Abstract
Bile acids are produced in the liver and excreted into the intestine, where their main function is to participate in lipid digestion. Ursodeoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA) have shown antiapoptotic, anti-inflammatory, and antioxidant effects in various models of neurodegenerative diseases. However, little is known about signaling pathways and molecular mechanisms through which these bile acids act as neuroprotectors, delaying translation to the clinical setting. We review evidence supporting a potentially therapeutic role for bile acids in retinal disorders, and the mechanisms and pathways involved in the cytoprotective effects of bile acids from the liver and the enterohepatic circulation to the central nervous system and the retina. As secondary bile acids are generated by the microbiota metabolism, bile acids might be a link between neurodegenerative retinal diseases and microbiota.
Introduction
Bile acids are produced in the liver and excreted into the intestine, where their main function is to participate in the emulsification, absorption, and digestion of lipids. They have a secondary role as a steroid hormone modulating various metabolic process, such as hepatic glucose metabolism and liver cell survival [1].
Traditional Asian medicine recommended the use of vertebrate and invertebrate bile for patients with visual disorders [2]. For more than 10 years, numerous studies have confirmed that the hydrophilic bile acids, ursodeoxycholic acid (UDCA) and tauroursodeoxycholic acid (TUDCA), are protective in diseases affecting the central nervous system and the retina [3]. However, there is no clinical indication for the use of bile acids in neurodegenerative diseases. Although antiapoptotic, anti-inflammatory, and antioxidant effects have been shown for these molecules, little is known about primary signaling pathways and molecular mechanisms through which bile acids act as neuroprotectants, delaying translation to the clinical setting. We review evidence supporting a potentially therapeutic role for bile acids in retinal disorders, and the mechanisms and pathways involved in the cytoprotective effects of bile acids from the liver and the enterohepatic circulation to the central nervous system and the retina.
The bile acids: Chemical structure and physiology
Bile acids are the major constituents of human bile [1]. They have a 24-carbon structure containing 5β-steroids, and their main role is the emulsification of lipids, a fundamental step for lipid absorption and digestion [4]. Primary bile acids, cholic acid (CA) and chenodeoxcholic acid (CDCA), are synthesized from cholesterol in the liver (Figure 1) via two main pathways, the classical and alternative pathways. The classical pathway is initiated by cholesterol 7α-hydroxylase (CYP7A1), which is regulated by the farnesoid X receptor (FXR). The alternative pathway can be initiated by different enzymes that are also expressed outside the liver [1].
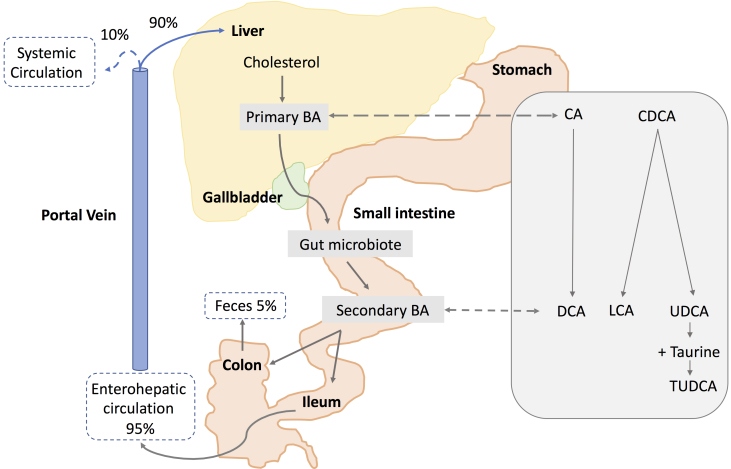
Figure 1.
Schematic representation of synthesis and circulation of bile acids. Primary bile acids (BAs), cholic acid (CA), and chenodeoxycholic acid (CDCA) are synthesized in the liver from cholesterol and stored in the gallbladder. Following food intake, bile acids are released into the small intestine. Secondary bile acids are produced by the gut microbiota from modifications of primary bile acids. Deoxycholic acid (DCA) is formed from CA. Lithocholic acid (LCA) and ursodeoxycholic acid (UDCA) are formed by CDCA. Taurine conjugation of UDCA forms tauroursodeoxycholic acid (TUDCA). About 95% of the bile acids are reabsorbed in the ileum, and 5% are lost in feces. The bile acids absorbed by the enterocytes are released into the portal vein and redirected to the liver for recycling (enterohepatic circulation). Only a small portion (10%) escapes the enterohepatic circulation and reaches the systemic circulation.
Bile acids are transported from the hepatocytes through the bile canaliculi and stored in the gallbladder. Following food intake, the presence of fats and proteins in the stomach results in the release of bile acids from the gallbladder into the duodenum. In the intestine, gut microbiota produces the secondary bile acids by modification of the primary bile acids, via 7α-dehydroxylation, deconjugation, and oxidation or epimerization of the hydroxyl groups at C-3, C-7, and C-12 (Figure 1). The secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA) are formed by dehydroxylation of CA and CDCA, respectively, performed by dehydratases of the anaerobic flora from the human colon. Epimerization of hydroxyl groups of CDCA by the hydroxysteroid dehydrogenases of intestinal bacteria leads to the formation of UDCA.
Bile acids are secreted as conjugated molecules with glycin or taurine, forming the bile salts, such as TUDCA, the taurine conjugate of UDCA. Bile acids are then redirected to the liver via the portal vein (enterohepatic circulation, Figure 1). Ninety-five percent of the unconjugated bile acids are reabsorbed into intestinal enterocytes by passive diffusion in the jejunum and colon, while conjugated bile acids are actively taken up in the ileum mainly via the apical sodium-dependent bile acid transporter (ASBT) [5]. The remaining 5% of the unconjugated bile acids are excreted via feces. Most bile acids absorbed by the enterocytes and released into the portal vein are redirected to the liver for recycling. The main bile acid transporters are summarized in Table 1. Less than 10% of bile acid reaches the systemic circulation [6]. Bile acids has been detected in plasma at a concentration range of nanograms per milliliter, as well as in the cerebrospinal fluid [7]. However, bile acid concentrations have not been measured thus far in ocular fluids.
Table 1. Receptors and transporters for bile acids.
| Membrane receptors | Bile acid affinity | Location |
|---|---|---|
| Takeda G-protein coupled receptor 5 (TGR5) |
LCA, DCA, CDCA, CA, TUDCA |
Liver, intestine, brain, eye (primary retinal ganglion cells) spleen, lung, monocytes |
| Sphingosine 1-phosphate receptor 2 (S1PR2) |
TCA, GCA, TDCA, GDCA, TUDCA |
liver, brain, eye (rat bipolar retinal cells, mouse retinal endothelial cells) heart, lung |
| α5β1 Ιντεγριν |
TUDCA |
Liver, brain, retina (human vessels, astrocytes) |
| Nuclear receptors |
|
|
| Farsenoid X receptor (FXR) |
LCA, DCA, CDCA, CA |
Liver, intestine, brain |
| Vitamin D receptor (VDR) |
LCA |
Intestine, liver, bone, kidneys, retina and cells (βcells, adipocytes, vascular smooth muscle cells, monocytes) |
| Pregnane X receptor (PXR) |
LCA |
Liver, intestine, brain, retina (RPE cells) |
| Glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) |
UDCA,TCA,GDCA, TUDCA |
Liver, brain, retina |
| Constitutive androstane receptor (CAR) |
CA |
Liver, brain, kidney, adrenals |
| Transporters |
|
|
| Apical sodium-dependent bile acid transporter (ASBT) |
Unconjugated and conjugated bile acids |
Intestine, cholangiocytes, brain |
| Sodium taurocholate cotransporting polypeptide (NTCP) |
Unconjugated and conjugated bile acids |
Liver, brain |
| Organic anion-transporting polypeptide (OATP) |
Unconjugated and conjugated bile acids |
Liver, Intestine, brain, retina |
| Multidrug resistant protein (MRP), 2, 3, 4 |
TCA, CA and conjugated bile acids |
Liver, Brain, retina |
| Bile salt export pump (BSEP) | Conjugated bile acids | Liver, brain |
LCA: lithocholic acid, DCA: deoxycholic acid, CDCA: chenodeoxcholic acid, CA: cholic acid, UDCA: ursodeoxycholic acid, TCA: taurocholic acid, GDCA: glycodeoxycholic acid, TUDCA: tauroursodeoxycholic acid, RPE: retinal pigment epithelium
Bile acid signaling occurs through nuclear receptors and cell membrane receptors [8] (Table 1), including the FXR, the vitamin D receptor (VDR), the pregnane X receptor (PXR), the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR), the constitutive androstane receptor (CAR), Takeda G protein-coupled receptor 5 (TGR5), the α5 β1 integrin, and the sphingosine-1-phosphate receptor 2 (s1PR2). The most studied bile acid receptors are FXR and TGR5. Both receptors are abundantly expressed in the enterohepatic circulation. Bile acids exert negative feedback regulation on their own synthesis mainly through the FXR [9]. Bile acids are also involved in the regulation of various metabolic processes.
