 4E), continuing into periglomerular endothelial cells of mature RCs, and on parietal epithelial cells that encapsulated the RC (Figures 4D and 4F). Analysis of phospho-SMAD and ERK staining, respectively, suggests evidence including but not limited to potential activation of BMP and FGF signaling within the vascular/mesangial population invading the glomerular cleft (Figures 4G and 4H). These data support potential roles for FBLN2, NTN4, and BMP4 in RC assembly from the initial stages of endothelial and interstitial recruitment (Figure 4I).
Figure 4.
Spatiotemporal Mapping of the Expression of Genes Encoding Podocyte-Expressed Factors and Predicted Endothelial and Interstitial Cell Interactions
(A–E) (A) In situ hybridization for FBLN2, NTN4, and BMP4 indicating gene expression (arrowheads) during intermediate phases of proximal fate to podocyte development. Arrowheads point to expression in developing podocytes that is absent in mature RCs, with the exception of NTN4, which is expressed in periglomerular vasculature and at lower levels in mature podocytes. Immunostaining in SSB and RC for (B) FBLN2, (C) HSPG2, (D) NTN4, (E) UNC5B, and indicated markers.
(F–H) (F) In situ hybridization of UNC5B. Phospho-SMAD1/5- (G) and phospho-ERK- (H) positive cells (arrowheads) showing labeling of infiltrating cells into the glomerular cleft in SSB and in RCs (scale bars, 25 μm). Dist, Distal Nephron; Med, Medial Nephron; Prox, Proximal Nephron; Pod, Podocytes; PE, Parietal Epithelium; SSB, S-Shaped Body; RC, Renal Corpuscle.
(I) Dot pots showing relative expression (blue color intensity) and relative number of positive nuclei (dot size) for extracellular matrix and cell signaling genes found within clusters identified in Figure 1A.
See also Figure S5.
Endothelial and Interstitial Cell Responses to Podocyte-Derived Expressed Factors
NTN4 and BMP4 are known to have multiple roles in kidney and glomerular disease and development (Dudley et al., 1999, Michos et al., 2007, Miyazaki et al., 2000, Oxburgh et al., 2005, Oxburgh et al., 2011, Raatikainen-Ahokas et al., 2000, Ueda et al., 2008, Wang et al., 2008), and non-glomerular associated Fbln2 expression has been reported in the mouse kidney (Pan et al., 1993); however, no specific role has been attached to any of these factors in coordinating glomerular endothelial or mesangial programs in the human kidney. To explore the function of these factors on human vascular and mesangial cell development, biologically relevant primary endothelial and interstitial cell types were isolated from the human fetal kidney by enzymatic dissociation of the cortical region and antibody specific enrichment (anti-VECAD1, endothelial; anti-PDGFRB, interstitial) (Figure 5A) and tested for high-affinity responses to nanomolar concentrations of specific factors informed by the previous literature. As expected from published studies (Barkefors et al., 2008), VEGFA promoted endothelial cell migration through a cell permeable membrane, reflecting VEGFA's known actions in facilitating early assembly of the glomerular vasculature (Figure 1), increased angiogenic tubule formation in Matrigel (Figures 5B and 5C), and enhanced endothelial cell proliferation (Figure S5B). Interestingly, FBLN2 and NTN4 but not BMP4 also increased endothelial cell migration and angiogenic tube formation but did not alter proliferation suggesting a partial overlap with VEGFA-stimulated activities (Figures 5B–5E, S5A, and S5B). NTN4 has been shown to elicit context-dependent angiogenic or anti-angiogenic responses consistent with observations here (Lambert et al., 2012, Nacht et al., 2009, Hoang et al., 2009). FBLN2 has been linked to cell adhesion (Pfaff et al., 1995), and the transient accumulation in the matrix surrounding migrating endothelial cells in the glomerular cleft is consistent with matrix interactions promoting adhesion and cell spreading (Figures 4B, 4D, and S5C). To examine FBLN2 action further, a scratch assay was performed on a confluent population of endothelial cells to determine if FBLN2 influences cell spreading at the leading edge, a sensitive and quantitative assay of general cell motility and cytoskeletal spreading in a homogeneous environment that was previously described (Yarrow et al., 2004). A marked increase in lamellar extensions was observed in the presence of FBLN2 and this response was abrogated by co-incubation with Endorepellin, an inhibitory subunit of HSPG2 that is thought to inhibit FBLN2-HSPG2 interactions and is generally anti-angiogenic (Poluzzi et al., 2016, Mongiat et al., 2003, Brown et al., 1997, Douglass et al., 2015) (Figures 5F and 5G). Additionally, endothelial cells preferentially adhered to an FBLN2-coated surface, an interaction that was strongly inhibited on addition of Endorepellin (Figures 5H and 5I). Together these support a role for podocyte-expressed FBLN2 acting at least in part through endothelial-produced HSPG2 in promoting outgrowth, migration, and adhesion of endothelial cells in glomerular morphogenesis. Interestingly, mouse Fbln2 does not show similar dynamic podocyte restricted activity in RC development and is instead restricted to the apical domain of tubular epithelia (Figure S5D). A closely related gene Fbln1 is expressed in the glomerular cleft in mouse but is widely detected, whereas FBLN1 is nearly absent in human kidney tissues suggesting a marked species difference in the regulation of both genes with respect to podocyte development (Figure S5D). Although VEGFA, FBLN2, and NTN4 may all act together at early stages of glomerular morphogenesis, only VEGFA and NTN4 are likely to play a continuing role in the maintenance of the glomerular network.
