Abstract
Osteogenesis imperfecta (OI) comprises a genetically heterogeneous group of skeletal fragility diseases. Here, we report on five independent families with a progressively deforming type of OI, in whom we identified four homozygous truncation or frameshift mutations in MESD. Affected individuals had recurrent fractures and at least one had oligodontia. MESD encodes an endoplasmic reticulum (ER) chaperone protein for the canonical Wingless-related integration site (WNT) signaling receptors LRP5 and LRP6. Because complete absence of MESD causes embryonic lethality in mice, we hypothesized that the OI-associated mutations are hypomorphic alleles since these mutations occur downstream of the chaperone activity domain but upstream of ER-retention domain. This would be consistent with the clinical phenotypes of skeletal fragility and oligodontia in persons deficient for LRP5 and LRP6, respectively. When we expressed wild-type (WT) and mutant MESD in HEK293T cells, we detected WT MESD in cell lysate but not in conditioned medium, whereas the converse was true for mutant MESD. We observed that both WT and mutant MESD retained the ability to chaperone LRP5. Thus, OI-associated MESD mutations produce hypomorphic alleles whose failure to remain within the ER significantly reduces but does not completely eliminate LRP5 and LRP6 trafficking. Since these individuals have no eye abnormalities (which occur in individuals completely lacking LRP5) and have neither limb nor brain patterning defects (both of which occur in mice completely lacking LRP6), we infer that bone mass accrual and dental patterning are more sensitive to reduced canonical WNT signaling than are other developmental processes. Biologic agents that can increase LRP5 and LRP6-mediated WNT signaling could benefit individuals with MESD-associated OI.
Keywords: MESD, osteogenesis imperfecta, WNT signaling
Main Text
Osteogenesis imperfecta (OI) is a group of inherited disorders characterized by bone fragility. Clinical severity ranges from non-deforming to progressively deforming to neonatally lethal.1 At present, mutations in 19 different genes have been associated with OI: COL1A1 (MIM: 120150), COL1A2 (MIM: 120160), IFITM5 (MIM: 614757), BMP1 (MIM: 112264), CREB3L1 (MIM: 616215), CRTAP (MIM: 605497), FKBP10 (MIM: 607063), MBTPS2 (MIM: 300294), P3H1 (MIM: 610339), PLOD2 (MIM: 301865), PPIB (MIM: 123841), SEC24D (MIM: 607186), SERPINF1 (MIM: 172860), SERPINH1 (MIM: 600943), SP7 (MIM: 606633), SPARC (MIM: 182120), TENT5A (MIM: 611357), TMEM38B (MIM: 611236), WNT1 (MIM: 164820). ∼85% of OI cases are caused by mutations in one of the two genes that encode type 1 collagen, COL1A1 or COL1A2. Extra-skeletal manifestations seen in individuals with OI, such as blue sclerae, dentinogenesis imperfecta, and ligamentous laxity, depend upon the specific gene that is mutated and the nature of the mutation.
Here we report the discovery of an additional autosomal-recessive OI-associated gene (MESD, mesoderm development gene, previously called MESDC2 [MIM: 607783]); we describe clinical findings in five affected individuals from five independent, unrelated families; and we provide data that support the likelihood that OI-associated MESD mutations produce hypomorphic alleles rather than complete loss-of-function alleles. This study was conducted in accordance with the Helsinki Declaration and was approved by the ethics committee of the University of Cologne, Germany and the ethics committee of the University Medical Center Göttingen, Germany. All guardians signed informed consent for the molecular analysis, for publication of the results and corresponding clinical information, including clinical photographs, and for skin biopsies, where applicable.
Individuals who participated in this study had been clinically diagnosed with a progressive deforming form of OI (Figure 1); radiographic findings in all included osteopenia, skeletal deformity, healed fractures, and newly-acquired fractures. All affected individuals were offspring of consanguineous unions and had no prior family history of skeletal fragility. We performed whole-exome sequencing in order to simultaneously interrogate all known OI-associated genes. We used either the NimbleGen SeqCap EZ Human Exome Library kit (Roche NimbleGen) or the Agilent Sure Select Human All Exon V6 kit (Agilent Technologies) for exome capture. Library preparation, sequencing, and data analysis were performed as previously described.2 Because of parental consanguinity, autosomal-recessive inheritance was considered likely, so for further study, we prioritized homozygous exonic and/or splicing variants whose reference population frequencies were <1% in gnomAD and ABraOM or <5% in in-house controls.
