Abstract
Aims
To characterise the clinical and topographical features of circumscribed choroidal haemangioma (CCH) and to visualise the patterns of tumour extent in the ocular fundus.
Methods
Data on the size, shape and location of 113 CCH were converted into a database of two-dimensional retinal charts by means of computer drawing software. The extent of the tumours was visualised by merging the charts and displaying the number of overlapping tumours on colour-coded maps.
Results
The mean largest tumour diameter was 7.2 mm (range, 2.5–11.0 mm), mean tumour height was 2.4 mm (range, 0.7–4.6 mm) and mean diameter/height ratio was 3.2 (range, 2.1–6.0). The mean distance from the posterior tumour margin to the foveola and optic disc margin was 1.7 mm (range, 0–15 mm) and 2.4 mm (range, 0–11 mm), respectively. The hemispheric location of the tumour centroid was temporal in 75 eyes (66%) and nasal in 38 (34%) (p=0.0005) and the distribution between the superior and inferior hemispheres was 68 (60%) and 45 (40%), respectively (p=0.03). The presence of subretinal fluid (SRF) was significantly associated with young age at diagnosis (p=0.0002), low tumour diameter/height ratio (p=0.0004), nasal hemisphere location (p=0.006) and close proximity to the optic disc (p=0.004).
Conclusions
The superotemporal quadrant close to the macula is the most frequent location of CCH. The tumours are generally characterised by a diameter/height ratio of >2. Tumours in young patients, with marked elevation, in nasal hemisphere and in proximity to the optic disc are associated with SRF exudation.
Keywords: circumscribed choroidal haemangioma, topography, distribution, location, imaging
Introduction
Circumscribed choroidal haemangioma (CCH) typically presents as a solitary, unilateral tumour in the posterior pole of the eye without any other ocular or systemic anomalies.1 Its true incidence is unknown, as most CCH are diagnosed if the patient becomes symptomatic or incidentally during routine examination. Jarrett et al discovered one CCH for every 15 cases of choroidal melanoma,2 which is broadly in line with clinical experience and expectations.
Ophthalmoscopically, CCH is seen as a round or oval, dome-shaped, orange-red mass. Over time, secondary changes may occur, such as overlying drusen, fibrous metaplasia and retinal pigment epithelium (RPE) atrophy or hyperplasia.1 3 On ultrasonography, CCH appears as an acoustically solid mass with high internal reflectivity.4 Fluorescein angiography shows gradually increasing fluorescence, while indocyanine green (ICG) angiography is characterised by rapid hyperfluorescence followed by relative hypofluorescence, so-called ‘washout’ phenomenon, in the very late phase.5 6 During early adulthood, CCH may cause visual loss and metamorphopsia due to subretinal fluid (SRF) exudation, macular oedema or macular elevation or tilting. Since the beginning of this century, photodynamic therapy has been considered the treatment of choice for symptomatic CCH.7 8
Despite its characteristic clinical appearance, it is vitally important to distinguish CCH from more serious conditions like choroidal melanoma and metastasis, which can be a particularly challenging in chronic lesions with metaplastic changes. The main objectives of the present study were to elicit detailed information on clinical and topographical features of CCH that could be of diagnostic significance.
Materials and methods
Study design and patients
This multicentre study was performed as a retrospective medical chart review of patients with CCH diagnosed between 2002 and 2017. Participating centres were the Department of Ophthalmic Oncology, Cole Eye Institute, Cleveland, Ohio, USA; Department of Ophthalmology, Haukeland University Hospital, Bergen, Norway and Shri Bhagwan Mahavir Vitreoretinal Services, Sankara Nethralaya, Chennai, Tamil Nadu, India. Only patients with medical records containing detailed information about the size, shape, appearance and exact location of the CCH were included. Other clinical information extracted from the records were gender, age at presentation, type and duration of symptoms, causes of referral and best-corrected visual acuity (BCVA). Ethical approval was obtained from all participating hospital ethics committees, and the study was conducted in accordance with the Declaration of Helsinki.