Figure 5.
Chemotactic, Angiogenic, and Mesangial-Inductive Effects of FBLN2, NTN4, and BMP4
(A) Isolation of primary HFK endothelial and interstitial cells, subculture.
(B) Schematic of migration assay indicating input cells migrating through permeable filter to chemoattracting factor in the lower chamber.
(C) Quantitation of endothelial, interstitial, or mixed migration across permeable membrane in response to factors indicated.
(D–G) (D) Schematic and quantification of endothelial tube assay in response to factors indicated with (E) representative images of tube formation and dotted line highlighting tubes. Scale bars, 0.5 mm. (F) Quantification and (G) representative images of endothelial or interstitial cell lamellar extension length and area (yellow) in response and to factors indicated (scale bars, 50 μm).
(H and I) (H) Representative images and (I) quantitation of endothelial cell adhesion to surfaces coated with factors indicated (scale bars, 20 μm).
(J) Pseudotemporal upregulation of indicated genes during Interstitial cluster INT1/2 differentiation. Immunohistochemical staining of phospho-SMAD1/5 and PDGFRB costaining in renal vesicles, S-Shaped Bodies, and RCs showing colocalization in mesangial cells. Immunofluorescent staining of PDGFRB, GATA3, MAFB, and REN showing PDGFRB+/GATA3+ interstitial cells recruited to SSBs. REN + cells identify juxtaglomerular cells (Scale bars, 50 μm).
(K and L) (K) Quantification and (L) representative images of BMP4 induction of GATA3 and PDGFRB with or without the presence of pSMAD or MAPK inhibition of interstitial cells after 12 h (scale bars, 25 μm).
Data are represented as mean ± SEM. One asterisk (*) indicates p value smaller than 0.05 (p < 0.05). Two asterisks (**) indicate p value smaller than 0.01 (p < 0.01). Three asterisks (***) indicate p value smaller than 0.001 (p < 0.001). See also Figure S5.
Podocyte-directed actions on the interstitial to mesangial cell transition are not well understood. Genetic studies in the mouse have shown endothelial cell-derived PDGFB acts through mesangial cell PDGFRB to promote mesangial cell-dependent formation of the glomerulus (Bjarnegård et al., 2004). PDGF pathway action plays a broader role in regulating pericytes, a myofibroblast-like cell type interacting with non-glomerular vasculature, which shares a common origin from interstitial progenitor cells (Kobayashi et al., 2014). To initially determine if PDGFRB + cortical interstitial cells show expected responses in vitro, we examined the effects of PDGFB. PDGFB addition enhanced interstitial cell migration across a cell permeable membrane (Figures 5B and 5C), and the proliferative stimulus invoked by PDGFB was inhibited by DMPQ (Lo et al., 2017), a specific PDGFRB inhibitor (Figures S5A and S5B). Thus, the in vitro system replicated PDGF/PDGFRB interactions. Interestingly, interstitial cells were also stimulated to migrate by FBLN2 similarly to endothelial cells by a yet unclear mechanism (Figure 5C). Interstitial trajectory analysis showed that interstitial progenitor cells generate different interstitial cell types (INT-1/INT-2/INT-3). In conjunction with the developmental progression, differential gene expression analysis predicted an upregulation of the BMP receptor BMPR1B and the transcriptional regulatory factor GATA3, involved in mesangial differentiation (Labastie et al., 1995) in INT-1/INT-2 cells (Figure 4H and Table S13), and an upregulation of BMP signaling inhibitors in INT-3 cells, a yet uncharacterized interstitial cell type (Tables S14 and S15). Both gain and loss of function of Bmp4 in mouse podocytes result in glomerular capillary defects suggesting that precise regulation of BMP signaling is critical in RC programs (Ueda et al., 2008).
Consistent with a role for BMP signaling in human glomerular development, cells in the glomerular cleft exhibited phospho-SMAD1/5 activity (Figures 5J and S5F). Linked nuclear GATA3 raised the possibility that GATA3 may be a target of BMP signaling. In support of this conjecture, exogenous BMP4 induced expression of ID1, an established BMP target (Hollnagel et al., 1999, Ying et al., 2003), in cultured interstitial cells, and elevated GATA3 levels (Figures 5K, 5L, and S5E). To examine a possible BMP4 GATA3 link, LDN-193189 was added to inhibit BMP type 1 receptor/phospho-SMAD activity in the presence of BMP4 (Brown et al., 2015, Cuny et al., 2008, Yu et al., 2008). As expected, LDN-193189 inhibited expression of the BMP-target ID1 (Figure S5E). In contrast, qPCR measurement indicated that GATA3 expression was unaltered suggesting that BMP4 elevation of GATA3 is independent of pSMAD1/5 activity (Figure S5E). BMPs have also been reported to act through Smad-independent ERK, p38, JNK, and SAPK MAPK pathways (Brown et al., 2015, Oxburgh et al., 2011, Leung-Hagesteijn et al., 2005, Herpin and Cunningham, 2007, Otani et al., 2007). To address this possibility, the UO126 MEK inhibitor was added to block ERK activity in the presence of BMP4. As expected, UO126 did not perturb BMP4-induced ID1 levels, but GATA3 levels were reduced (Figure S5E). However, UO126 alone was sufficient to increase GATA3 to a similar level as BMP4 treatment suggesting that multiple context-dependent signaling inputs may be involved in GATA3 regulation. BMPs are known to induce GATA3 (Peng et al., 2015, Lichtner et al., 2013) and act synergistically with p38 and ERK in some contexts (Brown et al., 2015, Xu et al., 2008, Shim et al., 2009). There are likely other sources of BMP4 (Raatikainen-Ahokas et al., 2000) and other signals that regulate GATA3 in the mesangium (Moriguchi et al., 2016, Van Esch et al., 2000). BMP7 is involved in kidney development and disease (Dudley et al., 1999, Fetting et al., 2014, Godin et al., 1998, Oxburgh et al., 2005) (Figures 4G, 4H, S5F, and S5G), is expressed in later podocyte development (GS7) (Figure 3B), and along with BMP4 can induce pERK1/2 and p38 in mouse mesangial cells (Otani et al., 2007) suggesting mesangial BMP responsiveness is likely conserved in mammals.
