Abstract
Purpose
Internet-based cognitive behavioral therapy (iCBT), with and without therapist support, is effective in reducing treatment-induced menopausal symptoms and perceived impact of hot flushes and night sweats (HF/NS) in breast cancer survivors. The aim of the current study was to evaluate the cost-utility, cost-effectiveness, and budget impact of both iCBT formats compared to a waiting list control group from the Dutch healthcare perspective.
Methods
A Markov model was constructed with a 5-year time horizon. Costs and health outcomes were measured alongside a randomized controlled clinical trial and included quality-adjusted life years (QALYs), overall levels of menopausal symptoms, and perceived impact of HF/NS. Uncertainty was examined using probabilistic and deterministic sensitivity analyses, together with a scenario analysis incorporating a different perspective.
Results
iCBT was slightly more expensive than the waiting list control, but also more effective, resulting in incremental cost-utility ratios of €23,331/QALY and €11,277/QALY for the guided and self-managed formats, respectively. A significant reduction in overall levels of menopausal symptoms or perceived impact of HF/NS resulted in incremental costs between €1460 and €1525 for the guided and €500–€753 for the self-managed format. The estimated annual budget impact for the Netherlands was €192,990 for the guided and €74,592 for the self-managed format.
Conclusion
Based on the current trial data, the results indicate that both guided and self-managed iCBT are cost-effective with a willingness-to-pay threshold of well below €30,000/QALY. Additionally, self-managed iCBT is the most cost-effective strategy and has a lower impact on healthcare budgets.
Keywords: Cost-effectiveness, Budget impact, Menopause, Breast cancer, Cognitive behavioral therapy, Internet-based
Introduction
Adjuvant treatments for breast cancer (BC), including chemotherapy, endocrine therapy, and oophorectomy can lead to treatment-induced menopausal symptoms [1, 2]. These symptoms, and in particular hot flushes and night sweats (HF/NS), negatively affect health-related quality of life (HRQL) [3–5] and cause some women to discontinue their endocrine treatments [6, 7]. Although medications such as gabapentin, clonidine, and antidepressants are moderately effective in reducing HF/NS, they are accompanied by bothersome side effects [8–11]. In contrast, cognitive behavioral therapy (CBT) programs are without side effects, are effective, and are favored by BC survivors [12–16].
CBT programs have often been delivered in group format [14–16]. However, BC survivors have reported practical and scheduling barriers to attending such group sessions [16]. Therefore, these programs have been translated into an online format [17, 18]. Our recent randomized controlled trial (RCT) comparing Internet-based CBT (iCBT), with and without therapist support, with a waiting list control group demonstrated that women allocated to iCBT experienced a greater reduction in overall levels of menopausal symptoms and perceived impact of HF/NS. Significant reductions in the frequency of HF/NS and improvement in sleep quality were also observed [19]. When asked about preferences for a specific format, only a minority of women showed a strong preference for guided (16%) or self-managed (21%) iCBT. Although the magnitude of the effects favored the guided over the self-managed iCBT group, the former is associated with higher costs due to the added therapist support.
The observed differences in effectiveness and costs between the iCBT formats and the reality of budget restrictions underscore the need for an economic evaluation to assist policymakers in deciding whether to allocate healthcare resources to this program. Moreover, it may also guide practitioners in choosing which specific format to adopt [20]. Although a previous study by Mewes et al. [21] indicated that face-to-face group-based CBT was cost-effective, it is unknown whether online-delivered CBT will lead to favorable cost-effectiveness ratios as well. Moreover, there are no studies reporting the budget impact of iCBT for treatment-induced menopausal symptoms, commonly used to estimate the impact on national, regional, or local health budget plans [22].
The objective of the current study was to evaluate the cost-utility and cost-effectiveness of guided and self-managed iCBT compared to a waiting list control group in terms of quality-adjusted life years (QALYs) and the primary clinical outcomes of the associated RCT (i.e., overall levels of menopausal symptoms and perceived impact of HF/NS), incorporating a healthcare perspective over a 5-year time period. An additional aim was to establish the estimated annual budget impact of implementing guided and/or self-managed iCBT in the Netherlands.
Methods
Research design and study sample
A detailed description of the design, interventions, and outcomes of the RCT is provided elsewhere [18, 19]. Briefly, from 2015 to 2017, an RCT was conducted to evaluate the efficacy of iCBT, with and without therapist support, for treatment-induced menopausal symptoms in BC survivors. Patients were recruited from 12 hospitals in the Netherlands. Upon return of the informed consent and the baseline questionnaire (T0), 254 patients were randomized to a guided iCBT group, self-managed iCBT group, or a waiting list control group. Follow-up assessments were administered at 10 weeks (T1) and 24 weeks post-randomization (T2). All institutional review boards approved the study.