Tumour data
Data regarding morphological and topographical characteristics of the CCH were obtained from the written records and by careful examination of available fundus photographs and drawings, ultrasonographic A-scans and B-scans, fluorescein and ICG angiograms, optical coherence tomography (OCT) images and magnetic resonance imaging (MRI) scans. Ultrasonographic measurements of tumour dimensions included the largest basal diameter and height. In order to discriminate between flat and more sphere-shaped CCH, the tumours were categorised according to their diameter/height ratio. Recorded tumour characteristics also included shape, colour, the presence of fibrous metaplasia, retinal lipid exudates, drusen and hyperplasia or atrophy of the RPE as well as OCT-confirmed SRF and retinal oedema.
Tumour location
The location of the CCH was determined according to their geometric centre (tumour centroid), which for the round and oval lesions corresponded to the midpoint of the largest tumour diameter. For irregular tumours, the location was judged by the centremost point. The CCH were then categorised according to their location in quadrants and hemispheres (defined by a horizontal and vertical axis through the foveal centre). The distances from the posterior tumour margin to the foveola and the optic disc margin were estimated clinically by using the horizontal diameter of the optic disc as a reference length of 1.75 mm.9 10
Fundus mapping
Based on the various imaging modalities, each CCH was drawn with azimuth equidistant projections on a standardised retinal drawing chart with a foveal centre surrounded by circles representing the equator, ora serrata and limbus.9 11 All drawings were done by one author (JK), and care was taken to correct for peripheral distortions when calculating retinal diagrams from tumour parameters.12 13 The drawing tools of the computer software PowerPoint (Microsoft, Redmond, Washington, USA) were then used to convert all fundus drawings into a database of identical two-dimensional retinal charts. The retinal charts of left eyes were flipped across the vertical axis, so that all eyes were displayed as right eyes. A custom-made Matlab program (The MathWorks, Natick, Massachusetts, USA), was used to merge, filter and finally convert the collection of digital tumour drawings into a map of the fundus, in which the number of overlapping CCH was indicated by different colours (online supplementary figure). Separate colour-coded maps were made for various subgroups of patients and tumours, where dark blue indicated areas without any tumours and dark red revealed the area with the maximum number of overlapping CCH.
bjophthalmol-2018-313388supp001.pdf (173.8KB, pdf)
Statistical analysis
The χ² test and Student’s t-test were used to compare the patients’ gender and age distribution between the participating centres. The χ² goodness-of-fit test was used to analyse the distribution of the CCH under the null hypothesis that they are uniformly distributed in the ocular fundus. For the comparison between two groups of patients or tumours with different characteristics (binary variables), the Fisher’s exact test and Mann–Whitney U-test were applied for categorical and continuous variables, respectively. The subgroups were assigned by the median split method. Statistical analyses of tumour location were performed under the assumption that each chart quadrant includes an equal area of the choroidal sphere. In one patient with two distinct CCH in the same eye, only the largest tumour was included in the statistical analyses. The Bonferroni correction for multiple testing was applied when necessary. The data were analysed using Graph Pad Prism software, V.7.0 (Graph Pad Software, San Diego, California, USA) and IBM SPSS Statistics, V.24 (SPSS, Chicago, Illinois, USA). All tests were two-sided, and statistical significance was defined as a p<0.05.
Results
Patient and tumour characteristics
We identified 113 patients (72 men and 41 women) with CCH who met our inclusion criteria. The median age at time of diagnosis was 58 years (mean, 57 years; range, 11–93 years). The right eye was involved in 62 patients and the left eye in 51 patients. Sixty patients were included from Cole Eye Institute, 33 from Haukeland University Hospital and 20 from Sankara Nethralaya. The gender distribution did not differ significantly between the three centres. The patients from Chennai were significantly younger (mean age, 49 years) compared with those from Cleveland and Bergen (mean age, 57 and 61 years, respectively) (p=0.008).
