Abstract
Purpose
Conbercept is a novel recombinant fusion protein designed as a decoy receptor for vascular endothelial growth factor (VEGF) and placental growth factor. The primary purpose was to investigate the effect and safety of conbercept, based on a practical protocol, in the eyes of patients with diabetic macular edema (DME), and the secondary aim was to evaluate the efficacy of low-dose triamcinolone acetonide in patients with refractory DME who had little response to conbercept.
Methods
In this retrospective clinical study, 89 treatment eyes from 76 patients with clinically significant DME were initially treated with one to three consecutive monthly intravitreal conbercept (IVC) injections, followed by retreatment with conbercept or switch therapy to triamcinolone acetonide (TA) based on a 6-month observation of the curative effect of IVC.
Results
Sixty eyes were initiated on conbercept treatment for DME throughout the entire 1-year assessment period. After at least three consecutive monthly IVC treatments, 29 eyes further received intravitreal triamcinolone acetonide (IVTA) injections at month 6. From baseline to 1 year, the mean number of conbercept injections in the IVC group (n=60) was 4.5±1.0, and the mean number of conbercept injections in the IVC plus IVTA group (n=29) was 3.1±0.3. The mean best-corrected visual acuity (BCVA) and central macular thickness (CMT) were statistically significantly improved at 1 and 3 months after IVC treatments in the IVC group, and gradually improved at 9 months after IVTA treatments in the IVC plus IVTA group. There were no severe complications or conbercept-related adverse ocular and systemic side effects.
Conclusions
Conbercept could be effective for visual and anatomic improvements in DME eyes with relatively fewer intravitreal injections and longer treatment intervals in clinical practice. Low-dose TA may be useful for patients with refractory DME resistant to anti-VEGF therapy.
Introduction
Diabetic retinopathy (DR), a common cause leading to vision loss or blindness worldwide, has been considered the specific microvascular complication of diabetes mellitus (DM). In patients with DR, visual function can be severely damaged by complications of diabetic macular edema (DME) or the occurrence of retinal neovascularization (which eventually results in vitreous hemorrhage (VH), tractional retinal detachment (TRD), and neovascular glaucoma), or both. DME is one of the most common phenotypes of diabetic maculopathy, and directly influences macular function (central visual dysfunction), which significantly affects the quality of life of patients with diabetes, and is associated with an increased risk of life-threatening microvascular diseases [1]. With the increasing incidence of diabetes, DME is gradually becoming a crucial societal health issue [2]. DME has been considered to be caused mainly by the hyperpermeability of retinal vessels leading to the extravasation of fluid and lipoproteins (hard exudate [HE]) into the retinal layers [3]. Vascular endothelial growth factor (VEGF) has been shown to be the key promoter of neovascularization and hyperpermeability in patients with DME [4-6].
The treatment of DME has involved different methods in the past decade, such as timely focal or grid laser photocoagulation therapy, or both [7], local steroid hormone injection [8], and vitrectomy surgery [9,10]. Despite the limited effectiveness for the preservation of vision in macular edema, some side effects cannot be ignored. Recent reports have shown that intravitreal injection with an anti-VEGF agent has emerged as the first-line therapy for DME [11,12]. Bevacizumab (Avastin; Genentech/Roche, South San Francisco, CA) and ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA) were the two most commonly used anti-VEGF agents in patients with DME or as adjuncts to vitrectomy for proliferative diabetic retinopathy (PDR), which have been found to bind VEGF-A only, or both. Aflibercept (Eylea; Regeneron Pharmaceuticals, Inc., Berlin, Germany) is a decoy receptor fusion protein composed of the second domain of human VEGF receptor 1 and the third domain of VEGF receptor 2, which are fused to the Fc domain of human immunoglobulin 1 (IgG1). In 2018, aflibercept received approval from the Chinese Food and Drug Administration (CFDA) to treat DME. Conbercept (KH902; Chengdu Kanghong Biotech Co., Ltd., Sichuan, China), a recent novel VEGF antagonist that binds to more molecular targets, has shown ideal effectiveness in retinal neovascularization and macular edema [13-15]. Conbercept is a humanized, recombinant fusion protein composed of the extracellular domain 2 of VEGF receptor 1 (Flt-1) and extracellular domains 3 and 4 of VEGF receptor 2 (KDR) combined with the Fc region of human IgG1, and is designed as a receptor decoy to block all isoforms of VEGF-A, VEGF-B, VEGF-C, and placenta growth factor (PIGF) [16]. Preclinical studies showed a higher binding affinity of conbercept to VEGF and a longer half-life in the vitreous body than other anti-VEGF agents [17,18]. Moreover, recent clinical trials showed a strong antiangiogenetic effect of conbercept on choroidal neovascularization (CNV) caused by age-related macular degeneration (AMD) and PDR. In addition, repeated intravitreal injections of KH902 present an excellent safety profile for retinal nerve fiber layer (RNFL) thickness in the aforementioned ocular neovascular disorders [19]. The primary purpose of the present study was to evaluate the safety and efficacy of the intravitreal injection of conbercept in patients with DME, and the secondary aim was to evaluate the efficacy of low-dose triamcinolone acetonide in patients with refractory DME who have little response to conbercept.