Discussion
The kidney's filter is a key target for re-building kidney function. The findings here inform on cell interactions that may facilitate this goal (Van Den Berg et al., 2018, Morizane et al., 2017). The single-nuclear transcriptional profiling reported here complements approaches from our group and others (Adam et al., 2017, Combes et al., 2019, Karaiskos et al., 2018, Lindström et al., 2018a, Lindström et al., 2018b, Lindström et al., 2018d, Menon et al., 2018, O'brien et al., 2018, Rutledge et al., 2017, Wilson and Humphreys, 2019, Wu et al., 2018a, Wu et al., 2019) to identify previously unappreciated factors mediating formation of the renal filter. FBLN2, NTN4, and BMP4 are expressed by developing podocytes concomitant with angioblast and interstitial cell recruitment expressing cognate interacting factors indicating related and potentially concerted actions in the establishment of the glomerular filter (Figure 1). The in vitro studies support their actions on endothelial or mesangial cell programs. Mutations for mouse Fibulins 1, 3, 4, and 5 have strong pleiotropic phenotypes including hemorrhages in multiple tissues and defects in the capillaries heart, lung, and kidney including impaired formation of glomerular capillaries and abnormal cell junctions in Fbln1 mutants (Kostka et al., 2001). However, in mouse, Fbln2 mutants are viable with no reported kidney phenotype and Fbln2 is localized quite differently in the mouse kidney (Olijnyk et al., 2014) when compared with its human ortholog. We hypothesize that developmentally restricted expression of FBLN2 may perform highly specialized functions directing glomerular vascularization in human kidney development replacing some of the functions of Fbln1 in mouse. Thus far, human FBLN2 has not been linked to human disease or kidney function (de Vega et al., 2009) but could be uncovered by genome-wide association study (GWAS)-powered investigations. However, FBLN2 has been shown to exhibit properties in non-kidney systems similar to those demonstrated here. FBLN2 regulates invasion, migration, and/or adhesion of astrocytes, keratinocytes, and cancer cells and is linked to wound healing cells (de Vega et al., 2009, Fontanil et al., 2017, Law et al., 2012, Olijnyk et al., 2014, Schaeffer et al., 2018). FBLN2 also shows alternate splice isoform abundance in mouse versus human (Grässel et al., 1999, Pfaff et al., 1995). In contrast, disruption of Bmp4 leads to mesangial and RC defects in the mouse (Ueda et al., 2008), suggesting that BMP4's function in human and mouse may be a conserved requirement for glomerulus development. A current goal for kidney translational research is to vascularize pluripotent-derived kidney tissues in vitro and replicate glomerular function but has thus far eluded our best efforts and impedes clinical application of kidney organoids. Thus, there is an acute need for knowledge of mechanisms sufficient to direct vascularization of kidney tissue types in vitro, and our work presented here has identified three potential candidates. Future studies will focus on regulation of FBLN2, NTN4, and BMP4 to induce in vitro assembly of glomerular capillary formation, maintenance, and function. In conclusion, these studies highlight the power of single-cell analysis in the understanding of human organogenesis, predicting cell interactions and distinct pathways of action not observed in the mouse model system.
Limitations of the Study
Deidentified primary human fetal kidney tissue for nuclei profiling, imaging, and in vitro culture were received on a case-by-case basis. Limitations on the availability of tissue samples and variability in sample age and sex precluded a detailed mechanistic follow-up of the preliminary findings of BMP4-mediated regulation of mesangial GATA3 expression. Additional studies will be essential to clarify the pathway(s) of action of the signaling activities identified here and how these factors may cooperate with a broader range of signals within the developing glomerular environment. Here, GATA3 upregulation within interstitial cells could facilitate unbiased systematic screening to broadly explore pathway actions in future work.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank all members of the McMahon and Zhang laboratories including Nils Lindstrom, Pietro Cippa, and Andrew Ransick for critical input. We thank Dr. Seth Ruffins for help with confocal imaging and deconvolution. We thank Mickey Huang for High-Content imaging. Work in A.P.M.’s laboratory was supported by grants from the National Institutes of Health (NIH) (DK107350, DK094526, DK110792). A.D.K. was supported by the NIH (5F32DK109616-02) and the University of Southern California (USC) Stem Cell postdoctoral fellowship from the Hearst Foundation.
Author Contributions
A.D.K., B.B.L., K.Z, and A.P.M. planned experiments and analyzed data. B.B.L., A.D.K., and Y.W. assembled the figures. B.B.L., A.D.K., S.C., Y.W., R.K.P., J.G, T.T., and J.A.M. collected data. M.E.T. and B.G. provided embryonic and fetal kidneys. A.D.K., B.B.L., K.Z. and A.P.M. wrote the manuscript incorporating input from all authors.