Eighty-nine patients (79%) had visual symptoms attributable to the CCH, with a median duration of 6 months (mean, 20 months; range, 1 month–20 years) prior to diagnosis. The most common presenting symptoms were blurred vision and scotoma (71%), metamorphopsia (10%) and photopsia (4%). The mean BCVA at presentation was 0.5 logMAR units (range, −0.1 to 3.0 logMAR), which is equivalent to a Snellen visual acuity of 20/63. The referral diagnoses were CCH (33%), unspecified choroidal tumour (31%), choroidal melanoma (12%), central serous chorioretinopathy (8%), age-related macular degeneration (6%), choroidal nevus (4%), choroidal metastasis (3%) and others (3%).
The median largest basal tumour diameter was 7.5 mm (mean, 7.2 mm; range, 2.5–11.0 mm) and the median tumour height was 2.2 mm (mean, 2.4 mm; range, 0.7–4.6 mm). All CCH appeared dome-shaped and moderately elevated with a median basal diameter/height ratio of 3.0 (mean, 3.2; range, 2.1–6.0). The lesion was round in 60 cases (53%), oval in 35 (31%) and irregular in 18 (16%). The colour of the tumour was judged as orange in 71 cases (63%), red in 18 (16%), orange-red in 17 (15%), grey in 5 (4%) and yellow in 2 (2%). Overlying pigmentary changes were noted in 33 CCH (29%), fibrous metaplasia in 26 (23%), RPE atrophy in 18 (16%), retinal lipid exudates in 16 (14%) and drusen in 9 (8%), whereas no tumours displayed orange pigment or retinal haemorrhages. SRF associated with the CCH was present in 76 eyes (67%) and retinal oedema in 48 (42%). SRF exudation from the tumour was significantly associated with young age at diagnosis (p=0.0002) and a low basal diameter/height ratio (p=0.0004).
Ancillary diagnostic testing
Ultrasonographic A-scans and B-scans were available for review in 110 patients (97%). High internal reflectivity on A-scan was noted in 103 cases (94%), while seven tumours (6%) showed medium to high internal reflectivity. In all cases, B-scan ultrasonography revealed acoustic solidity of the tumour.
Fluorescein angiograms were available in 67 patients (59%). All cases showed a gradually increasing fluorescence throughout the angiography, leading to a diffuse hyperfluorescence and staining of the tumour in the late phase. ICG angiograms were eligible for review in 101 cases (89%). In the very early arterial phase of the ICG angiography, all cases showed hyperfluorescence of intrinsic tumour vessels, which within about 30 s after injection turned into a strong and uniform fluorescence of the entire tumour (figure 1). In 43 eyes, where the angiographic procedure was prolonged into the late phases (20–40 min), all tumours demonstrated the characteristic ‘washout phenomenon’ with relative hypofluorescence (compared with the adjacent choroid) and a surrounding hyperfluorescent rim. In 22 of these eyes (51%), multiple, strongly hyperfluorescent punctate spots were seen over the lesion.
Figure 1.
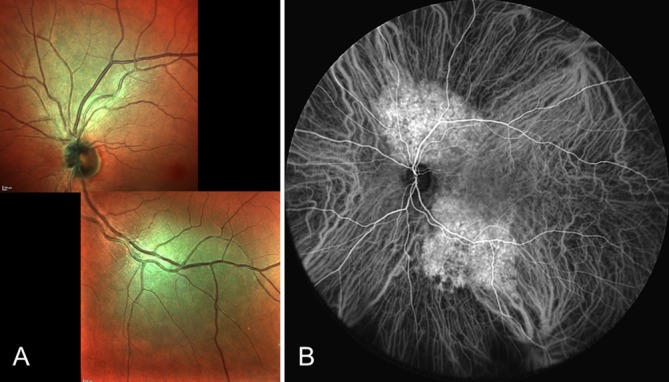
(A,B) Images of the left eye of a 63-year-old man showing two distinct circumscribed choroidal haemangiomas: one superior to the optic disc and one along the inferotemporal vascular arcade. (A) Multicolour scanning laser image (composite). (B) Ultra-widefield indocyanine green angiogram (40 s after injection).
Contrast-enhanced MRI was performed in 23 patients (20%). Four CCH (17%) were too small to be seen on MRI. All the other tumours demonstrated hyperintensity or isointensity on both T1-weighted and T2-weighted images when compared with the vitreous as well as strong and homogenous contrast (gadolinium) enhancement.