Methods
Study population
This retrospective study group comprised 89 eyes from 76 patients diagnosed with clinically significant DME who were recruited from the First Affiliated Hospital of Chongqing Medical University (Chongqing, China) between December 2015 and November 2016. Eligible participants consisted of patients with type 1 or type 2 diabetes mellitus (DM) aged older than or 18 years with one or both eyes selected in the clinical research. All patients with DME underwent an initially comprehensive ophthalmic examination, including best-corrected visual acuity (BCVA), intraocular pressure (IOP), slit-lamp biomicroscopy, color fundus photography, optical coherence tomography (OCT), and sodium fluorescein angiography (FA), before the intravitreal injection. The major exclusion criteria, including ocular and systemic factors, were as follows: (1) IOP greater than or 21 mmHg, glaucoma in the study eyes; intraocular treatment with macular laser photocoagulation, corticosteroids, and anti-VEGF agents within the previous 6 months; (2) patients during menstruation or those taking anticoagulative drugs; (3) patients suffering DME combined with other ocular problems, including uveitis, epiretinal membrane, and any history of pars plana vitrectomy. The study received approval from the Ethics Research Committee of Chongqing Medical University, and all patients provided informed consent before treatment. All patients provided the written informed consent before treatment. The tenets of the Declaration of Helsinki were upheld during all procedures of this study. All treatments in this study adhered to the association for research in vision and ophthalmology (ARVO) statement on patients in ophthalmic and vision research.
Study protocol
BCVA was measured in decimal values and converted to logMAR scores for all patients at every visit. The IOP values of patients were detected before and 1 day after the injection, and at the monthly follow-up visit for at least 3 months. Macular thickness was determined with a Heidelberg Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) examination to evaluate the degree of DME. All patients completed clinical assessments at baseline and at each monthly visit. The central macular thickness (CMT) was considered the space between the retinal internal limiting membrane and the RPE at the fovea. The exact values were automatically measured by the bundled software of the instrument. FA (Heidelberg Engineering) was performed on each eye to diagnose DME, and to identify the stages of DR with the injection of 10% sodium fluorescein solution in the antecubital vein. Intravitreal conbercept injection at a dose of 0.5 mg in 0.05 ml was performed using an aseptic technique in the ophthalmology operating room. Conbercept was pumped into a 1 ml syringe and injected into the vitreous body following the standard protocol [20]. All eyes included in this study were initially treated with one to three consecutive monthly intravitreal conbercept (IVC) injections followed by retreatment with conbercept or switch therapy to triamcinolone acetonide (TA). Intravitreal triamcinolone acetonide (IVTA) was initiated at the 6-month visit for persistent nonremission diabetic macular edema, after at least three consecutive monthly IVC injections.
Statistical analysis
Statistical analyses were performed with SPSS (Version 17.0, Chicago, IL). The data are presented as the mean ± standard deviation (SD). Two-tailed p values less than 0.05 were considered statistically significant. The Wilcoxon matched-pairs test with Bonferroni corrections were used to compare the significance of the changes in the BCVA and the CMT between baseline and each time point.
Results
Clinical features of study participants
Eighty-nine eyes from 76 southwestern Chinese patients with clinically significant DME underwent IVC injection in the present study. All participants completed at least 1 year of follow-up. Detailed clinical findings for the enrolled patients are presented in Table 1. The mean age of the patients comprising 46 men (52%) and 43 women (48%), was 59.6±8.50 years (range: 34 to 81 years). In total, 94% (84) of the patients had type 2 diabetes, and the mean duration of diabetes was 10.2±4.3 years. Of the 89 eyes examined, 16 had local photocoagulation for microaneurysms or no perfusion areas, or both, and 11 had panretinal photocoagulation (PRP) for longer than 12 months. Thirty-one patients underwent phacoemulsification cataracts and intraocular lens implantation.