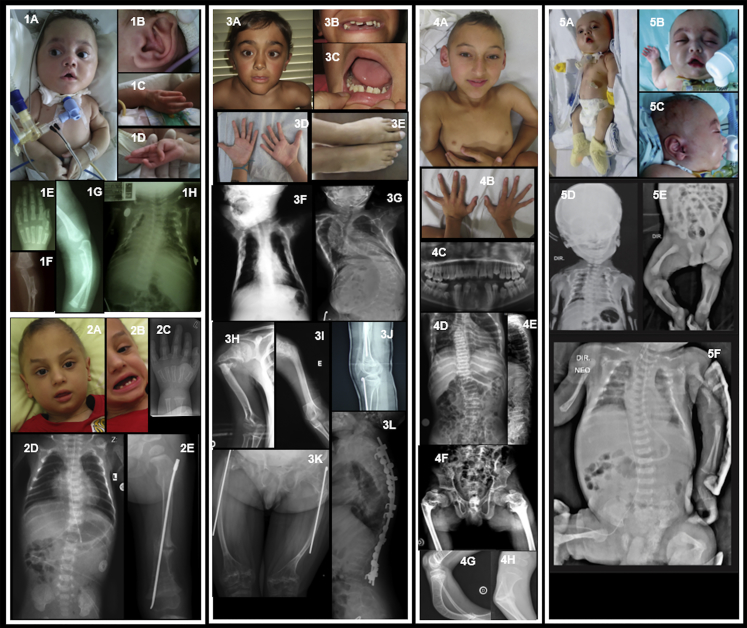
Figure 1.
Clinical and Radiological Features of Individuals with MESD Mutations
(1) Individual 1 from Brazil (Family 1 IV-5)
(1A–1B) Facial dysmorphic features include bluish sclerae, mid-face hypoplasia, arched eyebrows, tented upper lip; also note tracheostomy, narrow chest, and rhizomelia; posteriorly rotated ears.
(1C–1D) Long fingers with adducted thumbs and contractures of the fifth digits.
(1E) Radiograph of left hand showing long, tapering fingers.
(1F–1G) Fracture of left ulna and radius at 5 months, healing with callus formation.
(1H) Radiograph of chest at 5 months showing bell-shaped, narrow chest with multiple (healing) rib fractures.
(2) Individual 2 from Turkey (Family 2 IV-1)
(2A–2B) Facial dysmorphic features include tall forehead, arched eyebrows with sparse lateral thirds, micrognathia, posteriorly rotated ears, widely spaced teeth, and oligodontia.
(2C) Radiograph of left hand at 8 months showing long, tapering fingers.
(2D) Chest radiograph at 5 months showing bell-shaped thorax, thin ribs with healing posterior rib fractures.
(2E) Fracture of the left femur at age 4 years, post-surgical rodding.
(3) Individual 3 from Portugal (Family 3 V-2)
(3A–3C) Facial dysmorphic features include triangular facies, arched eyebrows, left convergent strabismus, midface hypoplasia, thin upper lip, pointed chin, widely spaced teeth, and oligodontia.
(3D) Hands showing long fingers with mild contractures.
(3E) Feet showing 2-3 partial cutaneous syndactyly with overlapping toes.
(3F–3G) Chest radiographs at 6 months and 12 years, respectively, showing multiple rib fractures and progressive scoliosis with vertebral compression fractures.
(3H–3I) Upper limb radiographs showing right and left humeral fractures, respectively.
(3J) Right knee at age 8 years, showing signs of cyclical pamidronate therapy, rodding of the tibia, and very gracile fibula.
(3K) Aged 16 years, thin femurs with bilateral rodding of the femoral shafts post-fracture.
(3L) Lateral spinal radiographs at 12 years, showing osteopenia, progressive vertebral compression fractures, and spinal fixation.
(4) Individual 4 from Brazil (Family 4 II-2)
(4A) He has minimal facial dysmorphic features, slightly upslanting palpebral fissures, and a pointed chin; sclerae are white; skeletal deformity with rhizomelic shortening is noted.
(4B) Impression of long fingers with mild interphalangeal contractures.
(4C) Panorex radiograph showing disorganized dentition, abnormal premolars, and missing lower jaw teeth (numbers 31, 32, and 41).
(4D–4E) Radiographs showing osteopenia, narrow chest, thin ribs with fractures, scoliosis, and vertebral compression fractures.