Fundus distribution of the tumours
There was a significant nasotemporal asymmetry in the distribution of the tumour centroids, as 75 (66%) were located in the temporal and 38 (34%) in the nasal hemisphere (p=0.0005). The distribution between the superior and inferior hemispheres was 68 (60%) and 45 (40%), respectively (p=0.03). All the 113 CCH were located posterior to the equator.
The hemispherical distribution of the tumours did not differ significantly between genders, patients in the age group <58 years and ≥58 years, right and left eyes or between CCH with a basal diameter/height ratio <3 and≥3 (continuous variables were divided by their median value). The presence of SRF was significantly associated with nasal hemisphere location of the CCH (p=0.006) and close proximity to the optic disc (p=0.004) (table 1).
Table 1.
Topographical distribution of the tumour centroids of 113 circumscribed choroidal haemangiomas in various fundus hemispheres and the distances from the posterior tumour margin to the foveola and optic disc margin, according to binary patient and tumour characteristics
| Binary variables | Eyes | Hemisphere (n) | Distance (mean±SD, mm) | ||||||||
| n | T | N | P* | U | L | P value* | Foveola | P value† | Optic disc | P value† | |
| Female | 41 | 31 | 10 | 0.15 | 22 | 19 | 0.32 | 1.6±2.8 | 0.33 | 2.8±2.6 | 0.33 |
| Male | 72 | 44 | 28 | 46 | 26 | 1.7±2.1 | 2.2±2.1 | ||||
| Age <58 years | 56 | 33 | 23 | 0.11 | 31 | 25 | 0.34 | 1.6±2.4 | 0.92 | 2.1±2.5 | 0.07 |
| Age≥58 years | 57 | 42 | 15 | 37 | 20 | 1.7±2.4 | 2.7±2.0 | ||||
| Right eye | 62 | 42 | 20 | 0.84 | 36 | 26 | 0.70 | 1.8±2.6 | 0.98 | 2.7±2.5 | 0.14 |
| Left eye | 51 | 33 | 18 | 32 | 19 | 1.6±2.0 | 2.0±2.0 | ||||
| Basal diameter/height ratio <3 | 50 | 32 | 18 | 0.69 | 27 | 23 | 0.25 | 1.7±1.7 | 0.27 | 2.4±2.4 | 0.78 |
| Basal diameter/height ratio ≥3 | 63 | 43 | 20 | 41 | 22 | 1.7±2.8 | 2.4±2.1 | ||||
| Presence of subretinal fluid | 76 | 44 | 32 | 0.006‡ | 47 | 29 | 0.68 | 1.8±2.0 | 0.01 | 2.0±2.2 | 0.004‡ |
| Absence of subretinal fluid | 37 | 31 | 6 | 21 | 16 | 1.4±3.0 | 3.2±2.1 | ||||
*P values calculated using the Fisher’s exact test.
†P values calculated using the Mann–Whitney U-test.
‡P values, which also remained statistically significant after Bonferroni correction for multiple comparisons.
L, lower; N, nasal; T, temporal; U, upper.
Patterns of tumour extent
The median distances from the nearest tumour margin to the foveola and to the optic disc margin were 1.0 mm (mean, 1.7 mm; range, 0–15.0 mm) and 2.0 mm (mean, 2.4 mm; range, 0–11.0 mm), respectively. The anterior tumour margin was located posterior to the equator in all cases.
A 63-year-old, previously healthy, Norwegian male had two asymptomatic and distinct CCH in his left eye; one juxtapapillary tumour (largest basal diameter 6.0 mm, height 1.0 mm) located superior to the optic disc and one (largest basal diameter 7.0 mm, height 1.8 mm) located along the inferotemporal vascular arcade (figure 1). Otherwise, all patients presented with a solitary, unilateral lesion.
The patterns of tumour extent, visualised by the computationally merged retinal charts, corresponded clearly with the regional distribution of the tumour centroids. The maximum number of overlapping tumours was 47, and this area was located temporal to the macular region at the boundary between the upper and lower hemisphere (figure 2). The lower nasal quadrant was the area with the lowest number of overlapping tumours.
Figure 2.