Through activation of the FXR and TGR5, bile acids regulate not only their own synthesis and enterohepatic circulation but also triglyceride, cholesterol, glucose, and energy homeostasis [10]. The PXR functions as a xenobiotic sensor that could protect the liver from bile acid toxicity during cholestasis [8]. Activation of PXR increases expression of cytochrome P450s that hydroxylate bile acids to less toxic, more hydrophilic bile acids that are subsequently excreted into the bile [8]. VDR activation participates in bile acid synthesis, conjugation, transport, and metabolism. It also plays a role as an intestine bile acid sensor protecting the gut from bile acid toxicity [8]. α5β1 integrins are the main receptors implicated in the mechanism of action of UDCA during cholestasis. UDCA, which in vivo is converted to its taurine conjugate TUDCA, is a mainstay for the treatment of cholestatic liver disease. It has been shown that TUDCA can directly activate intrahepatocytic α5β1 integrins, which trigger signal transduction pathways (focal adhesion kinase (FAK), src, Erk1/2, and p38) toward choleresis [11]. Finally, activation of S1PR2 in hepatocytes regulates bile acid synthesis and increases glycogen synthesis [8].
Cytoprotective effect of UDCA and TUDCA in the liver
Hydrophobic bile acids within the hepatocyte induce cell death during cholestasis, while hydrophilic bile acids are cytoprotective. UDCA, a hydrophilic bile acid used for the treatment of cholesterol gallstone dissolution, is currently considered the first choice therapy for several forms of cholestatic syndromes [12]. The cytoprotective effects of this molecule result, in part, from its ability to reduce apoptosis [9]. The antiapoptotic effects of UDCA and TUDCA have been demonstrated in rat liver and human hepatocytes. UDCA negatively modulates the mitochondrial pathway by inhibiting Bax translocation, the formation of reactive oxygen species (ROS), cytochrome c release, and caspase-3 activation [9]. Moreover, TUDCA inhibits apoptosis associated with endoplasmic reticulum (ER) stress by modulating intracellular calcium levels and inhibiting calpain and caspase-12 activation [9]. Importantly, nuclear translocation of UDCA mediated by nuclear steroid receptors (NSRs) was shown to be essential for its antiapoptotic properties [13]. UDCA interacts with NSRs, the glucocorticoid receptor (GR), and the mineralocorticoid receptor (MR) to reach the nucleus. Once in the nucleus, UDCA modulates the E2F-1/p53/Bax pathway, thus preventing apoptosis [14].
Neuroprotective effects of UDCA and TUDCA in neurodegenerative disorders
Numerous studies have reported neuroprotective effects of UDCA and TUDCA in various models of neurodegenerative diseases, including, Alzheimer disease (AD) [15-19], Parkinson disease (PD) [20-22], and Huntington’s disease (HD) [23]. In APP/PS1 mice, a murine model of AD, TUDCA prevented amyloid precursor protein processing and amyloid-β deposition [18], and significantly attenuated Aβ deposition in the brain after the onset of amyloid pathology [15]. In the rotenone model of PD, UDCA exerted antiapoptotic and anti-inflammatory effects [20]. It improved mitochondrial dysfunction and reduced nuclear factor-κB (NF-κB) expression and tumor necrosis factor (TNF) alpha levels [20]. Furthermore, UDCA prevented proapoptotic alterations in Bax and Bcl-2, and reduced the activities of caspase 8, 9, and 3. Similarly, TUDCA was neuroprotective in a mouse model of PD through the modulation of JNK activity, which plays a central role in dopaminergic neuronal death, the production of ROS, and the activation of the Akt prosurvival pathway, involving Bad phosphorylation and NF-κB activation [21]. TUDCA also reduced mitochondrial dysfunction that is characteristic of PD [22]. In a transgenic mouse model of HD, TUDCA improved the locomotor and sensorimotor abilities, together with a reduction in striatal cell apoptosis and intracellular huntingtin inclusion [23].
There are only three clinical studies evaluating the safety and efficacy of bile acids in neurodegenerative disorders, all of them reported in patients with amyotrophic lateral sclerosis (ALS) [24-26]; see Table 2. In a cohort of 18 patients with ALS, oral UDCA showed excellent tolerability and safety, and was accumulated in the cerebrospinal fluid in a dose-dependent manner [24]. In a crossover study, oral UDCA showed a beneficial effect on the rate of functional decline in patients with ALS [25]. More recently, in a double-blind placebo controlled study, oral TUDCA treatment slowed down the progression of ALS disease [26]. Conversely, endogenous bile acid levels appear to be suppressed in patients with neurodegenerative diseases. In patients with AD, plasma concentrations of cholic acid were lower compared with age-matched control subjects. Similarly, the taurocholic acid (TCA) level was significantly lower in the brain of patients with AD pathology [19].
Table 2. Clinical studies evaluating bile acids in neurodegenerations.
| Biliary acid | Study design | Number of patients | Disease | Dose | Route | Main results |
|---|---|---|---|---|---|---|
| UDCA |
Randomized, non-controlled |
18 |
Amyotrophic lateral sclerosis |
15, 30 or 50 mg/kg/day during 4 weeks |
Orally |
-Excellent safety and tolerability
-Cerebrospinal fluid penetration in a dose-dependent manner |
| UDCA |
Double-blind, placebo-controlled, randomized, crossover, single center, phase III trial |
63 (16 analyzed) |
Amyotrophic lateral sclerosis |
3.5 g/140 ml/day for 3 months |
Orally (solubilized) |
The rate of progression (assessed by the Appel ALS rating scale) was significantly
lower in patients treated with UDCA compared to placebo |
| TUDCA | Double-blind placebo- controlled, randomized, multi-center, phase II trial | 34 (29 analyzed) | Amyotrophic lateral sclerosis | 1 g day for 54 weeks | Orally | Deterioration of function (assed by the ALS Functional Rating Scale Revised) was significantly slower in TUDCA group compared to placebo |
ALS: Amyotrophic lateral sclerosis, UDCA: Ursodeoxycholic acid, TUDCA: Tauroursodeoxycholic acid
Although main receptors and bile acid transporters (Table 1) have been found in the brain [6,27], little is known about the primary signaling pathways mediating their neuroprotective effects [28]. Bile acids could act directly in the brain through binding to the central FXR and TGR5, or indirectly by intermediate agents released after interaction of bile acids with receptors in the gut, such as fibroblast growth factor 19 and glucagon-like peptide 1, both capable of signaling to the central nervous system [6].