Declaration of Interests
The authors declare no competing interests.
Published: October 25, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.09.029.
Contributor Information
Kun Zhang, Email: kzhang@bioeng.ucsd.edu.
Andrew P. McMahon, Email: amcmahon@med.usc.edu.
Supplemental Information
References
- Adam M., Potter A.S., Potter S.S. Psychrophilic proteases dramatically reduce single-cell RNA-seq artifacts: a molecular atlas of kidney development. Development. 2017;144:3625–3632. doi: 10.1242/dev.151142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkefors I., Le Jan S., Jakobsson L., Hejll E., Carlson G., Johansson H., Jarvius J., Park J.W., Li Jeon N., Kreuger J. Endothelial cell migration in stable gradients of vascular endothelial growth factor A and fibroblast growth factor 2: effects on chemotaxis and chemokinesis. J. Biol. Chem. 2008;283:13905–13912. doi: 10.1074/jbc.M704917200. [DOI] [PubMed] [Google Scholar]
- Becht E., Mcinnes L., Healy J., Dutertre C.A., Kwok I.W.H., Ng L.G., Ginhoux F., Newell E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2018;37:38–44. doi: 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- Berry R., Harewood L., Pei L., Fisher M., Brownstein D., Ross A., Alaynick W.A., Moss J., Hastie N.D., Hohenstein P. ESRRG functions in early branch generation of the ureteric bud and is essential for normal development of the renal papilla. Hum. Mol. Genet. 2011;20:917–926. doi: 10.1093/hmg/ddq530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnegård M., Enge M., Norlin J., Gustafsdottir S., Fredriksson S., Abramsson A., Takemoto M., Gustafsson E., Fässler R., Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- Boualia S.K., Gaitan Y., Tremblay M., Sharma R., Cardin J., Kania A., Bouchard M. A core transcriptional network composed of Pax2/8, Gata3 and Lim1 regulates key players of pro/mesonephros morphogenesis. Dev. Biol. 2013;382:555–566. doi: 10.1016/j.ydbio.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Brenner B.M., Lawler E.V., Mackenzie H.S. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- Brown J.C., Sasaki T., Göhring W., Yamada Y., Timpl R. The C-terminal domain V of perlecan promotes beta1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur. J. Biochem. 1997;250:39–46. doi: 10.1111/j.1432-1033.1997.t01-1-00039.x. [DOI] [PubMed] [Google Scholar]
- Brown A.C., Muthukrishnan S.D., Oxburgh L. A synthetic niche for nephron progenitor cells. Dev. Cell. 2015;34:229–241. doi: 10.1016/j.devcel.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am. J. Physiol. Renal. Physiol. 2005;288:F939–F952. doi: 10.1152/ajprenal.00369.2004. [DOI] [PubMed] [Google Scholar]
- Chen Z., Migeon T., Verpont M.C., Zaidan M., Sado Y., Kerjaschki D., Ronco P., Plaisier E. HANAC syndrome Col4a1 mutation causes neonate glomerular hyperpermeability and adult glomerulocystic kidney disease. J. Am. Soc. Nephrol. 2016;27:1042–1054. doi: 10.1681/ASN.2014121217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew C., Lennon R. Basement membrane defects in genetic kidney diseases. Front. Pediatr. 2018;6:11. doi: 10.3389/fped.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes A.N., Zappia L., Er P.X., Oshlack A., Little M.H. Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med. 2019;11:3. doi: 10.1186/s13073-019-0615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny G.D., Yu P.B., Laha J.K., Xing X., Liu J.F., Lai C.S., Deng D.Y., Sachidanandan C., Bloch K.D., Peterson R.T. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg. Med. Chem. Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl U., Sjödin A., Larue L., Radice G.L., Cajander S., Takeichi M., Kemler R., Semb H. Genetic dissection of cadherin function during nephrogenesis. Mol. Cell Biol. 2002;22:1474–1487. doi: 10.1128/mcb.22.5.1474-1487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega S., Iwamoto T., Yamada Y. Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol Life Sci. 2009;66:1890–1902. doi: 10.1007/s00018-009-8632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass S., Goyal A., Iozzo R.V. The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connect Tissue Res. 2015;56:381–391. doi: 10.3109/03008207.2015.1045297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley A.T., Godin R.E., Robertson E.J. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 1999;13:1601–1613. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremina V., Sood M., Haigh J., Nagy A., Lajoie G., Ferrara N., Gerber H.P., Kikkawa Y., Miner J.H., Quaggin S.E. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetting J.L., Guay J.A., Karolak M.J., Iozzo R.V., Adams D.C., Maridas D.E., Brown A.C., Oxburgh L. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development. 2014;141:17–27. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanil T., Álvarez-Teijeiro S., Villaronga M., Mohamedi Y., Solares L., Moncada-Pazos A., Vega J.A., García-Suárez O., Pérez-Basterrechea M., García-Pedrero J.M. Cleavage of Fibulin-2 by the aggrecanases ADAMTS-4 and ADAMTS-5 contributes to the tumorigenic potential of breast cancer cells. Oncotarget. 2017;8:13716–13729. doi: 10.18632/oncotarget.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini N., North K.E., Kopp J.B., Mckenzie L., Winkler C. NPHS2 gene, nephrotic syndrome and focal segmental glomerulosclerosis: a HuGE review. Genet. Med. 2006;8:63–75. doi: 10.1097/01.gim.0000200947.09626.1c. [DOI] [PubMed] [Google Scholar]