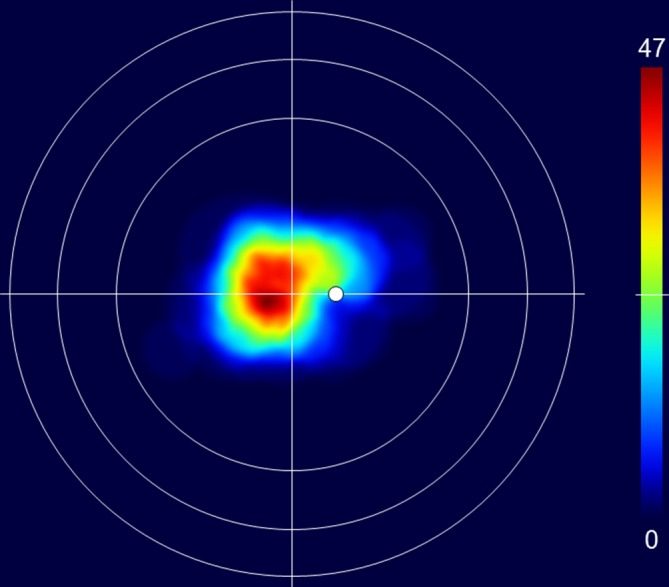
Merged fundus drawings showing the location of 113 CCH on a retinal chart with a foveal centre surrounded by circles representing the equator, ora serrata and limbus. The colours on the chart indicate the number of overlapping CCH according to the colour scale bar to the right. The top of the scale (dark red) represents the maximum number of overlapping tumours (47) and the bottom (dark blue) indicates no tumours. CCH, circumscribed choroidal haemangiomas.
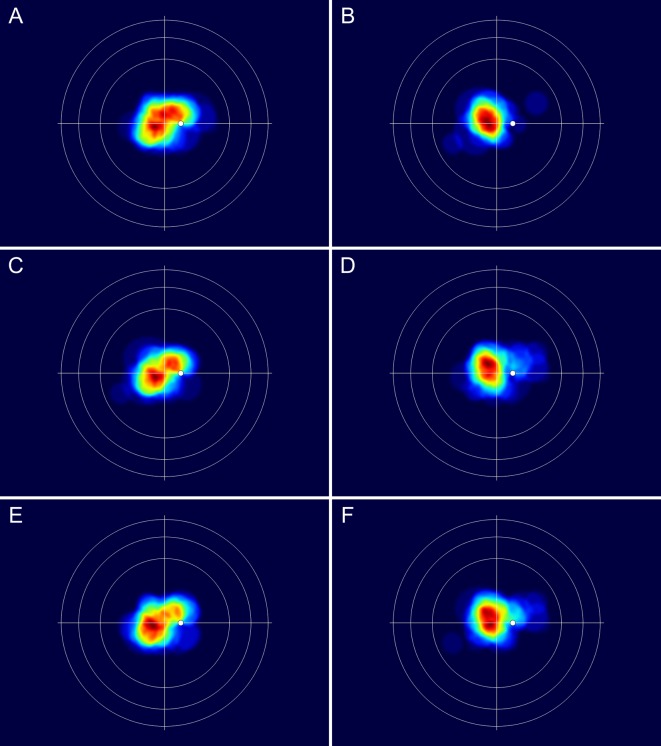
When comparing the merged retinal charts from patients with and without SRF exudation from the tumour, the differences in tumour location became evident. In eyes with tumour exudation, the CCH seemed to be located more nasally with a closer proximity to the upper part of the optic disc compared with those without exudation (figure 3A,B). Although not supported by the statistical subanalyses in table 1, similar differences in the patterns of tumour extent were observed between patients<58 years and those ≥58 years of age (figure 3C,D) and between tumours with a basal diameter/height ratio <3 and ≥3 (figure 3E,F).
Figure 3.