In the brain, it was shown that TUDCA binding to TGR5 microglia induced anti-inflammatory mediators by increasing intracellular cAMP levels [29]. Additionally, through MR binding in primary neurons, TUDCA counteracted amyloid beta-peptide-induced neuronal apoptosis [30]. Moreover, it has been reported that bile acids can modulate neurotransmitter activity [28]. For instance, UDCA was found to inhibit GABAergic currents and to serve as an antagonist for gamma-aminobutyric acid type A (GABAA) receptors expressed in human embryonic kidney (HEK)293 cells [31].
Neuroprotective effects of UDCA and TUDCA in retinal disorders
UDCA and TUDCA have shown neuroprotective effects in several models of retinal disease: photoreceptor degeneration, retinal ganglion cell (RGC) degeneration, diabetic retinopathy, and laser-induced choroidal neovascularization, at variable doses and routes of administration (Table 3). The known mechanisms of action of biliary acids in retinal disease models at the level of the RPE, photoreceptors, RGCs, and the blood–retinal barrier (BRB) are summarized in Figure 2. Antiapoptotic effects of biliary acids in retinal disease models have been described by suppression of caspase-dependent and independent pathways (apoptosis-inducing factor (AIF) release) or reduction of ER stress. Anti-inflammatory and antioxidant effects have also been reported, as well as preservation of the BRB.
Table 3. Urso- and Tauroursodeoxycholic acids in different models of retinal disease.
| Biliary acid | Study design | Model (Disease) | Dose/ Concentration | Route | References |
|---|---|---|---|---|---|
| TUDCA |
In vivo |
rd10 mouse (PR) and LIRD mouse |
500 mg/kg x2 or x4 |
Subcutaneous injection |
[2] |
| TUDCA |
In vivo |
rd10 mouse |
500 mg/ kg every 3 days (x8) |
Subcutaneous injection |
[37] |
| TUDCA |
In vivo |
rd1 mouse |
500 mg/ kg every 3 days (x5) |
Subcutaneous injection |
[86] |
| UDCA TUDCA |
In vivo |
CNV laser-treated rat |
TUDCA 500 mg/kg/day. UDCA 100 mg/kg/day
Before laser and for 14 days |
Intraperitoneal injection |
[60] |
| TUDCA |
In vivo |
P23H rat (PR) |
500 mg/kg once a week (x14) |
Intraperitoneal injection |
[39] |
| TUDCA |
In vivo |
Retinal detachment induced Brown Norway rats |
500 mg/kg daily X3 or X5 |
Intraperitoneal injection |
[43] |
| TUDCA |
In vivo |
rd10 mouse and LIRD mouse |
500 mg/kg x1 or every 3 day (x14) |
Intraperitoneal injection |
[36] |
| TUDCA |
In vivo |
BbsM390R/M390R mice
rd10 mouse
rd1 and rd16 mice |
500 mg/kg
twice a week, (x22)
every 3 days (x11)
daily (x24) |
Subcutaneous injection |
[38] |
| TUDCA |
In vivo |
Lrat–/– mouse (LCA) |
500 mg/kg every 3 days (x6) |
Subcutaneous injection |
[41] |
| TUDCA |
In vitro |
Retinal cell exposed to high glucose |
100 µM/day from D2-D7 |
- |
[55] |
| TUDCA |
In vitro |
STZ-induced (diabetic) rat retinas |
100 µM/day 7 days |
- |
[56] |
| TUDCA |
In vivo |
P23H rat |
500 mg/kg weekly (x14) |
Intraperitoneal injection |
[44] |
| TUDCA |
In vivo |
Lrat–/– mouse (LCA) |
500 mg/ kg every 3 days (x8) |
Subcutaneous injection |
[42] |
| TUDCA |
In vivo |
NMDA-induced damage in rat |
500 mg/kg/day (x 6) |
Intraperitoneal injection |
[47] |
| TUDCA |
In vitro |
Cat retinal ganglion cells |
0.5 μM, 25 ml, 5 min |
- |
[50] |
| TUDCA |
In vitro |
SD rats retinal explants cultured in advanced glycation end-products |
100 µM, 7 days |
- |
[87] |
| TUDCA |
In vitro |
ARPE-19, primary human RPE cells |
30–300 µM 1 h |
- |
[45] |
| TUDCA |
In vitro In vivo |
High glucose-induced HRMECs
STZ-induced (diabetic) rat |
5.0μM, 25.0μM and 125.0μM
250mg/kg/d and 500mg/kg/d |
|
[88] |
| TUDCA |
In vitro |
Leber hereditary optic neuropathy fibroblasts |
100 µM, 24 h |
|
[51] |
| TUDCA |
In vivo |
rd1 mouse |
500 mg/kg every 3 days (x15) |
Intraperitoneal injection |
[89] |
| UDCA |
In vivo |
STZ-induced (diabetic) mice |
100 mg/kg/d 6 weeks |
Intraperitoneal injection |
[57] |
| TUDCA |
In vivo |
P23H rat |
5.05±0.11 μg /mg microsphere |
Intravitreal injection |
[90] |
| UDCA |
In vivo |
STZ-induced (diabetic) mice |
15,30 mg/kg 1 months |
Oral |
[58] |
| TUDCA | In vivo | Optic nerve crush rat model | 100 mM TUDCA 14 days | Topically, 1 drop every 12 h | [49] |
TUDCA: Tauroursodeoxycholic acid, UDCA: ursodeoxycholic acid, LIRD: light-induced retinal degeneration; PR: Pigmentary Retinopathy, LCA: Leber congenital amaurosis
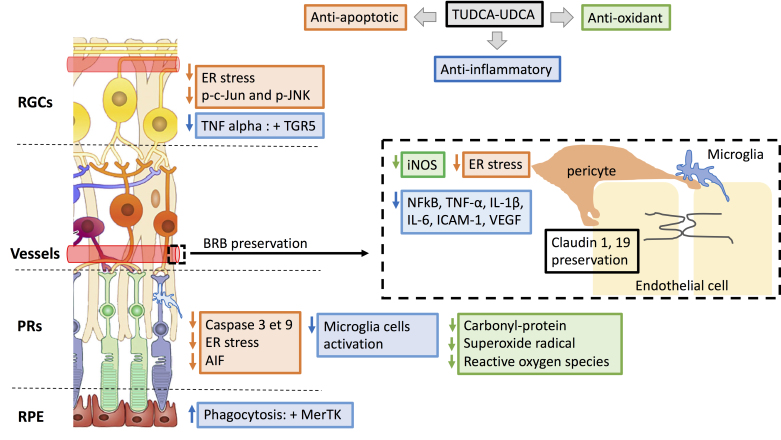
Figure 2.
Mechanisms involved in urso- and tauroursodeoxycholic acid neuroprotective effects in retinal disease. Antiapoptotic (orange), anti-inflammatory (blue), and antioxidant effects of biliary acids described in retinal disease models, at the level of retinal ganglion cells (RGCs), photoreceptors (PRs), RPE, and the blood–retinal barrier (BRB; vascular endothelial cells, pericytes, and microglia). ER: endoplasmic reticulum; AIF: apoptosis- inducing factor; NF-kB: nuclear factor-kappa B; TNF: tumor necrosis factor; IL: interleukin, ICAM: intercellular cell adhesion molecule; iNOS: inducible nitric oxide synthase; VEGF: vascular endothelial growth factor.
Photoreceptor degeneration
Photoreceptors are specialized neurons critical for vision. They are responsible for visual phototransduction, the first step in converting light energy into a neurosensory signal. Photoreceptor degeneration is present in several retinal diseases of different etiologies, including retinitis pigmentosa, Leber congenital amaurosis, and retinal detachment.