Table 1. Baseline characteristics of patients with DME clinical data and features.
| Characteristic | Total |
|---|---|
| No. of eyes(patients) |
89(76) |
| Age (mean ± SD) |
34–81(59.6±8.5) |
| Gender(Male/Female) |
46/43 |
| Duration of diabetes(years) |
10.2±4.3 |
| HbA1c (%) |
7.9±0.8 |
| Lens status (phakic/pseudophakic) |
58/31 |
| PRP eyes(%) |
11 |
| Focal retinal photocoagulation eyes(%) | 5 |
Plus–minus values are means ±SD.
Treatment of DME
Sixty eyes from 50 patients were initiated on conbercept treatment for DME throughout the entire 1-year assessment period. Twenty-nine IVC-treated eyes (from 26 patients) further received IVTA injections at month 6 due to persistent nonremission diabetic macular edema, after at least three consecutive monthly IVC injections. During the 12-month follow-up period, the mean number of conbercept injections in the IVC group (n=60) was 4.5±1.0, and the mean number of treatments in the IVC plus IVTA group (n=29) was 3.1±0.3. In the IVC group, two eyes (5%) received seven injections, six eyes (10%) received six injections, 22 eyes (36.7%) received five injections, 21 eyes (35%) received four injections, seven eyes (11.7%) received three injections, and two eyes (3.3%) received two injections, over the 12-month study period. In the IVC plus IVTA group, 26 eyes (90%) received three injections, and three eyes (10%) received four injections over the 6-month study period before IVTA treatment. A total of 29 eyes received one IVTA injection at month 6.
BCVA outcomes
The average levels of BCVA before and after the injections are presented in Figure 1 and Table 2. The mean BCVA for all participants at baseline was 0.74±0.27. There was an early and strong response of patients with DME to this anti-VEGF drug. The greater efficacy of conbercept started to become apparent as early as 4 weeks after the initiation of treatment. The BCVA was statistically significantly improved (p<0.001) at 1 month (0.69±0.24) after the first IVC injection compared with that at baseline. This improvement continued up to month 3 (0.65±0.24), and started to decrease at month 6 (0.68±0.26). Notably, 32.6% (29) of IVC-treated eyes were not sensitive to conbercept treatments within half a year. Starting at the 6-month visit, an IVTA injection was considered for persistent diabetic macular edema with no improvement, after at least three consecutive monthly IVC injections. As shown in Figure 1, this group showed great improvement (p<0.001) in the mean visual acuity by 9 months (0.65±0.24), after receiving IVTA treatments for 3 months, but moderately worse at 12 months (0.66±0.25). In the IVC group (60 eyes), the BCVA was gradually increased at 9 months (0.60±0.26), but not statistically significantly better at 12 months (0.62±0.26) after IVC treatment was reinitiated at the 6-month visit (0.62±0.27).
Figure 1.
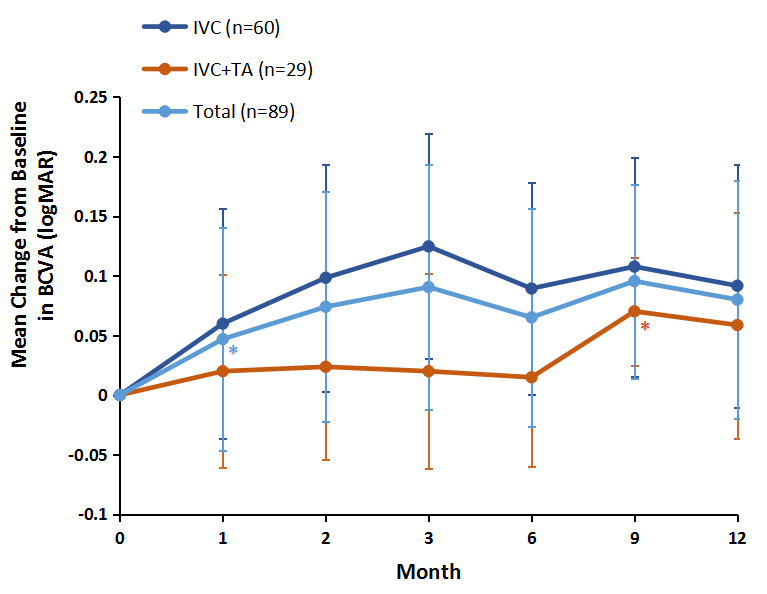
Mean change in best-corrected visual acuity (BCVA) from baseline to month 9. Intravitreal triamcinolone acetonide (TA) injections were administered at month 6 after at least three consecutive intravitreal conbercept (IVC) injections. Data are expressed as mean ± standard deviation (SD). Wilcoxon matched-pairs test with Bonferroni corrections were used to compare the significance of the changes between baseline and each time point. *p <0.001 relative to the baseline of the BCVA.