(A–F) Merged fundus drawings of circumscribed choroidal haemangiomas showing the patterns of tumour extent according to various patient and tumour characteristics. The retinal chart and colour scale details are as described in figure 2. Note that the maximum number of overlapping tumours differs between the images. The following numbers in parentheses refer to the maximum number of overlapping tumours: (A) tumours with subretinal fluid exudation (28), (B) tumours without subretinal fluid exudation (20), (C) age <58 years (24), (D) age ≥58 years (26), (E) tumour diameter/height-ratio<3 (21), (F) tumour diameter/height-ratio≥3 (28).
Discussion
This study provides detailed clinical and topographical data on a large cohort of CCH patients from three different continents. As CCH may mimic a malignancy and lead to unnecessary and potentially harmful treatment, it is critical to be aware of its diagnostic features. Only one-third of the patients were referred with the correct diagnosis. This is in accordance with the study by Shields et al, where the diagnosis of CCH was accurately suspected before referral only in 29% of cases.3 In the present study, the referral diagnoses were unspecified choroidal tumour, choroidal melanoma or choroidal metastasis in 46% of the patients, indicating that a malignant tumour was most commonly suspected by the referring physicians. Serous chorioretinopathy was the most common non-tumour differential diagnosis, which may be due to the subtle clinical appearance of the CCH combined with the frequent presence of SRF. In almost all cases (94%), the colour of the tumour was described as different tones of orange and red and thus very similar to the surrounding choroid. Prior to the diagnosis, the CCH had led to visual symptoms caused by SRF, retinal oedema or elevation or tilting of the macula in the majority of patients (79%). The remaining tumours were detected incidentally during routine examination or examination for other eye conditions.
Ancillary testing revealed remarkably recognisable and consistent results. Among the tumours tested, all showed high or medium to high internal reflectivity and acoustic solidity on ultrasonography, a gradually increasing fluorescence on fluorescein angiography, and early hyperfluorescence followed by late hypofluorescence on ICG angiography. All lesions examined by MRI showed strong contrast enhancement and hyperintensity or isointensity on the T1-weighted and T2-weighted images relative to the vitreous.
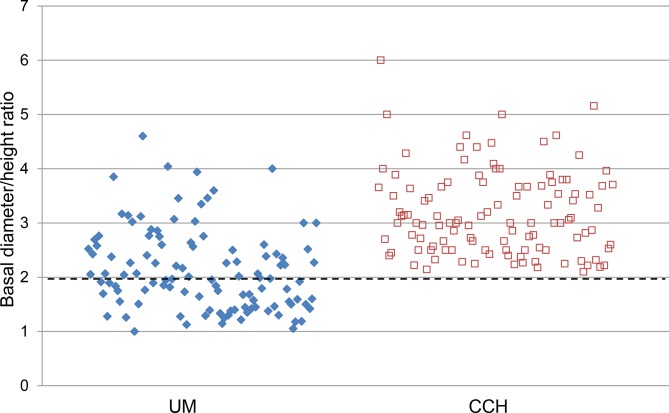
Seventy-two of the 113 patients (64%) were male, corresponding to a male-to-female ratio of 1.8:1. A male predominance of CCH has previously been reported,3 6 14 while others have found an even distribution between genders.15 All tumours were less than 5.0 mm in height, similar to observations in a clinicopathological study by Witschel and Font, wherein they reported that all tumours were less than 6.0 mm in height.15 In the present study, none of the CCH had a basal diameter/height ratio equal to or lower than 2. This differs considerably from the results of a previous study on 110 posterior uveal melanomas,16 where the basal diameter/height ratio was below 2 in 56 (51%) of the tumours (figure 4). A basal diameter/height ratio above 2 and height of less than 5.0 mm may therefore be added as a supportive diagnostic criterion for the discrimination between CCH and other intraocular tumours such as choroidal melanoma.
Figure 4.
Scatter plot showing the distribution of the basal diameter/height ratio for 110 posterior UM previously reported by Krohn et al 16 and for the 113 CCH in the present study. The basal diameter/height ratio for each UM and CCH are represented by blue diamonds and red squares, respectively. A small number of the plotted points have been slightly jittered to avoid overlap. Note that all the CCH have a basal diameter/height ratio above 2 (indicated by the dashed horizontal line). CCH, circumscribed choroidal haemangiomas; UM, uveal melanomas.