- Godin R.E., Takaesu N.T., Robertson E.J., Dudley A.T. Regulation of BMP7 expression during kidney development. Development. 1998;125:3473–3482. doi: 10.1242/dev.125.17.3473. [DOI] [PubMed] [Google Scholar]
- Grässel S., Sicot F.X., Gotta S., Chu M.L. Mouse fibulin-2 gene. Complete exon-intron organization and promoter characterization. Eur. J. Biochem. 1999;263:471–477. doi: 10.1046/j.1432-1327.1999.00523.x. [DOI] [PubMed] [Google Scholar]
- Harewood L., Liu M., Keeling J., Howatson A., Whiteford M., Branney P., Evans M., Fantes J., Fitzpatrick D.R. Bilateral renal agenesis/hypoplasia/dysplasia (BRAHD): postmortem analysis of 45 cases with breakpoint mapping of two de novo translocations. PLoS One. 2010;5:e12375. doi: 10.1371/journal.pone.0012375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin A., Cunningham C. Cross-talk between the bone morphogenetic protein pathway and other major signaling pathways results in tightly regulated cell-specific outcomes. FEBS J. 2007;274:2977–2985. doi: 10.1111/j.1742-4658.2007.05840.x. [DOI] [PubMed] [Google Scholar]
- Hoang S., Liauw J., Choi M., Guzman R.G., Steinberg G.K. Netrin-4 enhances angiogenesis and neurologic outcome after cerebral ischemia. J. Cereb. Blood Flow Metab. 2009;29:385–397. doi: 10.1038/jcbfm.2008.128. [DOI] [PubMed] [Google Scholar]
- Hollnagel A., Oehlmann V., Heymer J., Rüther U., Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J. Biol. Chem. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- Igarashi P., Somlo S. Genetics and pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- Karaiskos N., Rahmatollahi M., Boltengagen A., Liu H., Hoehne M., Rinschen M., Schermer B., Benzing T., Rajewsky N., Kocks C. A single-cell transcriptome atlas of the mouse glomerulus. J. Am. Soc. Nephrol. 2018;29:2060–2068. doi: 10.1681/ASN.2018030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Kwan K.M., Carroll T.J., Mcmahon A.P., Mendelsohn C.L., Behringer R.R. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132:2809–2823. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Mugford J.W., Krautzberger A.M., Naiman N., Liao J., Mcmahon A.P. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Reports. 2014;3:650–662. doi: 10.1016/j.stemcr.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaki F., Miyazaki Y., Niimura F., Matsusaka T., Ichikawa I., Motojima M. Foxc1 gene null mutation causes ectopic budding and kidney hypoplasia but not dysplasia. Cells Tissues Organs. 2013;198:22–27. doi: 10.1159/000351291. [DOI] [PubMed] [Google Scholar]
- Kostka G., Giltay R., Bloch W., Addicks K., Timpl R., Fässler R., Chu M.L. Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1-deficient mice. Mol. Cell Biol. 2001;21:7025–7034. doi: 10.1128/MCB.21.20.7025-7034.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T., Deng K., Hogan B.L. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–1395. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- Labastie M.C., Catala M., Gregoire J.M., Peault B. The GATA-3 gene is expressed during human kidney embryogenesis. Kidney Int. 1995;47:1597–1603. doi: 10.1038/ki.1995.223. [DOI] [PubMed] [Google Scholar]
- Lake B.B., Ai R., Kaeser G.E., Salathia N.S., Yung Y.C., Liu R., Wildberg A., Gao D., Fung H.L., Chen S. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science. 2016;352:1586–1590. doi: 10.1126/science.aaf1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake B.B., Codeluppi S., Yung Y.C., Gao D., Chun J., Kharchenko P.V., Linnarsson S., Zhang K. A comparative strategy for single-nucleus and single-cell transcriptomes confirms accuracy in predicted cell-type expression from nuclear RNA. Sci. Rep. 2017;7:6031. doi: 10.1038/s41598-017-04426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake B.B., Chen S., Sos B.C., Fan J., Kaeser G.E., Yung Y.C., Duong T.E., Gao D., Chun J., Kharchenko P.V., Zhang K. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 2018;36:70–80. doi: 10.1038/nbt.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert E., Coissieux M.M., Laudet V., Mehlen P. Netrin-4 acts as a pro-angiogenic factor during zebrafish development. J. Biol. Chem. 2012;287:3987–3999. doi: 10.1074/jbc.M111.289371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law E.W., Cheung A.K., Kashuba V.I., Pavlova T.V., Zabarovsky E.R., Lung H.L., Cheng Y., Chua D., Lai-Wan Kwong D., Tsao S.W. Anti-angiogenic and tumor-suppressive roles of candidate tumor-suppressor gene, Fibulin-2, in nasopharyngeal carcinoma. Oncogene. 2012;31:728–738. doi: 10.1038/onc.2011.272. [DOI] [PubMed] [Google Scholar]
- Leimeister C., Schumacher N., Gessler M. Expression of Notch pathway genes in the embryonic mouse metanephros suggests a role in proximal tubule development. Gene Expr. Patterns. 2003;3:595–598. doi: 10.1016/s1567-133x(03)00114-5. [DOI] [PubMed] [Google Scholar]