TUDCA suppressed caspase-dependent apoptosis mechanisms (terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and caspase 3 immunoreactivity; Figure 2) and preserved function and morphology of photoreceptors in several models of retinal degeneration in which apoptosis is the final common pathway of photoreceptor cell death [32-35], such as the light-induced retinal damage model [2,36], and three models of retinitis pigmentosa: the rd10 mouse [2,36-38], the P23H rat [39], and the Pde6b (rd1) mouse. When ER function is disrupted, unfolded proteins accumulate within the organelle, which is called ER stress. The unfolded protein response (UPR) may lead to apoptosis in the case of prolonged or severe ER stress [40]. In Lrat −/−, a murine model of Leber congenital amaurosis, S-opsin aggregation induces ER stress and subsequent cone degeneration [40]. In this model, TUDCA reduced not only caspase 3-mediated apoptosis but also ER stress markers (decrease in UPR CHOP and degradation of cone membrane associated proteins; Figure 2), thus preserving cone density [41,42]. In a mouse model of Bardet-Biedl syndrome type 1 (retinitis pigmentosa and obesity), TUDCA not only preserved retinal function and outer nuclear layer thickness but also prevented obesity [38].
In addition to apoptotic mechanisms, oxidative stress could play a role in photoreceptor cell death in various models of retinal degeneration, including light-induced retinal damage models and retinal detachment models [36,43]. TUDCA not only preserved photoreceptors from apoptosis but also reduced retinal oxidative stress markers, such as superoxide radical levels in a model of retinal light damage [36], and carbonyl-protein content in a rat model of retinal detachment [43] (Figure 2).
The beneficial effects of TUDCA also resulted from its anti-inflammatory activity as shown in P23H rats, in which the bile acid reduced the number and activation of microglia cells (Iba 1 and MHC-II, Figure 2) [44]. Interestingly, TUDCA enhanced phagocytosis of photoreceptors outer segments by RPE via the activation of Mer tyrosine kinase (MerTK) receptor activation (Figure 2), which has an important role in physiologic renewal of photoreceptor outer segments [45].
RGC degeneration
RGCs transmit visual information from the retina to the midbrain for processing and interpretation. Among diseases affecting RGCs, investigations have focused on glaucoma and Leber hereditary optic neuropathy.
Excessive stimulation of NMDA receptors could lead to RGC death by inducing a series of events, such as perturbation of Na+/K+ homeostasis, Ca2+ overload, mitochondrial dysfunction, and oxidative stress [46]. In a model of RGC excitotoxicity induced by the intravitreal administration of N-methyl-D-aspartate (NMDA) in rats, the systemic administration of TUDCA increased RGC survival and function [47].
In a rat optic nerve crush model, RGC death occurs by caspase dependent-apoptosis [48], and topical administration of TUDCA increased the density of RGCs compared to PBS-treated animals [49]. In cat wholemount retinas, TUDCA restored partially the retinal neurocircuitry deterioration and subsequent abnormal visual response of RGCs [50]. Critically, a metabolomic study performed on fibroblasts collected from patients with Leber hereditary optic neuropathy (LHON) revealed that elevation of markers of LHON-associated ER stress was reversed with TUDCA treatment (Figure 2) [51].
Diabetic retinopathy
All retinal cell types are affected by diabetic retinopathy (DR): endothelial cells and pericytes of retinal vessels, RPE cells, glial cells, and retinal neurons, including photoreceptors and RGCs. The exact cause of DR is unknown, but postulated mechanisms are hyperglycemia, advanced glycation end products (AGE), activation of cytokines, inflammation, and oxidative stress [52,53] that lead to microangiopathy, BRB breakdown, and cell death [54].
TUDCA decreased cell death in rat retinal neural cells exposed to elevated glucose concentration [55]. This was accompanied by decreased annexin V and TUNEL labeling, mitonuclear translocation of AIF, as well as by decreased oxidative stress (protein carbonyl and reactive oxygen species production; Figure 2) [55]. Additionally, Oshitari et al. [56] showed that the numbers of p-c-Jun- and p-JNK-immunopositive RGCs were higher, and the numbers of regenerating neurites were lower, in diabetic rat retinas and in retinas exposed to high glucose, which was partially improved with TUDCA (Figure 2). ER stress and inflammation occurring in diabetic retinopathy could also be suppressed by UDCA, thus preventing pericyte loss [57]. UPR markers and inflammatory cytokines, such as MPC-1 and TNF-α, were attenuated following UDCA treatment in streptozotocin (STZ)-induced diabetic mice, as well as in human retinal pericytes exposed to AGE or modified low-density lipoprotein (mLDL; Figure 2) [57]. Additionally, UDCA attenuates BRB breakdown during DR by reversing the reduced expression of claudin-1 and claudin 19 in STZ-treated diabetic mice (Figure 2) [58]. UDCA decreased retinal inflammation by reducing the nuclear translocation of p65 subunit of NF-κB in retinas from STZ-induced diabetic mice and the retinal expression of TNF-α, interleukin-1β (IL-1β), IL-6, intercellular cell adhesion molecule-1 (ICAM-1), inducible nitric oxide synthase (iNOS), and vascular endothelial growth factor (VEGF) in STZ-induced diabetic mice (Figure 2) [58].
More recently, it has been shown that intermittent fasting prevented diabetic retinopathy in db/db mice by restructuring the microbiota toward species producing TUDCA [59]. It was concluded that this confers retinal protection by TGR5 activation and subsequent suppression of TNF-α expression (Figure 2) [59].
Laser-induced choroidal neovascularization
Choroidal neovascularization (CNV) is a complication that leads to visual loss in several retinal diseases, including age-related macular degeneration, inflammatory retinal diseases, or myopia. Woo et al. [60] showed that TUDCA and UDCA, intraperitoneally administered, significantly suppressed laser-induced CNV formation in rats. This effect might be associated with anti-inflammatory action of the bile acids. However, the VEGF level in the retina was significantly lower only in the TUDCA-treated group compared to the control group, suggesting a different mechanism of action for UDCA.
UDCA and TUDCA signaling pathways in the retina
Receptors and transporters for bile acids have been identified in the retina (Table 1): TGR5 in primary retinal ganglion cells [59], S1PR2 in the inner nuclear layer of the rat retina, particularly in bipolar cells [61], and in mouse retinal endothelial cells [62], and α5β1 integrin in the vessels of the adult retina [63] and in astrocytes [64]. VDR is also expressed in the retina [65,66], and PXR has been found in RPE cells [67]. GR and MR have been described in the cells of the inner nuclear layer of the retina, particularly in Müller glial cells, and in amacrine cells [68,69] and in the RPE [67].
Among known transporters of bile acids, organic anion-transporting polypeptide (OATP) transporter has been described at the BRB [70,71], the neuroretina [72], and the RPE [73]. OATP1A2 is expressed in photoreceptor and amacrine cells, and OATP1B2 is found in the inner nuclear and plexiform layers [72]. Additionally, the multidrug resistance protein (MPR) 4 transporter has been detected in retinal vascular endothelial cells [74]. Conversely, other transporters of bile acid, such as ASBT, sodium taurocholate cotransporting polypeptide (NTCP), and bile salt export pump (BSEP), have not been described in the retina. The first described bile acid receptor, the FXR, has also not been reported in the retina, although a proteomic analysis of subretinal fluid revealed overexpression of the FXR pathway [75] in central serous chorioretinopathy compared with retinal detachment.
Although the machinery of bile acids has been partially found in the retina, specific interaction of UDCA and TUDCA with these receptors or transporters has been rarely explored. A recent report by Beli et al. suggested TGR5 activation by TUDCA in retinal ganglion cells. In addition, it has been shown that TUDCA could activate the MerTK receptor in RPE cells [45], and could directly interact with rhodopsin [76]. Interestingly, taurine, the constitutive amino acid of TUDCA molecule, is the most abundant amino acid in the retina [77]. Photoreceptors are particularly rich in taurine, and all retinal cells take up taurine from the extracellular milieu. High- and low-affinity Na+- and Cl−-dependent taurine transporters have been described in the retina. The principal transport protein is the high-affinity TauT transporter [78]. Additionally, it is known that treatment with taurine can prevent retinal neurodegeneration [79]. A taurine-specific receptor has not been yet identified, but it has been suggested that taurine neuroprotection could be mediated by GABA receptor stimulation [79]. Whether taurine and TUDCA share mechanisms of action remains to be elucidated. Finally, no UDCA receptor interaction has been described. It is known that UDCA does not bind to the FXR [9,80], and because UDCA is a non-conjugated bile acid, a different molecular mechanism of action might be expected.