CCH are usually solitary and unilateral, but bilateral CCH have been published.17–20 None of our patients had bilateral lesions. However, one otherwise healthy patient presented with two distinct CCH in the same eye, which, to the best of our knowledge, is the first reported case of multiple unilateral CCH (figure 1).
Previous studies have shown that the majority of CCH are located posterior to the equator and temporal to the optic disc,3 15 while information on their quadrantic distribution is scarce. The numerical distribution and the merged fundus drawings showed that the superotemporal quadrant close to the macula is the significantly most frequent location of CCH. SRF associated with the CCH was found in 67% of the eyes. A clear difference was observed when comparing the merged fundus drawings of patients with and without tumour exudation. In patients where SRF was present, the tumours were generally located more nasally and in close proximity or adjacent to the upper margin of the optic disc. A similar pattern of tumour extent was also found for the subgroup of patients with a low tumour diameter/height-ratio. This is in accordance with the findings that a short distance between the tumour and the optic disc and a low tumour diameter/height-ratio were significant risk factors for exudation. One may suspect that the presence of SRF per se can alter the perceived extent and size of the tumours. However, the tumour dimensions were mainly determined by ultrasonography, making it possible to discriminate between SRF and the tumour tissue. Still, fluid exudation can probably induce stromal oedema in the CCH and thereby increase its size. This confounding effect is reduced when using the tumour diameter/height-ratio for statistical analysis. A marked tumour elevation, indicated by a low tumour diameter/height-ratio, may suggest an increased blood flow and perfusion pressure in the haemangioma, which in turn can cause fluid exudation. The association between tumour exudation and proximity to the optic disc may also be explained by an elevated perfusion pressure within the tumour vasculature. The choroid and optic nerve head are supplied by 15–20 short posterior ciliary arteries that pierce the sclera in a circle very close to the optic nerve.21 It is tempting to hypothesise that the closer the CCH is located to the proximal segment of the posterior choroidal arteries, the higher the perfusion pressure may be within the tumour, and vice versa. Fluid exudation from the tumour was also significantly associated with young age at diagnosis, which probably reflects that non-symptomatic lesions are more often incidentally detected during examination for typical age-related eye diseases like cataract and macular degeneration. The main limitation of our study includes its retrospective design, which limited the availability of some data and prevented a standardisation of the examinations. Moreover, the data were collected from tertiary referral centres, thus indicating a possible bias toward more advanced cases.
In summary, both the distribution of the tumour centroids and the merged retinal charts demonstrated that the superotemporal quadrant close to the macula is the most frequent location of CCH. Variables significantly associated with SRF exudation were young age at diagnosis, marked tumour elevation, nasal hemisphere location and close proximity to the optic disc. The CCH were characterised by a diameter/height ratio above 2 and height of less than 5.0 mm, which may be useful for discrimination between CCH and other intraocular tumours such as choroidal melanoma. Awareness of the specific clinical features of CCH and appropriate selection and interpretation of ancillary testing are necessary to avoid misdiagnosis.