- Lejmi E., Bouras I., Camelo S., Roumieux M., Minet N., Leré-Déan C., Merkulova-Rainon T., Autret G., Vayssettes C., Clement O. Netrin-4 promotes mural cell adhesion and recruitment to endothelial cells. Vasc. Cell. 2014;6:1. doi: 10.1186/2045-824X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung-Hagesteijn C., Hu M.C., Mahendra A.S., Hartwig S., Klamut H.J., Rosenblum N.D., Hannigan G.E. Integrin-linked kinase mediates bone morphogenetic protein 7-dependent renal epithelial cell morphogenesis. Mol. Cell Biol. 2005;25:3648–3657. doi: 10.1128/MCB.25.9.3648-3657.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levéen P., Pekny M., Gebre-Medhin S., Swolin B., Larsson E., Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lichtner B., Knaus P., Lehrach H., Adjaye J. BMP10 as a potent inducer of trophoblast differentiation in human embryonic and induced pluripotent stem cells. Biomaterials. 2013;34:9789–9802. doi: 10.1016/j.biomaterials.2013.08.084. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Hellström M., Kalén M., Karlsson L., Pekny M., Pekna M., Soriano P., Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- Lindström N.O., De Sena Brandine G., Tran T., Ransick A., Suh G., Guo J., Kim A.D., Parvez R.K., Ruffins S.W., Rutledge E.A. Progressive recruitment of mesenchymal progenitors reveals a time-dependent process of cell fate acquisition in mouse and human nephrogenesis. Dev. Cell. 2018;45:651–660.e4. doi: 10.1016/j.devcel.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström N.O., Guo J., Kim A.D., Tran T., Guo Q., De Sena Brandine G., Ransick A., Parvez R.K., Thornton M.E., Basking L. Conserved and divergent features of mesenchymal progenitor cell types within the cortical nephrogenic niche of the human and mouse kidney. J. Am. Soc. Nephrol. 2018;29:806–824. doi: 10.1681/ASN.2017080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström N.O., Mcmahon J.A., Guo J., Tran T., Guo Q., Rutledge E., Parvez R.K., Saribekyan G., Schuler R.E., Liao C. Conserved and divergent features of human and mouse kidney organogenesis. J. Am. Soc. Nephrol. 2018;29:785–805. doi: 10.1681/ASN.2017080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström N.O., Tran T., Guo J., Rutledge E., Parvez R.K., Thornton M.E., Grubbs B., Mcmahon J.A., Mcmahon A.P. Conserved and divergent molecular and anatomic features of human and mouse nephron patterning. J. Am. Soc. Nephrol. 2018;29:825–840. doi: 10.1681/ASN.2017091036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Chen S., Boyle S., Zhu Y., Zhang A., Piwnica-Worms D.R., Ilagan M.X., Kopan R. The extracellular domain of Notch2 increases its cell-surface abundance and ligand responsiveness during kidney development. Dev. Cell. 2013;25:585–598. doi: 10.1016/j.devcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo H.M., Hwang T.L., Wu W.B. A phenanthrene derivative, 5,7-dimethoxy-1,4-phenanthrenequinone, inhibits cell adhesion molecule expression and migration in vascular endothelial and smooth muscle cells. Pharmacology. 2017;99:291–302. doi: 10.1159/000457802. [DOI] [PubMed] [Google Scholar]
- Lu X., Le Noble F., Yuan L., Jiang Q., De Lafarge B., Sugiyama D., Bréant C., Claes F., De Smet F., Thomas J.L. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- Menon R., Otto E.A., Kokoruda A., Zhou J., Zhang Z., Yoon E., Chen Y.C., Troyanskaya O., Spence J.R., Kretzler M., Cebrián C. Single-cell analysis of progenitor cell dynamics and lineage specification in the human fetal kidney. Development. 2018;145:1–14. doi: 10.1242/dev.164038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michos O., Gonçalves A., Lopez-Rios J., Tiecke E., Naillat F., Beier K., Galli A., Vainio S., Zeller R. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development. 2007;134:2397–2405. doi: 10.1242/dev.02861. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Oshima K., Fogo A., Hogan B.L., Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J. Clin. Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat M., Sweeney S.M., San Antonio J.D., Fu J., Iozzo R.V. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Yu L., Otsuki A., Ainoya K., Lim K.C., Yamamoto M., Engel J.D. Gata3 hypomorphic mutant mice rescued with a yeast artificial chromosome transgene suffer a glomerular mesangial cell defect. Mol. Cell Biol. 2016;36:2272–2281. doi: 10.1128/MCB.00173-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane R., Lam A.Q., Freedman B.S., Kishi S., Valerius M.T., Bonventre J.V. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol. 2015;33:1193–1200. doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane R., Miyoshi T., Bonventre J.V. Concise review: kidney generation with human pluripotent stem cells. Stem Cells. 2017;35:2209–2217. doi: 10.1002/stem.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motojima M., Kume T., Matsusaka T. Foxc1 and Foxc2 are necessary to maintain glomerular podocytes. Exp. Cell Res. 2017;352:265–272. doi: 10.1016/j.yexcr.2017.02.016. [DOI] [PubMed] [Google Scholar]
- Mundel P., Heid H.W., Mundel T.M., Krüger M., Reiser J., Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacht M., St Martin T.B., Byrne A., Klinger K.W., Teicher B.A., Madden S.L., Jiang Y. Netrin-4 regulates angiogenic responses and tumor cell growth. Exp. Cell Res. 2009;315:784–794. doi: 10.1016/j.yexcr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Narlis M., Grote D., Gaitan Y., Boualia S.K., Bouchard M. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J. Am. Soc. Nephrol. 2007;18:1121–1129. doi: 10.1681/ASN.2006070739. [DOI] [PubMed] [Google Scholar]