Clinical trials of bile acids for neurodegeneration
Although there is increasing evidence supporting a potential therapeutic role for bile acids in neurodegenerative disorders, their benefit in a clinical setting remains poorly explored. Only three clinical studies evaluating the safety and efficacy of oral bile acids have been reported, in patients with ALS [24-26] (Table 2). To date, there is no clinical study reporting the evaluation of bile acids in retinal disorders. Registered clinical trials of bile acids for neurodegeneration are summarized in Table 4, none of which has results available to date.
Table 4. Registered trials of the bile acids for neurodegenerations.
| Status | Study title | ClinicalTrials.gov Identifier | Condition | Study design | Intervention |
|---|---|---|---|---|---|
| Completed |
Ursodeoxycholic Acid for Rhegmatogenous Retinal Detachment |
NCT02841306 |
Rhegmatogenous Retinal Detachment |
Phase 1 clinical trial
Non-randomized
Parallel Assignment
Open labeled |
UDCA
26 participants |
| Recruiting |
Trial of Ursodeoxycholic Acid (UDCA) for Parkinson Disease: The “UP” Study |
NCT03840005 |
Parkinson Disease |
Phase 2 clinical trial Placebo Controlled, Randomized
Double Blind |
UDCA
30 participants |
| Not yet recruiting |
Brain Bioenergetics in Parkinson Disease and Response to Repeated Oral UDCA Treatment |
NCT02967250 |
Parkinson Disease |
Phase 1 clinical trial
Non-randomized
Open labeled
Single Group Assignment |
UDCA
20 participants |
| Unknown status |
Ursodiol in Huntington's Disease |
NCT00514774 |
Huntington Disease |
Phase 1 clinical trial Randomized
Parallel Assignment Double Blind |
UDCA
21 participants |
| Recruiting |
Safety and Efficacy of TUDCA as add-on Treatment in Patients Affected by Amyotrophic Lateral Sclerosis |
NCT03800524 |
Amyotrophic Lateral Sclerosis |
Phase 3 clinical trial Placebo Controlled, Randomized
Double Blind |
TUDCA
440 participants |
| Recruiting |
A Trial of Bile Acid Supplementation in Patients With Multiple Sclerosis |
NCT03423121 |
Progressive Multiple Sclerosis |
Phase 1–2 clinical trial Placebo Controlled, Randomized
Double Blind |
TUDCA
60 participants |
| Recruiting |
Study to Assess the Safety and Biologic Activity of AMX0035 for the Treatment of Alzheimer Disease |
NCT03533257 |
Alzheimer Disease |
Phase 2 clinical trial Placebo Controlled, Randomized
Double Blind |
AMX0035 (TUDCA and Phenylbutyrate) 100 participants |
| Active, non- recruiting |
AMX0035 in Patients With Amyotrophic Lateral Sclerosis |
NCT03127514 |
Amyotrophic Lateral Sclerosis |
Phase 2 clinical trial Placebo Controlled, Randomized
Double Blind |
AMX0035 (TUDCA and Phenylbutyrate) 132 participants |
| Enrolling by invitation | Open Label Extension Study of AMX0035 in Patients With Amyotrophic Lateral Sclerosis | NCT03488524 | Amyotrophic Lateral Sclerosis | Phase 2 clinical trial Single Group Assignment Non-randomized Open label | AMX0035 (TUDCA and Phenylbutyrate) 132 participants |
Discussion
Consistent evidence has shown the protective role of TUDCA and UDCA in retinal disorders. Importantly, TUDCA treatment in photoreceptor and RGC degeneration models not only inhibited apoptosis but also promoted cell survival and function [37,47]. TUDCA was shown to protect from caspase-dependent [2,43] and independent (AIF) apoptosis [55] and from ER stress-mediated apoptosis [41,42]. Additionally, anti-inflammatory and antioxidant effects have been reported for TUDCA in photoreceptor degeneration models [36,43,55]. How bile acids interact with retinal cells remains imperfectly understood although a direct interaction of TUDCA with TRG5 has been described in RGCs [59].
The cytoprotective effect of UDCA has been less extensively explored, mostly in models of diabetic retinopathy, where UDCA preserved the BRB and exerted antiapoptotic (ER stress mediated), anti-inflammatory, and antioxidant mechanisms [57,58]. Comparative efficacy of TUDCA and UDCA has been explored only in a laser-induced CNV model, showing similar effects but probably by different mechanisms [60].
Although UDCA and TUDCA have been found in cerebrospinal fluid after oral administration [24], no clinical studies have evaluated the ocular biodistribution of bile acids. Moreover, little is known about the role of endogenous circulating bile acids in the retina. Secondary bile acids are present in the systemic circulation after being absorbed by the intestine and released in the portal vein [5]. The mechanisms that regulate bile acids levels in the systemic circulation have not been fully explored, and the role of the microbiota seems central, as it is responsible for the transformation of primary bile acids to secondary neuroprotectant ones [5]. The levels of circulating secondary bile acids could result from increased production by the microbiota or from and increased intestine permeability, known to be also influenced by the microbiota [81]. In the cerebrospinal fluid, the levels of TUDCA are proportional to the circulating levels [6], but in the retina and the ocular media, the levels of bile acids have not been evaluated.
As studies increasingly link neurodegenerative disease to the state of the microbiota [82-85], we hypothesize that the effects of alterations to the microbiome on circulating bile acids may induce or exacerbate neurodegenerative processes, and specifically, retinal degeneration. Thus, therapeutic use of bile acids in retinal disease should be further investigated.
Acknowledgments
Authors acknowledge support from the Abraham J and Phyllis Katz Foundation, USA; Fondation de l’avenir, France; Association Française des Amblyopes Unilatéraux, France; NIH R01EY028859 and VA I01RX002806, USA. Authors have no commercial interests in the subject of the manuscript or in entities discussed in the manuscript.