Table 2. Best-corrected visual acuity outcomes.
| Characteristic | Total | IVC | IVC+TA |
|---|---|---|---|
| No. of eyes (patients) |
89(76) |
60(50) |
29(26) |
| BCVA at baseline (logMAR) |
0.74±0.27 |
0.71±0.29 |
0.81±0.20 |
| BCVA at 6 mon (logMAR) |
0.68±0.26 |
0.62±0.27 |
0.79±0.18 |
| P value |
<0.001* |
<0.001* |
0.34 |
| BCVA at 1 yr (logMAR) |
0.66±0.25 |
0.62±0.26 |
0.75±0.19 |
| Mean improvement from baseline |
-0.08±0.09 |
-0.09±0.10 |
-0.06±0.09 |
| to 1 yr |
|
|
|
| P value | <0.001* | <0.001* | 0.011 |
Effect on central macular thickness
The improvement in the CMT over 1 year of follow-up is shown in Figure 2 and Table 3. The mean CMT, statistically significantly decreased by 100±74.0 μm with conbercept and TA, was 356±77.0 μm at month 12, compared to 456±128 μm at baseline (p<0.001). In the IVC injection group, the mean CMT was reduced most statistically significantly at the 1-month visit (from 454±133 μm to 353±80.0 μm), and reached the minimum thickness (335±72.0 μm) by month 3. Despite the obvious regression at 6 months (359±83.0 μm), these improvements were basically sustained until the last evaluation time point at 12 months (351±80.0 μm). In the IVTA plus IVC injection group, the mean CMT was statistically significantly reduced by 94±71 μm at 12 months (p<0.001). Although the visual acuity was worse (p=0.034) at 12 months (0.62±0.26) than at 9 months (0.60±0.26), the mean CMT continued to decrease from month 6 to month 12 after the IVTA treatment.
Figure 2.
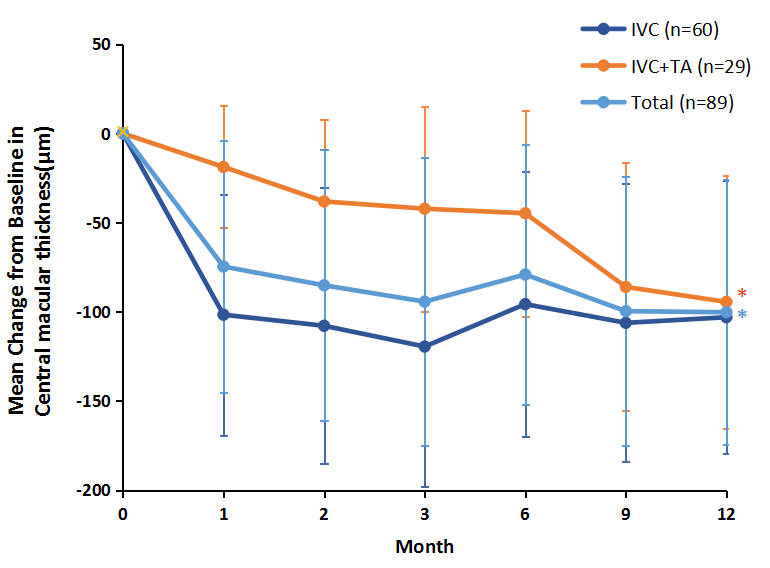
Mean change in central macular thickness (CMT) from baseline to month 9. Intravitreal triamcinolone acetonide (TA) injections were administered at month 6 after at least three consecutive intravitreal conbercept (IVC) injections. Data are expressed as mean ± standard deviation (SD). Wilcoxon matched-pairs test with Bonferroni corrections were used to compare the significance of the changes between baseline and each time point. *P <0.001 relative to the baseline of the CMT.
Table 3. CMT outcomes.
| Characteristic | Total | IVC | IVC+TA |
|---|---|---|---|
| No. of eyes (patients) |
89(76) |
60(50) |
29(26) |
| CMT at baseline (µm) |
456±128 |
454±133 |
460±121 |
| CMT at 6 mon (µm) |
377±87 |
359±83 |
415±83 |
| CMT at 1 year (µm) |
356±77 |
351±80 |
365±71 |
| Mean change in CMT from |
|
|
|
| baseline to month 6 (µm) |
-79±73 |
-96±74 |
-45±58 |
| Mean change in CMT from |
|
|
|
| month 6 to 1 year (µm) |
-21±35 |
-7±29 |
-50±29 |
| Mean change in CMT from |
|
|
|
| baseline to 1 year (µm) |
-100±74 |
-103±76 |
-94±71 |
| CMT improvement>100 µm at 1 year — no. (%) |
38 |
25 |
13 |
| CMT ≤300 µm at 1 year — no. (%) | 27(30) | 18(20) | 9(10) |
Plus–minus values are means ±SD.