(4F–4H) Radiographs showing skeletal deformity and evidence of fractures of the femoral necks and tibiae.
(5) Individual 5 from Brazil (Family 5 IV-1)
(5A–5C) Facial dysmorphic features include round face, prominent eyes with long lashes and epicanthic folds, small nose with bulbous tip, tented upper lip, high and narrow palate, retrognathia, and posteriorly rotated ears; also note narrow thorax with inverted nipples, rhizomelic shortening of limbs, and long fingers with contractures.
(5D–5F) Radiographs showing generalized osteopenia, multiple fractures (clavicles, ribs, left humerus, femur), narrow thorax, and bowing of femurs bilaterally.
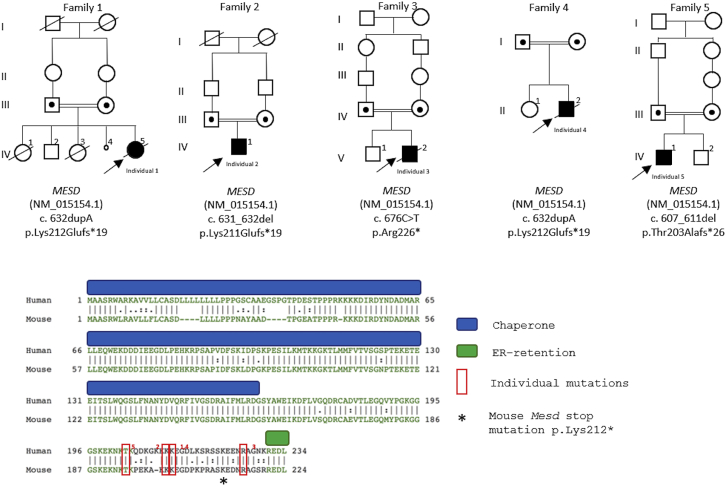
None of the affected individuals had likely pathogenic variants in any of the 19 genes which, when mutated, are known to cause OI. Instead, the affected individuals were each homozygous for a mutation in the third and final exon of MESD (GenBank: NM_015154.1), which is located downstream of the chaperone domain and upstream of the ER-retention domain (Table 1; Figure 2). These MESD variants were heterozygous in each parent and were not common polymorphisms in the Exome Variant Server, 1000 genomes, dbSNP, ExAC, or gnomAD databases. We found four different MESD mutations in the five affected individuals (c.607_611del [p.Thr203Alafs∗26], c.631_632del [p.Lys211Glufs∗19], c.632dupA [p.Lys212Glufs∗19] found in two persons, and c.676C>T [p.Arg226∗]). Notably, there are no high-quality loss-of-function variants annotated for MESD in the gnomAD database, while 7.9 such variants were calculated to be expected according to constraint metrics, indicating a high degree of loss-of-function intolerance of the gene (pLI = 0.91, observed/expected ratio = 0).
Table 1.
Clinical Features of Individuals with Homozygous Mutations in MESD
| Findings | Individual 1 | Individual 2 | Individual 3 | Individual 4 | Individual 5 |
|---|---|---|---|---|---|
| mutation in MESD (homozygous) | c.632dupA p.Lys212Glufs∗19 |
c.631_632del p.Lys211Glufs∗19 |
c.676C>T p.Arg226∗ |
c.632dupA p.Lys212Glufs∗19 |
c.607_611del p.Thr203Alafs∗26 |
| country of origin | Brazil | Turkey | Portugal | Brazil | Brazil |
| gender | female | male | male | male | male |
| consanguinity | yes | yes | yes | yes | yes |
| age at first visit (years) | after birth | 3 years, 4 months | 4 years, 6 months | 10 years | 6 months |
| age at last visit (years) | 1 year, 5 months (death) | 8 years, 4 months | 11 years, 1 months | 12 years, 2 months | 7 months (death) |
| age at start of bisphosphonate treatment | 15 days | 3 years, 5 months | 3 years, 6 months | 5 years | not treated |
| age at end of bisphosphonate treatment | NA | NA | approx. 11 years | regular use since 2015 not treated from 2012–2014 |
not treated |
| birth weight and birth length | W = 2,135 g (−2.35 SD), L = 45 cm (−1.93 SD) (39 WG) |
W = 1,550 g (−2.8 SD) L = 43 cm (−2.58 SD) (34 WG) |
W = 3,730 g (+0.38 SD) L = 51 cm (+0.38 SD) (37 WG) |
W = 2,730 g (−1.35 SD) L = 52 cm (+0.76 SD) (term) |
W = 2,465 g (−1.73 SD) L = 42 cm (−2.94 SD) (37 WG) |
| confirmed prenatal fractures | yes | no | no | no | yes |
| age at first fracture (months) | prenatal | 11 months | 24 months | 24 months | prenatal |
| color of sclera | bluish | white | white | white | bluish |
| eye abnormalities | NFA | no | no | no | no |
| dentinogenesis imperfecta | no | no | no | no | NA |
| disorganized dentition/clinical oligodontia | NA | yes | yes | yes | NA |
| radiographic evidence of oligodontia | NA | NA | NA | yes | NA |
| hypermobility of joints | no | no | NA | no | no |
| cardiac impairments | patent foramen ovale with a small left to right shunt | NA | NA | NA | no |
| hearing impairment | no | slight conductive hearing loss due to adenoid hyperplasia | no | NA | NA |
| old fractures of extremities | yes | yes (five fractures) | yes (right clavicle 2 years left femur 3 years 6 months right femur 4 years) |
yes | yes (fractures of both humeri, ribs, femur, and clavicle) |
| vertebral/thoracic cage fractures | no | yes (multiple fractures of vertebrae, ribs, and both scapulae) | vertebral compression fractures | yes (vertebrae and ribs) | difficult to assess (multiple fractures of ribs). |