- O'brien L.L., Guo Q., Bahrami-Samani E., Park J.S., Hasso S.M., Lee Y.J., Fang A., Kim A.D., Guo J., Hong T.M. Transcriptional regulatory control of mammalian nephron progenitors revealed by multi-factor cistromic analysis and genetic studies. PLoS Genet. 2018;14:e1007181. doi: 10.1371/journal.pgen.1007181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olijnyk D., Ibrahim A.M., Ferrier R.K., Tsuda T., Chu M.L., Gusterson B.A., Stein T., Morris J.S. Fibulin-2 is involved in early extracellular matrix development of the outgrowing mouse mammary epithelium. Cell. Mol. Life Sci. 2014;71:3811–3828. doi: 10.1007/s00018-014-1577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H., Otsuka F., Inagaki K., Takeda M., Miyoshi T., Suzuki J., Mukai T., Ogura T., Makino H. Antagonistic effects of bone morphogenetic protein-4 and -7 on renal mesangial cell proliferation induced by aldosterone through MAPK activation. Am. J. Physiol. Renal. Physiol. 2007;292:F1513–F1525. doi: 10.1152/ajprenal.00402.2006. [DOI] [PubMed] [Google Scholar]
- Oxburgh L., Dudley A.T., Godin R.E., Koonce C.H., Islam A., Anderson D.C., Bikoff E.K., Robertson E.J. BMP4 substitutes for loss of BMP7 during kidney development. Dev. Biol. 2005;286:637–646. doi: 10.1016/j.ydbio.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Oxburgh L., Brown A.C., Fetting J., Hill B. BMP signaling in the nephron progenitor niche. Pediatr. Nephrol. 2011;26:1491–1497. doi: 10.1007/s00467-011-1819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R.W., Wozney J.M., Gelbart W.M. Human BMP sequences can confer normal dorsal-ventral patterning in the Drosophila embryo. Proc. Natl. Acad. Sci. U S A. 1993;90:2905–2909. doi: 10.1073/pnas.90.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T.C., Sasaki T., Zhang R.Z., Fässler R., Timpl R., Chu M.L. Structure and expression of fibulin-2, a novel extracellular matrix protein with multiple EGF-like repeats and consensus motifs for calcium binding. J. Cell Biol. 1993;123:1269–1277. doi: 10.1083/jcb.123.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.S., Valerius M.T., Mcmahon A.P. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- Park J., Shrestha R., Qiu C., Kondo A., Huang S., Werth M., Li M., Barasch J., Suszták K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science. 2018;360:758–763. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T., Zhu G., Dong Y., Zeng J., Li W., Guo W., Chen Y., Duan M., Hocher B., Xie D. BMP4: a possible key factor in differentiation of auditory neuron-like cells from bone-derived mesenchymal stromal cells. Clin. Lab. 2015;61:1171–1178. doi: 10.7754/clin.lab.2015.150217. [DOI] [PubMed] [Google Scholar]
- Pfaff M., Sasaki T., Tangemann K., Chu M.L., Timpl R. Integrin-binding and cell-adhesion studies of fibulins reveal a particular affinity for alpha IIb beta 3. Exp. Cell Res. 1995;219:87–92. doi: 10.1006/excr.1995.1208. [DOI] [PubMed] [Google Scholar]
- Piscione T.D., Wu M.Y., Quaggin S.E. Expression of hairy/enhancer of split genes, Hes1 and Hes5, during murine nephron morphogenesis. Gene Expr. Patterns. 2004;4:707–711. doi: 10.1016/j.modgep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Plachov D., Chowdhury K., Walther C., Simon D., Guenet J.L., Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110:643–651. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- Poluzzi C., Iozzo R.V., Schaefer L. Endostatin and endorepellin: a common route of action for similar angiostatic cancer avengers. Adv. Drug Deliv. Rev. 2016;97:156–173. doi: 10.1016/j.addr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Mao Q., Tang Y., Wang L., Chawla R., Pliner H.A., Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatikainen-Ahokas A., Hytönen M., Tenhunen A., Sainio K., Sariola H. BMP-4 affects the differentiation of metanephric mesenchyme and reveals an early anterior-posterior axis of the embryonic kidney. Dev. Dyn. 2000;217:146–158. doi: 10.1002/(SICI)1097-0177(200002)217:2<146::AID-DVDY2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ranganathan P., Mohamed R., Jayakumar C., Brands M.W., Ramesh G. Deletion of UNC5B in kidney epithelium exacerbates diabetic nephropathy in mice. Am. J. Nephrol. 2015;41:220–230. doi: 10.1159/000381428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli S., Heidet L., Sich M., Henger A., Kretzler M., Gubler M.C., Antignac C. Early glomerular filtration defect and severe renal disease in podocin-deficient mice. Mol. Cell Biol. 2004;24:550–560. doi: 10.1128/MCB.24.2.550-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P., Esni F., Sjödin A., Larue L., Carlsson L., Gullberg D., Takeichi M., Kemler R., Semb H. A potential role of R-cadherin in striated muscle formation. Dev. Biol. 1997;187:55–70. doi: 10.1006/dbio.1997.8602. [DOI] [PubMed] [Google Scholar]