Changes in IOP and cataracts
The elevation in IOP was noted only in the IVC plus IVTA group after treatment with IVTA at month 6. None of the IVC injection eyes showed statistically significant elevation within the follow-up 12 months. The average IOP values in the IVTA injection group (29 eyes) at the baseline visit and the last follow-up visit were 15.1±1.70 and 17.7±1.40 mmHg, respectively. The average IOP values at the baseline visit and the last follow-up visit were 15.1±1.70 and 17.7±1.40 mmHg, respectively. The IOP values were higher than 21 mmHg in four eyes at month 7, and in one eye at month 8. The TA-induced ocular hypertension in these five eyes (five patients) was subsequently effectively controlled to normal with a topical beta blocker (Betaxolol eye drops, Alcon-Couvreur, Puurs, Belgium). A total of six eyes showed posterior subcapsule opacification or aggravation of cataract at month 12, with the corresponding decline in BCVA.
Safety of intravitreal conbercept injection
A total of 89 eyes received intravitreal conbercept injections two or more times (an average of 4.0 injections, range 2.0–7.0). Within 1 year of follow-up, no serious injection complications or conbercept-related adverse ocular and systemic side effects were found in any of the patients in the study. None of the patients experienced retinal tears, vitreous hemorrhage, retinal detachment, endophthalmitis, uveitis, or elevation in IOP. No patients suffered cardiovascular and cerebrovascular accidents. The relatively common side effects included a slight subconjunctival hemorrhage at the site of injection (observed in 12 patients). There was no detectable difference concerning nonperfusion area in the macular from baseline to each follow-up visit.
Discussion
Conbercept was designed as a recombinant fusion protein that targets and binds to all VEGF-A and VEGF-B isoforms, and placental growth factors. Although several studies have confirmed the effect of conbercept for the treatment of several VEGF-related retinal diseases [21,22], similar studies concerning DME are still limited. In the present study, the primary purpose was to investigate the effect and safety of conbercept based on a practical protocol in the eyes of patients with DME, and the secondary aim was to evaluate the efficacy of low-dose triamcinolone acetonide on patients with refractory DME who showed little response to conbercept. The results showed a statistically significant improvement in BCVA and the central macular thickness after multiple IVC injections, with a mean number of IVC injections of 4.5±1.0. There were no complications throughout the entire IVC injection period. Moreover, low-dose triamcinolone acetonide was effective in patients with refractory DME, although this treatment was accompanied by several side effects. These results collectively suggest that intravitreous conbercept could significantly improve vision in eyes with center-involved diabetic macular edema, with no need for monthly treatment. Low-dose TA may be useful for the eyes of patients with refractory DME with poor initial response to anti-VEGF therapy, especially with the lack of other forms of sustained-release intravitreal steroids approved for the treatment of DME in China.
Since the discovery of anti-VEGF agents, tremendous effort has been devoted to exploring their clinical efficacy and safety for different retinal diseases, including DME and PDR [23-25]. In multiple representative clinical trials of DME, distinct dosages and administration frequencies of anti-VEGF drugs were reported in different populations. In the REVEAL and RESOLVE studies, 0.5 mg ranibizumab was administered for three consecutive monthly intravitreal injections, and then on pro re nata (PRN) basis in Asian and non-Asian patients with DME, respectively. Ranibizumab in the Treatment of Visual Impairment in Diabetic Macular Edema (REVEAL study). Safety and Efficacy of Ranibizumab in Diabetic Macular Edema (RESOLVE Study). The patients received approximately seven ranibizumab injections over 12 months [26,27]. In the VISTA and VIVID studies, more frequent injections were performed on patients with DME, and the mean number of anti-VEGF antibody (aflibercept) injections was nine to 12 times per year [28]. Study of Intravitreal Aflibercept Injection in Patients with Diabetic Macular Edema (VISTA study). Intravitreal Aflibercept Injection in Vision Impairment due to DME (VIVID study). However, prolonged monthly anti-VEGF therapy for patients with DME was revealed to lead to a possible increased risk for death, and potentially, for cerebrovascular incidents [29]. In addition, these participants from the phase III clinical study did not need to pay for the treatments. Given the expensive price per dose of conbercept (approximately US$1,000) without medical insurance in China, frequent or monthly injections of anti-VEGF agents are not affordable for the majority of patients with DME. There is an urgent demand for us to explore a more cost-effective and safer treatment based on a practical protocol. Patients with unilateral or bilateral DME received one to three consecutive monthly administration, followed by individualized PRN therapy, in this study. Of the total 89 eyes, 60 eyes (67.4%) received a mean of 4.5 IVC injections throughout the 1-year period. Conbercept showed a longer treatment interval in the results. The number of intravitreal injections was similar to those in the PRIDE study, another representative study of a practical protocol for treating DME. Ranibizumab for Visual Impairment due to Diabetic Macular Edema: Real-World Evidence in the Italian Population (PRIDE Study). In the PRIDE study, an average of 4.0 intravitreal ranibizumab (IVR) injections were delivered over an 18-month visit [30]. Recently, another clinical study from Japan showed that the mean IVR was 2.6, and the mean IVA was 2.7, for the 6-month experimental period for DME therapy [12].