| retarded gross motor function | yes | yes | yes | yes | yes |
L = length; NA = not available; NFA = not formally assessed; SD = standard deviation; WG = weeks of gestation; W = weight
Figure 2.
Pedigrees of the Five MESD-Associated OI Individuals, Their MESD Mutations, and the Location of these Mutations in the MESD Protein
Upper panel: Family pedigrees and mutations in MESD for each affected individual.
Lower panel: Alignment of human MESD with mouse MESD, showing important chaperone activity and ER-retention domains; the first amino acid residues affected by the four different affected individual mutations are noted with red rectangles and the amino acid residue converted to a termination codon in the mouse Mesd expression construct is asterisked.
Salient clinical features in the individuals with MESD mutations are summarized in Table 1, and comprehensive clinical information is provided in the case reports in the Supplemental Data. Two individuals died from respiratory insufficiency before 18 months of age. The oldest affected individuals, when assessed at the ages of 10 years and 16 years, respectively, were non-ambulatory. Bluish sclerae were noted in the youngest individuals, but not in the older ones. None had dentinogenesis imperfecta, but the three older individuals had disorganized dentition and/or oligodontia (Figure 1). Global developmental delay or evidence of intellectual disability (ID) was present in three individuals (Table 1).
MESD mutations have not previously been associated with a human disease phenotype. In mice, homozygous loss-of-function Mesd mutations cause embryonic lethality during gastrulation.3 Mouse embryos homozygous for deletions spanning Mesd can already be distinguished from WT embryos at embryonic day E7.5, when they show lack of mesoderm and failure to form a primitive streak.3, 4, 5 These embryos do not survive beyond E11.5.3 Functional studies of MESD and its Drosophila ortholog, Boca, indicate that these proteins are endoplasmic reticulum (ER) chaperones for canonical Wingless-related integration site (WNT) signaling receptors (LRP5 and LRP6 in mammals and Arrow in Drosophila).5, 6 In the absence of MESD, LRP5 and LRP6 cannot traffic to the cell surface.5, 6, 7, 8, 9
The likelihood that MESD mutations cause OI in these five individuals is supported by prior research demonstrating the importance of canonical WNT signaling in skeletal development.2, 10, 11, 12, 13, 14, 15, 16, 17, 18 Specifically, LRP5 loss-of-function mutations are seen in the autosomal-recessive skeletal fragility disorder Osteoporosis-Pseudoglioma (OPPG) syndrome and in autosomal dominant juvenile osteoporosis.10, 11 Additionally, WNT1 loss-of-function mutations have been found in autosomal-recessive OI and in dominant early onset osteoporosis.2, 12, 13 Other data in support of the importance of canonical WNT signaling in skeletal development derive from human GWAS studies and from studies in mice with germline and conditional knockout alleles of canonical WNT signaling pathway components. For example, loci identified by GWAS for bone mineral density and fracture risk include WNT pathway members.14, 15 In mice, bone-forming osteoblasts fail to differentiate when canonical WNT signaling is ablated.16, 17, 18
Because embryonic lethality occurs in mice completely lacking MESD3, 4, 5 or both LRP5 and LRP6,19 we hypothesized that the MESD mutations responsible for human OI represent hypomorphic rather than amorphic alleles. Several lines of evidence support this speculation. First, OI-associated MESD mutations occur in the last exon, rather than throughout the gene, thereby making them likely to escape nonsense-mediated mRNA decay. Second, all mutations truncate or frameshift the protein after the 202nd amino acid residue, which is carboxy-terminal to the protein’s chaperone activity domain.20 Third, the OI-associated mutations all remove a highly conserved ER-retention domain;5 the inability to retain or recycle MESD in the ER could reduce the protein’s efficiency in serving as a chaperone.