Footnotes
Contributors: JK and ADS planned and designed the study. JK drafted the manuscript, and all authors contributed to data acquisition and analysis, revision of the manuscript and approval of the final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: Ethics approval was provided by the Regional Committee for Medical and Health Research Ethics, Western Norway and the Institutional Review Boards of Cleveland Clinic and Vision Research Foundation, Sankara Nethralaya, Chennai.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Singh AD, Kaiser PK. Uveal vascular tumors : Singh AD, Damato BE, Pe’er J, Clinical ophthalmic oncology. Philadelphia, PA: Saunders Elsevier, 2007: 289–99. [Google Scholar]
- 2. Jarrett WH, Hagler WS, Larose JH, et al. . Clinical experience with presumed hemangioma of the choroid: radioactive phosphorus uptake studies as an aid in differential diagnosis. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 1976;81:862–70. [PubMed] [Google Scholar]
- 3. Shields CL, Honavar SG, Shields JA, et al. . Circumscribed choroidal hemangioma: clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology 2001;108:2237–48. 10.1016/S0161-6420(01)00812-0 [DOI] [PubMed] [Google Scholar]
- 4. Verbeek AM, Koutentakis P, Deutman AF. Circumscribed choroidal hemangioma diagnosed by ultrasonography. A retrospective analysis of 40 cases. Int Ophthalmol 1995;19:185–9. [DOI] [PubMed] [Google Scholar]
- 5. Arevalo JF, Shields CL, Shields JA, et al. . Circumscribed choroidal hemangioma: characteristic features with indocyanine green videoangiography. Ophthalmology 2000;107:344–50. 10.1016/S0161-6420(99)00051-2 [DOI] [PubMed] [Google Scholar]
- 6. Schalenbourg A, Piguet B, Zografos L. Indocyanine green angiographic findings in choroidal hemangiomas: a study of 75 cases. Ophthalmologica 2000;214:246–52. 10.1159/000027499 [DOI] [PubMed] [Google Scholar]
- 7. Barbazetto I, Schmidt-Erfurth U. Photodynamic therapy of choroidal hemangioma: two case reports. Graefes Arch Clin Exp Ophthalmol 2000;238:214–21. 10.1007/s004170050346 [DOI] [PubMed] [Google Scholar]
- 8. Gupta M, Singh AD, Rundle PA, et al. . Efficacy of photodynamic therapy in circumscribed choroidal haemangioma. Eye 2004;18:139–42. 10.1038/sj.eye.6700597 [DOI] [PubMed] [Google Scholar]
- 9. Straatsma BR, Landers MB, Kreiger AE, et al. . Topography of the adult human retina. UCLA Forum Med Sci 1969;8:379–410. [PubMed] [Google Scholar]
- 10. Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci 1988;29:1151–8. [PubMed] [Google Scholar]
- 11. Li W, Judge H, Gragoudas ES, et al. . Patterns of tumor initiation in choroidal melanoma. Cancer Res 2000;60:3757–60. [PubMed] [Google Scholar]
- 12. Mosier MA. Retinal cartography. Can J Ophthalmol 1982;17:219–22. [PubMed] [Google Scholar]
- 13. Borodkin MJ, Thompson JT. Retinal cartography. An analysis of two-dimensional and three-dimensional mapping of the retina. Retina 1992;12:273–80. [PubMed] [Google Scholar]
- 14. Sanborn GE, Augsburger JJ, Shields JA. Treatment of circumscribed choroidal hemangiomas. Ophthalmology 1982;89:1374–80. 10.1016/S0161-6420(82)34635-7 [DOI] [PubMed] [Google Scholar]
- 15. Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol 1976;20:415–31. 10.1016/0039-6257(76)90067-9 [DOI] [PubMed] [Google Scholar]
- 16. Krohn J, Frøystein T, Dahl O. Posterior uveal melanoma. Distribution of the sites of origin and patterns of tumour extent in the ocular fundus. Br J Ophthalmol 2008;92:751–6. 10.1136/bjo.2007.133025 [DOI] [PubMed] [Google Scholar]
- 17. Schepens CL, Schwartz A. Intraocular tumors. I. Bilateral hemangioma of the choroid. AMA Arch Ophthalmol 1958;60:72–83. [PubMed] [Google Scholar]
- 18. Perri P, Incorvaia C, Costagliola C, et al. . Bilateral circumscribed haemangioma of the choroid not associated with systemic vascular syndrome. Br J Ophthalmol 2001;85:1260–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tran HV, Schalenbourg A, Zografos L. Bilateral circumscribed choroidal hemangioma in an otherwise healthy individual. Retin Cases Brief Rep 2007;1:149–52. 10.1097/01.ICB.0000279646.84077.b4 [DOI] [PubMed] [Google Scholar]
- 20. Rahman W, Horgan N, Hungerford J. Circumscribed choroidal haemangioma mimicking chronic central serous chorioretinopathy. Journal Français d'Ophtalmologie 2013;36:e37–40. 10.1016/j.jfo.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 21. Torczynski E. Choroid and suprachoroid : Jakobiec FA, Ocular anatomy, embryology, and teratology. Philadelphia, PA: Harper & Row, 1982: 553–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2018-313388supp001.pdf (173.8KB, pdf)