- Rutledge E.A., Benazet J.D., Mcmahon A.P. Cellular heterogeneity in the ureteric progenitor niche and distinct profiles of branching morphogenesis in organ development. Development. 2017;144:3177–3188. doi: 10.1242/dev.149112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer J., Tannahill D., Cioni J.M., Rowlands D., Keynes R. Identification of the extracellular matrix protein Fibulin-2 as a regulator of spinal nerve organization. Dev. Biol. 2018;442:101–114. doi: 10.1016/j.ydbio.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Schwab K., Hartman H.A., Liang H.C., Aronow B.J., Patterson L.T., Potter S.S. Comprehensive microarray analysis of Hoxa11/Hoxd11 mutant kidney development. Dev. Biol. 2006;293:540–554. doi: 10.1016/j.ydbio.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Sharif B., Barua M. Advances in molecular diagnosis and therapeutics in nephrotic syndrome and focal and segmental glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 2018;27:194–200. doi: 10.1097/MNH.0000000000000408. [DOI] [PubMed] [Google Scholar]
- Shim J.H., Greenblatt M.B., Xie M., Schneider M.D., Zou W., Zhai B., Gygi S., Glimcher L.H. TAK1 is an essential regulator of BMP signalling in cartilage. EMBO J. 2009;28:2028–2041. doi: 10.1038/emboj.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sison K., Eremina V., Baelde H., Min W., Hirashima M., Fantus I.G., Quaggin S.E. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J. Am. Soc. Nephrol. 2010;21:1691–1701. doi: 10.1681/ASN.2010030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Taguchi A., Nishinakamura R. Nephron reconstitution from pluripotent stem cells. Kidney Int. 2015;87:894–900. doi: 10.1038/ki.2014.358. [DOI] [PubMed] [Google Scholar]
- Takasato M., Er P.X., Chiu H.S., Maier B., Baillie G.J., Ferguson C., Parton R.G., Wolvetang E.J., Roost M.S., Lopes S.M., Little M.H. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2016;536:238. doi: 10.1038/nature17982. [DOI] [PubMed] [Google Scholar]
- Ueda H., Miyazaki Y., Matsusaka T., Utsunomiya Y., Kawamura T., Hosoya T., Ichikawa I. Bmp in podocytes is essential for normal glomerular capillary formation. J. Am. Soc. Nephrol. 2008;19:685–694. doi: 10.1681/ASN.2006090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg C.W., Ritsma L., Avramut M.C., Wiersma L.E., Van Den Berg B.M., Leuning D.G., Lievers E., Koning M., Vanslambrouck J.M., Koster A.J. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports. 2018;10:751–765. doi: 10.1016/j.stemcr.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H., Groenen P., Nesbit M.A., Schuffenhauer S., Lichtner P., Vanderlinden G., Harding B., Beetz R., Bilous R.W., Holdaway I. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406:419–422. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- Wang W., Reeves W.B., Ramesh G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am. J. Physiol. Renal. Physiol. 2008;294:F739–F747. doi: 10.1152/ajprenal.00508.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P.D. Polycystic kidney disease: new understanding in the pathogenesis. Int. J. Biochem. Cell Biol. 2004;36:1868–1873. doi: 10.1016/j.biocel.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Wilson P.C., Humphreys B.D. Kidney and organoid single-cell transcriptomics: the end of the beginning. Pediatr. Nephrol. 2019:1–7. doi: 10.1007/s00467-018-4177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Malone A.F., Donnelly E.L., Kirita Y., Uchimura K., Ramakrishnan S.M., Gaut J.P., Humphreys B.D. Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J. Am. Soc. Nephrol. 2018;29:2069–2080. doi: 10.1681/ASN.2018020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Tamayo P., Zhang K. Visualizing and interpreting single-cell gene expression datasets with similarity weighted nonnegative embedding. Cell Syst. 2018;7:656–666.e4. doi: 10.1016/j.cels.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Kirita Y., Donnelly E.L., Humphreys B.D. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J. Am. Soc. Nephrol. 2019;30:23–32. doi: 10.1681/ASN.2018090912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han J., Ito Y., Bringas P., Deng C., Chai Y. Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev. Cell. 2008;15:322–329. doi: 10.1016/j.devcel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida-Asanuma E., Asanuma K., Kim K., Donnelly M., Young Choi H., Hyung Chang J., Suetsugu S., Tomino Y., Takenawa T., Faul C., Mundel P. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am. J. Pathol. 2007;171:415–427. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarrow J.C., Perlman Z.E., Westwood N.J., Mitchison T.J. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004;4:21. doi: 10.1186/1472-6750-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Sanes J.R., Miner J.H. Identification and expression of mouse netrin-4. Mech. Dev. 2000;96:115–119. doi: 10.1016/s0925-4773(00)00369-5. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Nichols J., Chambers I., Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Yu P.B., Deng D.Y., Lai C.S., Hong C.C., Cuny G.D., Bouxsein M.L., Hong D.W., Mcmanus P.M., Katagiri T., Sachidanandan C. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat. Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.Z., Pan T.C., Zhang Z.Y., Mattei M.G., Timpl R., Chu M.L. Fibulin-2 (FBLN2): human cDNA sequence, mRNA expression, and mapping of the gene on human and mouse chromosomes. Genomics. 1994;22:425–430. doi: 10.1006/geno.1994.1404. [DOI] [PubMed] [Google Scholar]
- Zhong J., Yang H.C., Fogo A.B. A perspective on chronic kidney disease progression. Am. J. Physiol. Renal. Physiol. 2017;312:F375–F384. doi: 10.1152/ajprenal.00266.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.