Although fewer IVC injections were administered to the participants in the present study, relatively stable vision and structural improvement were acquired in the majority of patients at the last follow-up visit. The most significant improvement in BCVA and CMT occurred at month 3 after one to three consecutive monthly administrations. This peak time point of change was consistent with that of other studies in which patients received a loading phase of three consecutive injections for 3 months [31]. At the 12-month follow-up, the mean BCVA of the IVC group had increased from 0.71±0.29 logMAR to 0.62±0.26 logMAR, and the mean CMT had remarkably improved to more than 100 μm compared with the baseline level.
Notably, one-third of eyes (29 eyes) showed little change in BCVA or CMT after at least three consecutive monthly IVC treatments for half a year. These patients were further switched from anti-VEGF to steroid therapy at month 6, with one low-dose intravitreal TA injection as an ongoing therapy for DME in this retrospective study. The data suggested that corticosteroids induced a significant improvement in important visual function (BCVA) and macular morphology (CMT) with the peak value at 3 months. The effect was persistent, and then gradually declined at month 6 after the TA injection. These results are consistent with several previous reports showing the effectiveness of glucocorticoids, including TA, as well as the Intravitreal Dexamethasone Implant (Ozurdex®), for treating DME [32]. The present results collectively illustrate that the pathogenesis of DME is related not only to VEGF dependency but also to other underlying mechanisms suppressed by corticosteroids. We hypothesize that a high concentration of inflammatory factors, such as interleukin 6 (IL-6), IL-8, and IL-10, have a crucial effect on the pathogenesis of DME, and these cytokines were previously detected in the aqueous fluid of patients with DME [33,34]. Five eyes showed aggravating cataracts at the last follow-up visit after IVTA injection, which was in accordance with the final decline in BCVA. Nonetheless, we should not ignore the sustained improvement of the CMT value at month 12. This result also confirmed that intravitreal corticosteroids may represent an effective treatment for refractory DME, especially for patients with pseudophakic.
There were several limitations in the present study. It was a retrospective design with a relatively short-term follow-up period, and a limited number of participants. More prospective studies with a longer period and more appropriate time points for the treatment of DME are needed to verify the most efficient administration mode. Moreover, this study excluded patients who simultaneously received laser photocoagulation therapy during the 12-month follow-up, and these combination therapies are needed for comparison with the effects of conbercept in further studies.
In conclusion, this study revealed that conbercept is effective and safe for the treatment of a majority of patients with DME. Importantly, compared to previous phase III clinical studies, the present results showed fewer intravitreal conbercept injections and longer treatment intervals in clinical practice. Furthermore, a low dose of TA was effective for the treatment of patients with refractory DME resistant to anti-VEGF therapy.
Acknowledgments
The authors would like to thank the help of all their colleagues. Funding: This work was supported by the Science and Technology Project Foundation of Chongqing ( cstc2016jcyjA0597), the Science and Health Joint Medical Research Project Foundation of Chongqing (2019MSXM004), National Natural Science Foundation Project (grant no 81400390), National Natural Science Foundation Project (grant no 81870673), Chongqing Key Laboratory of Ophthalmology (CMTC, 2008CA5003). Author Contributions: The following authors were involved in the design of the study (Xuedong Zhang, Qingyun Zhou); collection (Chao Guo, Ailing You, Desai Wang), analysis (Qingyun Zhou), and interpretation (Qingyun Zhou) of the data; and preparation (Wenyan Wang), revision (Qingyun Zhou,), and approval of the manuscript (Qingyun Zhou, Xuedong Zhang).