References
- 1.Monte MJ, Marin JJG, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804–16. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boatright JH, Moring AG, McElroy C, Phillips MJ, Do VT, Chang B, Hawes NL, Boyd AP, Sidney SS, Stewart RE, Minear SC, Chaudhury R, Ciavatta VT, Rodrigues CMP, Steer CJ, Nickerson JM, Pardue MT. Tool from ancient pharmacopoeia prevents vision loss. Mol Vis. 2006;12:1706–14. [PubMed] [Google Scholar]
- 3.Vang S, Longley K, Steer CJ, Low WC. The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases. Glob Adv Health Med. 2014;3:58–69. doi: 10.7453/gahmj.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonin F, Arends IWCE. Latest development in the synthesis of ursodeoxycholic acid (UDCA): a critical review. Beilstein J Org Chem. 2018;14:470–83. doi: 10.3762/bjoc.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24:1803–23. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- 6.Mertens KL, Kalsbeek A, Soeters MR, Eggink HM. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front Neurosci. 2017;11:617. doi: 10.3389/fnins.2017.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinicke M, Schröter J, Müller-Klieser D, Helmschrodt C, Ceglarek U. Free oxysterols and bile acids including conjugates - Simultaneous quantification in human plasma and cerebrospinal fluid by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2018;1037:245–55. doi: 10.1016/j.aca.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 8.Copple BL, Li T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res. 2016;104:9–21. doi: 10.1016/j.phrs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaral JD, Viana RJS, Ramalho RM, Steer CJ, Rodrigues CMP. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 2009;50:1721–34. doi: 10.1194/jlr.R900011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32(Suppl 2):S237–45. doi: 10.2337/dc09-S355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gohlke H, Schmitz B, Sommerfeld A, Reinehr R, Häussinger D. α5 β1-integrins are sensors for tauroursodeoxycholic acid in hepatocytes. Hepatology. 2013;57:1117–29. doi: 10.1002/hep.25992. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–67. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Solá S, Amaral JD, Castro RE, Ramalho RM, Borralho PM, Kren BT, Tanaka H, Steer CJ, Rodrigues CMP. Nuclear translocation of UDCA by the glucocorticoid receptor is required to reduce TGF-beta1-induced apoptosis in rat hepatocytes. Hepatology. 2005;42:925–34. doi: 10.1002/hep.20870. [DOI] [PubMed] [Google Scholar]
- 14.Solá S, Castro RE, Kren BT, Steer CJ, Rodrigues CMP. Modulation of nuclear steroid receptors by ursodeoxycholic acid inhibits TGF-beta1-induced E2F-1/p53-mediated apoptosis of rat hepatocytes. Biochemistry. 2004;43:8429–38. doi: 10.1021/bi049781x. [DOI] [PubMed] [Google Scholar]
- 15.Dionísio PA, Amaral JD, Ribeiro MF, Lo AC, D’Hooge R, Rodrigues CMP. Amyloid-β pathology is attenuated by tauroursodeoxycholic acid treatment in APP/PS1 mice after disease onset. Neurobiol Aging. 2015;36:228–40. doi: 10.1016/j.neurobiolaging.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 16.Lo AC, Callaerts-Vegh Z, Nunes AF, Rodrigues CMP, D’Hooge R. Tauroursodeoxycholic acid (TUDCA) supplementation prevents cognitive impairment and amyloid deposition in APP/PS1 mice. Neurobiol Dis. 2013;50:21–9. doi: 10.1016/j.nbd.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Ramalho RM, Nunes AF, Dias RB, Amaral JD, Lo AC, D’Hooge R, Sebastião AM, Rodrigues CMP. Tauroursodeoxycholic acid suppresses amyloid β-induced synaptic toxicity in vitro and in APP/PS1 mice. Neurobiol Aging. 2013;34:551–61. doi: 10.1016/j.neurobiolaging.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Nunes AF, Amaral JD, Lo AC, Fonseca MB, Viana RJS, Callaerts-Vegh Z, D’Hooge R, Rodrigues CMP. TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-β deposition in APP/PS1 mice. Mol Neurobiol. 2012;45:440–54. doi: 10.1007/s12035-012-8256-y. [DOI] [PubMed] [Google Scholar]
- 19.Pan X, Elliott CT, McGuinness B, Passmore P, Kehoe PG, Hölscher C, McClean PL, Graham SF, Green BD. Metabolomic Profiling of Bile Acids in Clinical and Experimental Samples of Alzheimer’s Disease. Metabolites. 2017;7:28. doi: 10.3390/metabo7020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelkader NF, Safar MM, Salem HA. Ursodeoxycholic Acid Ameliorates Apoptotic Cascade in the Rotenone Model of Parkinson’s Disease: Modulation of Mitochondrial Perturbations. Mol Neurobiol. 2016;53:810–7. doi: 10.1007/s12035-014-9043-8. [DOI] [PubMed] [Google Scholar]
- 21.Castro-Caldas M, Carvalho AN, Rodrigues E, Henderson CJ, Wolf CR, Rodrigues CMP, Gama MJ. Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Mol Neurobiol. 2012;46:475–86. doi: 10.1007/s12035-012-8295-4. [DOI] [PubMed] [Google Scholar]
- 22.Rosa AI, Fonseca I, Nunes MJ, Moreira S, Rodrigues E, Carvalho AN, Rodrigues CMP, Gama MJ, Castro-Caldas M. Novel insights into the antioxidant role of tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2171–81. doi: 10.1016/j.bbadis.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Keene CD, Rodrigues CMP, Eich T, Chhabra MS, Steer CJ, Low WC. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc Natl Acad Sci USA. 2002;99:10671–6. doi: 10.1073/pnas.162362299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parry GJ, Rodrigues CMP, Aranha MM, Hilbert SJ, Davey C, Kelkar P, Low WC, Steer CJ. Safety, tolerability, and cerebrospinal fluid penetration of ursodeoxycholic Acid in patients with amyotrophic lateral sclerosis. Clin Neuropharmacol. 2010;33:17–21. doi: 10.1097/WNF.0b013e3181c47569. [DOI] [PubMed] [Google Scholar]
- 25.Min J-H, Hong Y-H, Sung J-J, Kim S-M, Lee JB, Lee K-W. Oral solubilized ursodeoxycholic acid therapy in amyotrophic lateral sclerosis: a randomized cross-over trial. J Korean Med Sci. 2012;27:200–6. doi: 10.3346/jkms.2012.27.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elia AE, Lalli S, Monsurrò MR, Sagnelli A, Taiello AC, Reggiori B, La Bella V, Tedeschi G, Albanese A. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur J Neurol. 2016;23:45–52. doi: 10.1111/ene.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillin M, DeMorrow S. Effects of bile acids on neurological function and disease. FASEB J. 2016;30:3658–68. doi: 10.1096/fj.201600275R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackerman HD, Gerhard GS. Bile Acids in Neurodegenerative Disorders. Front Aging Neurosci. 2016;8:263. doi: 10.3389/fnagi.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanguas-Casás N, Barreda-Manso MA, Nieto-Sampedro M, Romero-Ramírez L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J Cell Physiol. 2017;232:2231–45. doi: 10.1002/jcp.25742. [DOI] [PubMed] [Google Scholar]
- 30.Solá S, Amaral JD, Borralho PM, Ramalho RM, Castro RE, Aranha MM, Steer CJ, Rodrigues CMP. Functional modulation of nuclear steroid receptors by tauroursodeoxycholic acid reduces amyloid beta-peptide-induced apoptosis. Mol Endocrinol. 2006;20:2292–303. doi: 10.1210/me.2006-0063. [DOI] [PubMed] [Google Scholar]
- 31.Yanovsky Y, Schubring SR, Yao Q, Zhao Y, Li S, May A, Haas HL, Lin J-S, Sergeeva OA. Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA receptor block. PLoS One. 2012;7:e42512. doi: 10.1371/journal.pone.0042512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang CJ, Lai WW, Edward DP, Tso MO. Apoptotic photoreceptor cell death after traumatic retinal detachment in humans. Arch Ophthalmol Chic Ill. 1960;1995:880–6. doi: 10.1001/archopht.1995.01100070054025. [DOI] [PubMed] [Google Scholar]