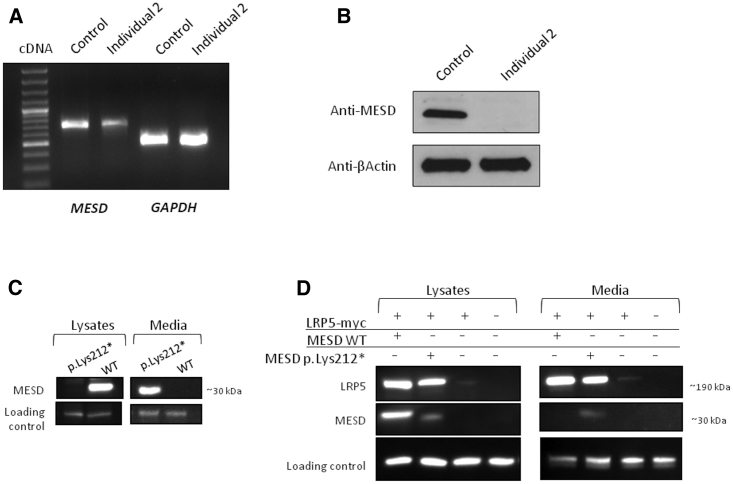
To test this hypothesis, we studied fibroblasts from Individual 2 (Family 2 IV-1) and control fibroblasts, and we expressed wild-type (WT) and mutant MESD in HEK293T cells. We were able to RT-PCR amplify spliced MESD mRNA transcripts from WT-derived and Individual 2-derived fibroblast cultures. Thus, at least one MESD mutation does not cause nonsense-mediated mRNA decay (Figure 3A). Since the other mutations reported here are located in the same (and final) exon of MESD, likely the same will hold true for them. Also, an antibody whose epitope is near the amino-terminal domain of MESD and should be able to recognize WT and OI-associated mutant MESD only detected MESD in cell lysate from WT fibroblasts (Figure 3B); this is consistent with mutant MESD failing to remain intracellular when its ER-retention domain is lost. Because endogenous MESD levels can be rate limiting when LRP5 or LRP6 is overexpressed,5 we performed transient transfection assays to determine whether mutant MESD retains an ability to chaperone LRP5. We used a previously described FLAG-epitope-tagged mouse Mesd expression construct that can chaperone human LRP5 in HEK293T cells.21 We performed site-directed mutagenesis to modify this Mesd construct to create a truncation mutation (Quikchange Lightning Site-Directed Mutagenesis Kit, Agilent Technologies). The alteration p.Lys212∗ retains the chaperone activity domain and deletes the ER-retention domain, as do the OI-associated mutations (Figure 2 lower panel). Because lack of the ER-retention domain could allow mutant MESD to traffic outside the cell, we then examined the cell lysates and conditioned medium of transiently transfected HEK293T cells. In agreement with what we previously observed in control and Individual 2-derived fibroblasts, WT but not mutant MESD was detected in the cell lysate. In conditioned medium, only mutant and not WT MESD was detected (Figure 3C). These findings are consistent with the mutant MESD being synthesized but not retained within the ER.
Figure 3.
Individual 2-Derived and Transiently Transfected Cell Cultures Showing That OI-Associated MESD Protein Is Expressed and Capable of Serving as a Chaperone for LRP5, but Unable to Be Retained within Endoplasmic Reticulum of the Cell
(A) Agarose gel electrophoretic separation and fluorescence staining of RT-PCR amplimers spanning from exon 1 to exon 3 obtained from wild type (WT, control) and MESD-associated OI (Individual 2) cultured fibroblasts. Spliced MESD amplimers are detected in both samples; spliced GAPDH amplimers serve as a positive control.