References
- 1.Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246–58. doi: 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poh S, Mohamed Abdul RB, Lamoureux EL, Wong TY, Sabanayagam C. Metabolic syndrome and eye diseases. Diabetes Res Clin Pract. 2016;113:86–100. doi: 10.1016/j.diabres.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Singh A, Stewart JM. Pathophysiology of diabetic macular edema. Int Ophthalmol Clin. 2009;49:1–11. doi: 10.1097/IIO.0b013e31819fd164. [DOI] [PubMed] [Google Scholar]
- 4.Blum A, Socea D, Ben-Shushan RS, Keinan-Boker L, Naftali M, Segol G, Tamir S. A decrease in VEGF and inflammatory markers is associated with diabetic proliferative retinopathy. Eur Cytokine Netw. 2012;23:158–62. doi: 10.1684/ecn.2012.0321. [DOI] [PubMed] [Google Scholar]
- 5.Morera Y, Gonzalez R, Lamdan H, Perez L, Gonzalez Y, Aguero J, Castro J, Romero JC, Etchegoyen AY, Ayala M, Gavilondo JV. Vaccination with a mutated variant of human Vascular Endothelial Growth Factor (VEGF) blocks VEGF-induced retinal neovascularization in a rabbit experimental model. Exp Eye Res. 2014;122:102–9. doi: 10.1016/j.exer.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Wisniewska-Kruk J, Klaassen I, Vogels IM, Magno AL, Lai CM, Van Noorden CJ, Schlingemann RO, Rakoczy EP. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp Eye Res. 2014;122:123–31. doi: 10.1016/j.exer.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Aiello LP, Edwards AR, Beck RW, Bressler NM, Davis MD, Ferris F, Glassman AR, Ip MS, Miller KM. Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2010;117:946–53. doi: 10.1016/j.ophtha.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ercalik NY, Yenerel NM, Imamoglu S, Kumral ET, Vural ET. Combined Intravitreal Ranibizumab and Sub-Tenon Injection of Triamcinolone for the Treatment of Diabetic Macular Edema with Retinal Detachment. J Ocul Pharmacol Ther. 2016;32:225–9. doi: 10.1089/jop.2015.0092. [DOI] [PubMed] [Google Scholar]
- 9.Doi N, Sakamoto T, Sonoda Y, Yasuda M, Yonemoto K, Arimura N, Uchino E, Ishibashi T. Comparative study of vitrectomy versus intravitreous triamcinolone for diabetic macular edema on randomized paired-eyes. Graefe’s archive for clinical and experimental ophthalmology = Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2012;250:71–8. doi: 10.1007/s00417-011-1777-7. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto T, Hitani K, Tsukahara I, Yamamoto S, Kawasaki R, Yamashita H, Takeuchi S. Early postoperative retinal thickness changes and complications after vitrectomy for diabetic macular edema. Am J Ophthalmol. 2003;135:14–9. doi: 10.1016/s0002-9394(02)01819-6. [DOI] [PubMed] [Google Scholar]
- 11.Martin DF, Maguire MG. Treatment choice for diabetic macular edema. N Engl J Med. 2015;372:1260–1. doi: 10.1056/NEJMe1500351. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu N, Oshitari T, Tatsumi T, Takatsuna Y, Arai M, Sato E, Baba T, Yamamoto S. Comparisons of Efficacy of Intravitreal Aflibercept and Ranibizumab in Eyes with Diabetic Macular Edema. BioMed Res Int. 2017;2017:1747108. doi: 10.1155/2017/1747108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Hua R. Therapeutic Efficacy of Conbercept for Inflammatory Choroidal Neovascularization. J Ocul Pharmacol Ther. 2018;34:235–6. doi: 10.1089/jop.2017.0097. [DOI] [PubMed] [Google Scholar]
- 14.Qu J, Cheng Y, Li X, Yu L, Ke X. EFFICACY OF INTRAVITREAL INJECTION OF CONBERCEPT IN POLYPOIDAL CHOROIDAL VASCULOPATHY: Subgroup Analysis of the Aurora Study. Retina. 2016;36:926–37. doi: 10.1097/IAE.0000000000000875. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Tan Y, Li C, Liu Y, Lu G. Observation of curative effect of intravitreal injection of conbercept in wet age-related macular degeneration: Optical coherence tomography analysis after injection. Microsc Res Tech. 2018 doi: 10.1002/jemt.22989. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Xu G, Wang Y, Xu X, Liu X, Tang S, Zhang F, Zhang J, Tang L, Wu Q, Luo D, Ke X. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121:1740–7. doi: 10.1016/j.ophtha.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Sun X. Profile of conbercept in the treatment of neovascular age-related macular degeneration. Drug Des Devel Ther. 2015;9:2311–20. doi: 10.2147/DDDT.S67536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Zhang J, Yan M, Li H, Yang C, Yu D. Recombinant anti-vascular endothelial growth factor fusion protein efficiently suppresses choridal neovasularization in monkeys. Mol Vis. 2008;14:37–49. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Yang X, Jin H, Qu Y, Zhang Y, Liu K, Xu X. Changes in Retinal Nerve Fiber Layer Thickness after Multiple Injections of Novel VEGF Decoy Receptor Conbercept for Various Retinal Diseases. Sci Rep. 2016;6:38326. doi: 10.1038/srep38326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiello LP, Brucker AJ, Chang S, Cunningham ET, Jr, D’Amico DJ, Flynn HW, Jr, Grillone LR, Hutcherson S, Liebmann JM, O’Brien TP, Scott IU, Spaide RF, Ta C, Trese MT. Evolving guidelines for intravitreous injections. Retina. 2004;24:S3–19. doi: 10.1097/00006982-200410001-00002. [DOI] [PubMed] [Google Scholar]
- 21.Jin E, Bai Y, Luo L, Huang L, Zhu X, Ding X, Qi H, Zhao M. Serum Levels of Vascular Endothelial Growth Factor before and after Intravitreal Injection of Ranibizumab or Conbercept for Neovascular Age-Related Macular Degeneration. Retina. 2017;37:971–7. doi: 10.1097/IAE.0000000000001274. [DOI] [PubMed] [Google Scholar]
- 22.Peng Y, Zhang X, Mi L, Liu B, Zuo C, Li M, Wen F. Efficacy and safety of conbercept as a primary treatment for choroidal neovascularization secondary to punctate inner choroidopathy. BMC Ophthalmol. 2017;17:87. doi: 10.1186/s12886-017-0481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rouvas A, Petrou P, Ntouraki A, Douvali M, Ladas I, Vergados I. Intravitreal ranibizumab (Lucentis) for branch retinal vein occlusion-induced macular edema: nine-month results of a prospective study. Retina. 2010;30:893–902. doi: 10.1097/IAE.0b013e3181cd4894. [DOI] [PubMed] [Google Scholar]
- 24.Stewart MW. A Review of Ranibizumab for the Treatment of Diabetic Retinopathy. Ophthalmol Ther. 2017;6:33–47. doi: 10.1007/s40123-017-0083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amstutz CA, Fleischhauer J, Zweifel S, Barthelmes D. Klin Monatsbl Augenheilkd. 2015;232:533–7. doi: 10.1055/s-0035-1545673. Long-Term Outcome in Patients with Intravitreal Anti-VEGF Therapy for Exudative AMD. [DOI] [PubMed] [Google Scholar]
- 26.Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, Ma Z, Ohji M, Tan N, Cha SB, Shamsazar J, Yau CL. The REVEAL Study: Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy in Asian Patients with Diabetic Macular Edema. Ophthalmology. 2015;122:1402–15. doi: 10.1016/j.ophtha.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, Heier JS, Terasaki H, Kaiser PK, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Korobelnik JF. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. •••;122:2044–52. doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Avery RL, Gordon GM. Systemic Safety of Prolonged Monthly Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2016;134:21–9. doi: 10.1001/jamaophthalmol.2015.4070. [DOI] [PubMed] [Google Scholar]
- 30.Menchini U, Bandello F, De Angelis V, Ricci F, Bonavia L, Viola F, Muscianisi E, Nicolo M. Ranibizumab for Visual Impairment due to Diabetic Macular Edema: Real-World Evidence in the Italian Population (PRIDE Study). J Ophthalmol. 2015;2015:324841. doi: 10.1155/2015/324841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, Rong A, Xu W, Niu Y, Wang Z. Comparison of 12-month therapeutic effect of conbercept and ranibizumab for diabetic macular edema: a real-life clinical practice study. BMC Ophthalmol. 2017;17:158. doi: 10.1186/s12886-017-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacella F, Romano MR, Turchetti P, Tarquini G, Carnovale A, Mollicone A, Mastromatteo A, Pacella E. An eighteen-month follow-up study on the effects of Intravitreal Dexamethasone Implant in diabetic macular edema refractory to anti-VEGF therapy. Int J Ophthalmol. 2016;9:1427–32. doi: 10.18240/ijo.2016.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohn HJ, Han DH, Kim IT, Oh IK, Kim KH, Lee DY, Nam DH. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011;152:686–94. doi: 10.1016/j.ajo.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Kim M, Kim Y, Lee SJ. Comparison of aqueous concentrations of angiogenic and in fl ammatory cytokines based on optical coherence tomography patterns of diabetic macular edema. Indian J Ophthalmol. 2015;63:312–7. doi: 10.4103/0301-4738.158069. [DOI] [PMC free article] [PubMed] [Google Scholar]