- 33.Viringipurampeer IA, Gregory-Evans CY, Metcalfe AL, Bashar E, Moritz OL, Gregory-Evans K. Cell Death Pathways in Mutant Rhodopsin Rat Models Identifies Genotype-Specific Targets Controlling Retinal Degeneration. Mol Neurobiol. 2019;56:1637–52. doi: 10.1007/s12035-018-1192-8. [DOI] [PubMed] [Google Scholar]
- 34.Sizova OS, Shinde VM, Lenox AR, Gorbatyuk MS. Modulation of cellular signaling pathways in P23H rhodopsin photoreceptors. Cell Signal. 2014;26:665–72. doi: 10.1016/j.cellsig.2013.12.008. Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenzel A, Grimm C, Samardzija M, Remé CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Oveson BC, Iwase T, Hackett SF, Lee SY, Usui S, Sedlak TW, Snyder SH, Campochiaro PA, Sung JU. Constituents of bile, bilirubin and TUDCA, protect against oxidative stress-induced retinal degeneration. J Neurochem. 2011;116:144–53. doi: 10.1111/j.1471-4159.2010.07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips MJ, Walker TA, Choi H-Y, Faulkner AE, Kim MK, Sidney SS, Boyd AP, Nickerson JM, Boatright JH, Pardue MT. Tauroursodeoxycholic acid preservation of photoreceptor structure and function in the rd10 mouse through postnatal day 30. Invest Ophthalmol Vis Sci. 2008;49:2148–55. doi: 10.1167/iovs.07-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drack AV, Dumitrescu AV, Bhattarai S, Gratie D, Stone EM, Mullins R, Sheffield VC. TUDCA slows retinal degeneration in two different mouse models of retinitis pigmentosa and prevents obesity in Bardet-Biedl syndrome type 1 mice. Invest Ophthalmol Vis Sci. 2012;53:100–6. doi: 10.1167/iovs.11-8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-Sánchez L, Lax P, Pinilla I, Martín-Nieto J, Cuenca N. Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Invest Ophthalmol Vis Sci. 2011;52:4998–5008. doi: 10.1167/iovs.11-7496. [DOI] [PubMed] [Google Scholar]
- 40.Zhang SX, Sanders E, Fliesler SJ, Wang JJ. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Exp Eye Res. 2014;125:30–40. doi: 10.1016/j.exer.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang T, Baehr W, Fu Y. Chemical Chaperone TUDCA Preserves Cone Photoreceptors in a Mouse Model of Leber Congenital Amaurosis. Invest Ophthalmol Vis Sci. 2012;53:3349–56. doi: 10.1167/iovs.12-9851. Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Y, Zhang T. Pathophysilogical mechanism and treatment strategies for Leber congenital amaurosis. Adv Exp Med Biol. 2014;801:791–6. doi: 10.1007/978-1-4614-3209-8_99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantopoulos D, Murakami Y, Comander J, Thanos A, Roh M, Miller JW, Vavvas DG. Tauroursodeoxycholic acid (TUDCA) protects photoreceptors from cell death after experimental retinal detachment. PLoS One. 2011;6:e24245. doi: 10.1371/journal.pone.0024245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noailles A, Fernández-Sánchez L, Lax P, Cuenca N. Microglia activation in a model of retinal degeneration and TUDCA neuroprotective effects. J Neuroinflammation. 2014;11:186. doi: 10.1186/s12974-014-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murase H, Tsuruma K, Shimazawa M, Hara H. TUDCA Promotes Phagocytosis by Retinal Pigment Epithelium via MerTK Activation. Invest Ophthalmol Vis Sci. 2015;56:2511–8. doi: 10.1167/iovs.14-15962. [DOI] [PubMed] [Google Scholar]
- 46.Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–93. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 47.Gómez-Vicente V, Lax P, Fernández-Sánchez L, Rondón N, Esquiva G, Germain F, de la Villa P, Cuenca N. Neuroprotective Effect of Tauroursodeoxycholic Acid on N-Methyl-D-Aspartate-Induced Retinal Ganglion Cell Degeneration. PLoS One. 2015;10:e0137826. doi: 10.1371/journal.pone.0137826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas CN, Berry M, Logan A, Blanch RJ, Ahmed Z. Caspases in retinal ganglion cell death and axon regeneration. Cell Death Dis. 2017;3:17032. doi: 10.1038/cddiscovery.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitamura Y, Bikbova G, Baba T, Yamamoto S, Oshitari T. In vivo effects of single or combined topical neuroprotective and regenerative agents on degeneration of retinal ganglion cells in rat optic nerve crush model. Sci Rep. 2019;9:101. doi: 10.1038/s41598-018-36473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia H, Nan Y, Huang X, Gao J, Pu M. Effects of Tauroursodeoxycholic Acid and Alpha-Lipoic-Acid on the Visual Response Properties of Cat Retinal Ganglion Cells: An In Vitro Study. Invest Ophthalmol Vis Sci. 2015;56:6638–45. doi: 10.1167/iovs.15-17301. [DOI] [PubMed] [Google Scholar]
- 51.Chao de la Barca JM, Simard G, Amati-Bonneau P, Safiedeen Z, Prunier-Mirebeau D, Chupin S, Gadras C, Tessier L, Gueguen N, Chevrollier A, Desquiret-Dumas V, Ferré M, Bris C, Kouassi Nzoughet J, Bocca C, Leruez S, Verny C, Miléa D, Bonneau D, Lenaers G, Martinez MC, Procaccio V, Reynier P. The metabolomic signature of Leber’s hereditary optic neuropathy reveals endoplasmic reticulum stress. Brain. 2016;139:2864–76. doi: 10.1093/brain/aww222. [DOI] [PubMed] [Google Scholar]
- 52.Pardue MT, Allen RS. Neuroprotective strategies for retinal disease. Prog Retin Eye Res. 2018;65:50–76. doi: 10.1016/j.preteyeres.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan T-T, Li X-F, Sun Y-M, Li Y-B, Su Y. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Biomed Pharmacother. 2015;74:145–7. doi: 10.1016/j.biopha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, Rothschild P-R, Omri S, Gélizé E, Jonet L, Delaunay K, De Kozak Y, Berdugo M, Zhao M, Crisanti P, Behar-Cohen F. Mechanisms of macular edema: Beyond the surface. Prog Retin Eye Res. 2018;63:20–68. doi: 10.1016/j.preteyeres.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Gaspar JM, Martins A, Cruz R, Rodrigues CMP, Ambrósio AF, Santiago AR. Tauroursodeoxycholic acid protects retinal neural cells from cell death induced by prolonged exposure to elevated glucose. Neuroscience. 2013;253:380–8. doi: 10.1016/j.neuroscience.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 56.Oshitari T, Bikbova G, Yamamoto S. Increased expression of phosphorylated c-Jun and phosphorylated c-Jun N-terminal kinase associated with neuronal cell death in diabetic and high glucose exposed rat retinas. Brain Res Bull. 2014;101:18–25. doi: 10.1016/j.brainresbull.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Chung Y-R, Choi JA, Koh J-Y, Yoon YH. Ursodeoxycholic Acid Attenuates Endoplasmic Reticulum Stress-Related Retinal Pericyte Loss in Streptozotocin-Induced Diabetic Mice. J Diabetes Res. 2017;2017:1763292. doi: 10.1155/2017/1763292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ouyang H, Mei X, Zhang T, Lu B, Ji L. Ursodeoxycholic acid ameliorates diabetic retinopathy via reducing retinal inflammation and reversing the breakdown of blood-retinal barrier. Eur J Pharmacol. 2018;840:20–7. doi: 10.1016/j.ejphar.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 59.Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, Prasad R, Bhatwadekar A, White FA, Townsend SD, Chan L, Ryan CN, Morton D, Moldovan EG, Chu F-I, Oudit GY, Derendorf H, Adorini L, Wang XX, Evans-Molina C, Mirmira RG, Boulton ME, Yoder MC, Li Q, Levi M, Busik JV, Grant MB. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes. 2018;67:1867–79. doi: 10.2337/db18-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo SJ, Kim JH, Yu HG. Ursodeoxycholic acid and tauroursodeoxycholic acid suppress choroidal neovascularization in a laser-treated rat model. J Ocul Pharmacol Ther. 2010;26:223–9. doi: 10.1089/jop.2010.0012. [DOI] [PubMed] [Google Scholar]