(B) Immunodetection of MESD following SDS-PAGE in cell lysates from control and Individual 2 fibroblasts. A polyclonal antibody that detects an epitope near the amino-terminal domain of MESD should recognize both WT and mutant MESD. However, only WT MESD is present in the cell lysate.
(C) Representative immuno-blot following SDS-PAGE of FLAG epitope-tagged WT or mutant (p.Lys212∗) MESD in cell lysate and conditioned medium from transiently transfected HEK293T cells. WT MESD is only detected in the cell lysate, whereas mutant MESD is only detected in the conditioned medium.
(D) Representative immuno-blots of the same membrane following SDS-PAGE using anti-myc antibody (for LRP5), anti-FLAG antibody (for MESD), anti-beta actin antibody (as a loading control for the cell lysate), and a non-specific cross-reacting band (as a loading control for the conditioned medium) of cell lysate and conditioned medium from HEK293T cells that were transiently transfected with expression vectors for myc-epitope tagged LRP5 (LRP5-myc), FLAG-epitope tagged WT MESD (MESD WT), or FLAG-epitope tagged mutant MESD (MESD p.Lys212∗). Note co-expression of LRP5-myc with either WT or mutant MESD increases the amount of immuno-detectable LRP5 in cell lysate and in conditioned medium, compared to when LRP5-myc is expressed alone. Also note that mutant MESD becomes detectable at low levels in cell lysate from HEK293T cells when transiently co-expressed with LRP5-myc. Mutant MESD is 13 amino acid residues shorter than WT MESD, which is why it migrates faster during SDS-PAGE.
We next determined whether the mutant protein could still chaperone LRP5. We co-expressed WT, or mutant, MESD with a Myc-epitope-tagged human LRP5 that lacks a transmembrane domain.21 Both WT and mutant MESD increased the abundance of epitope-tagged LRP5 in the conditioned medium (Figure 3D). Taken together, these data indicate that mutant MESD retains the ability to chaperone and traffic LRP5, though it may do so less efficiently than WT MESD because it fails to be retained in the ER.
The oligodontia, observed in Individual 4 for whom we have panorex films, may distinguish MESD-associated OI from other causes of OI. Reduced LRP6 signaling is the likely cause of oligodontia in this individual because oligodontia also occurs in persons who are heterozygous for LRP6 loss-of-function mutations.22 It is interesting that individuals with OI-associated MESD mutations do not have the eye defects seen in individuals who have the autosomal-recessive OPPG syndrome and who lack LRP5. Given that individuals with MESD-associated OI do not phenocopy humans or mice that are completely deficient for either LRP5 or LRP6, it seems likely that the skeletal fragility phenotype in those with MESD mutations is due to partial reductions in LRP5 and LRP6 signaling. This mechanism would be consistent with data from mice, in which partial reductions in LRP5 and LRP6 signaling affect bone mass without affecting other organ systems.23 At present, we do not know the relationship between the MESD mutation and the ID we observed in several affected persons. These individuals had no variants in known ID genes. Interestingly, ID is also a variable clinical feature of WNT1-associated recessive OI.24
In the individuals described here, we attempted to increase bone strength and reduce deformity by treating with bisphosphonates, which have been useful in other types of OI. Unfortunately, we did not observe beneficial effects in the four individuals treated (see Case Reports in Supplemental Data). Bisphosphonates function primarily to reduce bone catabolism, rather than to promote bone anabolism. Since the skeletal fragility in these individuals is a likely consequence of low bone formation due to reduced WNT signaling, therapies that can enhance WNT signaling may have therapeutic efficacy. In this regard, it should be noted that Evenity (romozosumab-aqqg; Amgen) has recently gained FDA approval for increasing bone formation and bone mass in individuals with age-related osteoporosis. This therapeutic monoclonal antibody works by neutralizing sclerostin, an endogenous LRP5 and LRP6 inhibitor. Sclerostin neutralization in the LRP5 knockout mouse model of the OPPG syndrome produced significant increases in bone mass and bone strength.25, 26 This same response could occur in individuals with MESD-associated OI.
In conclusion, we identified MESD as an additional locus for autosomal-recessive OI. The likely mechanism of effect for the four identified MESD truncating and frameshift mutations is the lack of ER retention, which reduces but does not eliminate the protein’s role as a chaperone for the WNT receptors LRP5 and LRP6. However, since MESD when bound to LRP5 can block WNT signaling,27 it is possible that the abnormal trafficking of mutant MESD outside of the cell causes a neomorphic effect. All five of the individuals with MESD-associated OI have a progressive deforming phenotype. Oligodontia may be a feature that separates this type of OI from the other known causes; however, too few individuals have been studied to reliably draw this conclusion. MESD joins a list of other WNT pathway components in which mutations can negatively affect bone mass accrual and bone strength. Therapies that enhance canonical WNT signaling in bone may significantly benefit these individuals.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We thank the families for their enthusiastic participation, and we are grateful to the physicians who were involved in the clinical care of these families. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2067/1-390729940 (B.W.) and is supported by following grants: São Paulo Research Foundation (FAPESP) grant 2015/22145-6 (C.A.M.); Swiss National Science Foundation grant 31003A-173183 (C.G., M.R.); FAPESP and Centro de Pesquisa, Inovação e Difusão (CEPID) grant 2013/08028-1 and Brazilian National Council for Scientific and Technological Development (CNPq) grant 304130/2016-8 (D.R.B.); FAPESP grant 2015/22145-6 (D.P.C.); CNPq grant 236670/2012-3 (E.R.V.); NIAMS grants 5R01-AR053237-12 and R01-AR064231 (M.L.W.); and DFG grant FOR 2722 (O.S.).
Published: September 26, 2019
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2019.08.008.
Web Resources
1000 Genomes Project, https://www.genome.gov
Arquivo Brasileiro Online de Mutações (ABraOM), abraom.ib.usp.br
Exome Aggregation Consortium (ExAC), exac.broadinstitute.org
Exome Variant Server, https://evs.gs.washington.edu
Genome Aggregation Database (gnomAD), https://gnomad.broadinstitute.org
OMIM, https://omim.org/
Single Nucleotide Polymorphism Database (dbSNP), https://www.ncbi.nlm.nih.gov
Supplemental Data
References
- 1.Marini J.C., Forlino A., Bächinger H.P., Bishop N.J., Byers P.H., Paepe A., Fassier F., Fratzl-Zelman N., Kozloff K.M., Krakow D. Osteogenesis imperfecta. Nat. Rev. Dis. Primers. 2017;3:17052. doi: 10.1038/nrdp.2017.52. [DOI] [PubMed] [Google Scholar]
- 2.Keupp K., Beleggia F., Kayserili H., Barnes A.M., Steiner M., Semler O., Fischer B., Yigit G., Janda C.Y., Becker J. Mutations in WNT1 cause different forms of bone fragility. Am. J. Hum. Genet. 2013;92:565–574. doi: 10.1016/j.ajhg.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holdener B.C., Faust C., Rosenthal N.S., Magnuson T. msd is required for mesoderm induction in mice. Development. 1994;120:1335–1346. doi: 10.1242/dev.120.5.1335. [DOI] [PubMed] [Google Scholar]
- 4.Wines M.E., Shi Y., Lindor M., Holdener B.C. Physical localization of the mesoderm development (mesd) functional region. Genomics. 2000;68:322–329. doi: 10.1006/geno.2000.6264. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh J.C., Lee L., Zhang L., Wefer S., Brown K., DeRossi C., Wines M.E., Rosenquist T., Holdener B.C. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell. 2003;112:355–367. doi: 10.1016/s0092-8674(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 6.Culi J., Mann R.S. Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell. 2003;112:343–354. doi: 10.1016/s0092-8674(02)01279-5. [DOI] [PubMed] [Google Scholar]
- 7.Culi J., Springer T.A., Mann R.S. Boca-dependent maturation of beta-propeller/EGF modules in low-density lipoprotein receptor proteins. EMBO J. 2004;23:1372–1380. doi: 10.1038/sj.emboj.7600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Chen J., Lu W., McCormick L.M., Wang J., Bu G. Mesd binds to mature LDL-receptor-related protein-6 and antagonizes ligand binding. J. Cell Sci. 2005;118:5305–5314. doi: 10.1242/jcs.02651. [DOI] [PubMed] [Google Scholar]
- 9.Koduri V., Blacklow S.C. Requirement for natively unstructured regions of mesoderm development candidate 2 in promoting low-density lipoprotein receptor-related protein 6 maturation. Biochemistry. 2007;46:6570–6577. doi: 10.1021/bi700049g. [DOI] [PubMed] [Google Scholar]