- 61.Porter H, Qi H, Prabhu N, Grambergs R, McRae J, Hopiavuori B, Mandal N. Characterizing Sphingosine Kinases and Sphingosine 1-Phosphate Receptors in the Mammalian Eye and Retina. Int J Mol Sci. 2018;19:3885. doi: 10.3390/ijms19123885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506–16. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brem RB, Robbins SG, Wilson DJ, O’Rourke LM, Mixon RN, Robertson JE, Planck SR, Rosenbaum JT. Immunolocalization of integrins in the human retina. Invest Ophthalmol Vis Sci. 1994;35:3466–74. [PubMed] [Google Scholar]
- 64.Neumann C, Garreis F, Paulsen F, Hammer CM, Birke MT, Scholz M. Osteopontin is induced by TGF-β2 and regulates metabolic cell activity in cultured human optic nerve head astrocytes. PLoS One. 2014;9:e92762. doi: 10.1371/journal.pone.0092762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Craig TA, Sommer S, Sussman CR, Grande JP, Kumar R. Expression and regulation of the vitamin D receptor in the zebrafish, Danio rerio. J Bone Miner Res. 2008;23:1486–96. doi: 10.1359/JBMR.080403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taverna MJ, Selam J-L, Slama G. Association between a protein polymorphism in the start codon of the vitamin D receptor gene and severe diabetic retinopathy in C-peptide-negative type 1 diabetes. J Clin Endocrinol Metab. 2005;90:4803–8. doi: 10.1210/jc.2004-2407. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Lu M, Sun X, Li C, Kuang X, Ruan X. Expression and activity of p-glycoprotein elevated by dexamethasone in cultured retinal pigment epithelium involve glucocorticoid receptor and pregnane X receptor. Invest Ophthalmol Vis Sci. 2012;53:3508–15. doi: 10.1167/iovs.11-9337. [DOI] [PubMed] [Google Scholar]
- 68.Gallina D, Zelinka C, Fischer AJ. Glucocorticoid receptors in the retina, Müller glia and the formation of Müller glia-derived progenitors. Development. 2014;141:3340–51. doi: 10.1242/dev.109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao M, Célérier I, Bousquet E, Jeanny J-C, Jonet L, Savoldelli M, Offret O, Curan A, Farman N, Jaisser F, Behar-Cohen F. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122:2672–9. doi: 10.1172/JCI61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kubo Y, Akanuma S-I, Hosoya K-I. Recent advances in drug and nutrient transport across the blood-retinal barrier. Expert Opin Drug Metab Toxicol. 2018;14:513–31. doi: 10.1080/17425255.2018.1472764. [DOI] [PubMed] [Google Scholar]
- 71.Akanuma S, Hirose S, Tachikawa M, Hosoya K-I. Localization of organic anion transporting polypeptide (Oatp) 1a4 and Oatp1c1 at the rat blood-retinal barrier. Fluids Barriers CNS. 2013;10:29. doi: 10.1186/2045-8118-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao B, Vavricka SR, Meier PJ, Stieger B. Differential cellular expression of organic anion transporting peptides OATP1A2 and OATP2B1 in the human retina and brain: implications for carrier-mediated transport of neuropeptides and neurosteriods in the CNS. Pflugers Arch. 2015;467:1481–93. doi: 10.1007/s00424-014-1596-x. [DOI] [PubMed] [Google Scholar]
- 73.Chan T, Zhu L, Madigan MC, Wang K, Shen W, Gillies MC, Zhou F. Human organic anion transporting polypeptide 1A2 (OATP1A2) mediates cellular uptake of all-trans-retinol in human retinal pigmented epithelial cells. Br J Pharmacol. 2015;172:2343–53. doi: 10.1111/bph.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tagami M, Kusuhara S, Imai H, Uemura A, Honda S, Tsukahara Y, Negi A. MRP4 knockdown enhances migration, suppresses apoptosis, and produces aggregated morphology in human retinal vascular endothelial cells. Biochem Biophys Res Commun. 2010;400:593–8. doi: 10.1016/j.bbrc.2010.08.109. [DOI] [PubMed] [Google Scholar]
- 75.Kowalczuk L, Matet A, Dor M, Bararpour N, Daruich A, Dirani A, Behar-Cohen F, Thomas A, Turck N. Proteome and Metabolome of Subretinal Fluid in Central Serous Chorioretinopathy and Rhegmatogenous Retinal Detachment: A Pilot Case Study. Transl Vis Sci Technol. 2018;7:3. doi: 10.1167/tvst.7.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lobysheva E, Taylor CM, Marshall GR, Kisselev OG. Tauroursodeoxycholic acid binds to the G-protein site on light activated rhodopsin. Exp Eye Res. 2018;170:51–7. doi: 10.1016/j.exer.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ripps H, Shen W. Review: taurine: a “very essential” amino acid. Mol Vis. 2012;18:2673–86. [PMC free article] [PubMed] [Google Scholar]
- 78.Sivakami S, Ganapathy V, Leibach FH, Miyamoto Y. The gamma-aminobutyric acid transporter and its interaction with taurine in the apical membrane of the bovine retinal pigment epithelium. Biochem J. 1992;283:391–7. doi: 10.1042/bj2830391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hadj-Saïd W, Fradot V, Ivkovic I, Sahel J-A, Picaud S, Froger N. Taurine Promotes Retinal Ganglion Cell Survival Through GABAB Receptor Activation. Adv Exp Med Biol. 2017;975:687–701. doi: 10.1007/978-94-024-1079-2_54. [DOI] [PubMed] [Google Scholar]
- 80.Howard WR, Pospisil JA, Njolito E, Noonan DJ. Catabolites of cholesterol synthesis pathways and forskolin as activators of the farnesoid X-activated nuclear receptor. Toxicol Appl Pharmacol. 2000;163:195–202. doi: 10.1006/taap.1999.8869. [DOI] [PubMed] [Google Scholar]
- 81.Jena PK, Sheng L, Di Lucente J, Jin L-W, Maezawa I, Wan Y-JY. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity. FASEB J. 2018;32:2866–77. doi: 10.1096/fj.201700984RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCombe PA, Henderson RD, Lee A, Lee JD, Woodruff TM, Restuadi R, McRae A, Wray NR, Ngo S, Steyn FJ. Gut microbiota in ALS: possible role in pathogenesis? Expert Rev Neurother. 2019;•••:1–21. doi: 10.1080/14737175.2019.1623026. [DOI] [PubMed] [Google Scholar]
- 83.Sasmita AO. Modification of the gut microbiome to combat neurodegeneration. Rev Neurosci. 2019 doi: 10.1515/revneuro-2019-0005. In press. [DOI] [PubMed] [Google Scholar]
- 84.Miraglia F, Colla E. Microbiome, Parkinson’s Disease and Molecular Mimicry. Cells. 2019;8:222. doi: 10.3390/cells8030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kowalski K, Mulak A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J Neurogastroenterol Motil. 2019;25:48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boatright JH, Nickerson JM, Moring AG, Pardue MT. Bile acids in treatment of ocular disease. J Ocul Biol Dis Infor. 2009;2:149–59. doi: 10.1007/s12177-009-9030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bikbova G, Oshitari T, Baba T, Yamamoto S. Altered Expression of NF- κ B and SP1 after Exposure to Advanced Glycation End-Products and Effects of Neurotrophic Factors in AGEs Exposed Rat Retinas. J Diabetes Res. 2015;2015:543818. doi: 10.1155/2015/543818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C-F, Yuan J-R, Qin D, Gu J-F, Zhao B-J, Zhang L, Zhao D, Chen J, Hou X-F, Yang N, Bu W-Q, Wang J, Li C, Tian G, Dong Z-B, Feng L, Jia X-B. Protection of tauroursodeoxycholic acid on high glucose-induced human retinal microvascular endothelial cells dysfunction and streptozotocin-induced diabetic retinopathy rats. J Ethnopharmacol. 2016;185:162–70. doi: 10.1016/j.jep.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 89.Lawson EC, Bhatia SK, Han MK, Aung MH, Ciavatta V, Boatright JH, Pardue MT. Tauroursodeoxycholic Acid Protects Retinal Function and Structure in rd1 Mice. Adv Exp Med Biol. 2016;854:431–6. doi: 10.1007/978-3-319-17121-0_57. [DOI] [PubMed] [Google Scholar]
- 90.Fernández-Sánchez L, Bravo-Osuna I, Lax P, Arranz-Romera A, Maneu V, Esteban-Pérez S, Pinilla I, Puebla-González MDM, Herrero-Vanrell R, Cuenca N. Controlled delivery of tauroursodeoxycholic acid from biodegradable microspheres slows retinal degeneration and vision loss in P23H rats. PLoS One. 2017;12:e0177998. doi: 10.1371/journal.pone.0177998. [DOI] [PMC free article] [PubMed] [Google Scholar]