- 10.Gong Y., Slee R.B., Fukai N., Rawadi G., Roman-Roman S., Reginato A.M., Wang H., Cundy T., Glorieux F.H., Lev D., Osteoporosis-Pseudoglioma Syndrome Collaborative Group LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 11.Hartikka H., Mäkitie O., Männikkö M., Doria A.S., Daneman A., Cole W.G., Ala-Kokko L., Sochett E.B. Heterozygous mutations in the LDL receptor-related protein 5 (LRP5) gene are associated with primary osteoporosis in children. J. Bone Miner. Res. 2005;20:783–789. doi: 10.1359/JBMR.050101. [DOI] [PubMed] [Google Scholar]
- 12.Fahiminiya S., Majewski J., Mort J., Moffatt P., Glorieux F.H., Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J. Med. Genet. 2013;50:345–348. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- 13.Laine C.M., Joeng K.S., Campeau P.M., Kiviranta R., Tarkkonen K., Grover M., Lu J.T., Pekkinen M., Wessman M., Heino T.J. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N. Engl. J. Med. 2013;368:1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrada K., Styrkarsdottir U., Evangelou E., Hsu Y.H., Duncan E.L., Ntzani E.E., Oei L., Albagha O.M., Amin N., Kemp J.P. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012;44:491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trajanoska K., Rivadeneira F. The genetic architecture of osteoporosis and fracture risk. Bone. 2019;126:2–10. doi: 10.1016/j.bone.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Day T.F., Guo X., Garrett-Beal L., Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Glass D.A., 2nd, Bialek P., Ahn J.D., Starbuck M., Patel M.S., Clevers H., Taketo M.M., Long F., McMahon A.P., Lang R.A., Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Hill T.P., Später D., Taketo M.M., Birchmeier W., Hartmann C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev. Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Kelly O.G., Pinson K.I., Skarnes W.C. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Liu C.C., Li Q., Nowak C., Bu G., Wang J. Two structural and functional domains of MESD required for proper folding and trafficking of LRP5/6. Structure. 2011;19:313–323. doi: 10.1016/j.str.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ai M., Holmen S.L., Van Hul W., Williams B.O., Warman M.L. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol. Cell. Biol. 2005;25:4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massink M.P., Créton M.A., Spanevello F., Fennis W.M., Cune M.S., Savelberg S.M., Nijman I.J., Maurice M.M., van den Boogaard M.J., van Haaften G. Loss-of-function mutations in the WNT co-receptor LRP6 cause autosomal-dominant oligodontia. Am. J. Hum. Genet. 2015;97:621–626. doi: 10.1016/j.ajhg.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmen S.L., Giambernardi T.A., Zylstra C.R., Buckner-Berghuis B.D., Resau J.H., Hess J.F., Glatt V., Bouxsein M.L., Ai M., Warman M.L., Williams B.O. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J. Bone Miner. Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- 24.Aldinger K.A., Mendelsohn N.J., Chung B.H., Zhang W., Cohn D.H., Fernandez B., Alkuraya F.S., Dobyns W.B., Curry C.J. Variable brain phenotype primarily affects the brainstem and cerebellum in patients with osteogenesis imperfecta caused by recessive WNT1 mutations. J. Med. Genet. 2016;53:427–430. doi: 10.1136/jmedgenet-2015-103476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang M.-K., Kramer I., Keller H., Gooi J.H., Collett C., Jenkins D., Ettenberg S.A., Cong F., Halleux C., Kneissel M. Reversing LRP5-dependent osteoporosis and SOST deficiency-induced sclerosing bone disorders by altering WNT signaling activity. J. Bone Miner. Res. 2014;29:29–42. doi: 10.1002/jbmr.2059. [DOI] [PubMed] [Google Scholar]
- 26.Kedlaya R., Veera S., Horan D.J., Moss R.E., Ayturk U.M., Jacobsen C.M., Bowen M.E., Paszty C., Warman M.L., Robling A.G. Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome. Sci. Transl. Med. 2013;5:211ra158. doi: 10.1126/scitranslmed.3006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin C., Lu W., Zhai L., Bethea T., Berry K., Qu Z., Waud W.R., Li Y. Mesd is a general inhibitor of different Wnt ligands in Wnt/LRP signaling and inhibits PC-3 tumor growth in vivo. FEBS Lett. 2011;585:3120–3125. doi: 10.1016/j.febslet.2011.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.