Intervention and waiting list control group
All women randomized to the intervention groups had access to a 6-week iCBT program. A strong emphasis was placed on HF/NS, but other symptoms were also addressed. Women in the guided iCBT group received an additional telephone intake and weekly online feedback from a therapist. The average time-investment per therapist was 3 h per patient. Participants allocated to the waiting list control group received usual care, which did not involve any form of care aimed at coping with menopausal symptoms.
Measures
Measurement and valuation of outcomes
HRQOL was assessed using the 36-item Short Form Health Survey (SF-36) [23, 24]. To obtain utilities, scores on the eight scales were transformed into a single EQ-5D utility score using the mapping algorithm of Ara and Brazier [25]. An additional algorithm was used to verify reliability of this conversion [26]. The EQ-5D utility scores can range between 0 and 1, with higher scores indicating better health. To calculate QALYs, we multiplied the derived utility scores with years of life (mortality rates) in the relevant health states.
The menopause-specific measures included overall levels of menopausal symptoms, as assessed by the Functional Assessment of Cancer Treatment-Endocrine Symptoms (FACT-ES) [27], and the perceived impact of HF/NS as assessed by the problem rating subscale of the Hot Flush Rating Scale (HFRS) [28]. A clinically significant improvement was defined as a 0.5 standard deviation (SD) improvement for both measures [19, 27, 29, 30].
Measurement and valuation of costs
Costs of the iCBT program were related to the online platform and therapist support. The online costs for the guided iCBT program were dependent on the number of therapists, irrespective of the number of patients, whereas the number of patients determined the online costs for the self-managed format. Valuations of the resources used were based on cost information provided by two potential providers of iCBT in the Netherlands, and invoices obtained during the RCT (e.g., hourly therapist rates).
Direct healthcare costs were measured during the RCT by the Dutch iMTA Medical Consumption Questionnaire (iMCQ) [31]. Healthcare costs included the average number of visits to a range of healthcare providers (general practitioner, medical specialist, psychologist/psychiatrist, social worker, physiotherapist, lymphedema therapist, dietitian, and a practitioner of complementary alternative medicine). Valuation of visits to healthcare providers was based on the Dutch costing manual for economic evaluations [32, 33]. Mean per patient resource use and valuation can be found in Table 1. Both types of costs (intervention and healthcare utilization) were applied in the healthcare perspective.
Table 1.
Input cost parameters in the MARKOV model
| Parameters | Mean | Standard error | Distribution | Sources |
|---|---|---|---|---|
| Utilities | ||||
| Menopausal symptoms | 0.83 | 0.013 | Beta | [19] |
| Reduction in menopausal symptoms | 0.85 | 0.017 | Beta | [19] |
| Recurrence | 0.73 | 0.020 | Beta | [54] |
| Transition probabilities | ||||
| Menopausal symptoms to reduction in menopausal symptoms (guided iCBT) | 0.44 | – | Dirichlet | [19] |
| Menopausal symptoms to reduction in menopausal symptoms (self-managed iCBT) | 0.39 | – | Dirichlet | [19] |
| Menopausal symptoms to reduction in menopausal symptoms (waitlist control group iCBT) | 0.23 | – | Dirichlet | [19] |
| To recurrence from either state of menopausal symptoms or reduction in menopausal symptoms | 0.01 | – | Beta | [35] |
| Recurrence to death | 0.04 | – | Beta | [36] |
| Background mortality (age 47 to 51) | 0.0007–0.0012 | – | Fixed | |
| Intervention costsa, b | ||||
| Online platform costs (guided iCBT) | € 12.59 | – | – | Practice |
| Online platform costs (self-managed iCBT) | € 33.28 | – | – | Practice |
| Training costs therapists | € 9.42 | – | – | Practice |
| Hourly rate therapist support (in total 3 h needed to support patient) | € 135.00 | – | – | Practice |
| Total costs guided iCBT per patient without overhead costs | € 157.01 | – | – | Practice |
| Total costs self-managed iCBT per patient without overhead costs | € 33.28 | – | – | Practice |
| Total costs guided iCBT per patient with 44% overhead costs | € 226.09 | ± 20% | Gamma | Practice |
| Total costs self-managed iCBT per patient with 44% overhead costs | € 47.92 | ± 20% | Gamma | Practice |
| Health care costs | ||||
| Health state: menopausal symptoms | ||||
| General practitioner | € 48.70 | ± 20% | Gamma | [19, 33] |
| Medical specialist | € 152.00 | ± 20% | Gamma | [19, 33] |
| Psychologist or psychiatrist | € 35.20 | ± 20% | Gamma | [19, 33] |
| Social worker | € 3.25 | ± 20% | Gamma | [19, 33] |
| Physiotherapist | € 207.78 | ± 20% | Gamma | [19, 33] |
| Lymphedema therapist | € 106.01 | ± 20% | Gamma | [19, 33] |
| Dietitian | € 18.74 | ± 20% | Gamma | [19, 33] |
| Alternative medicine | € 8.96 | ± 20% | Gamma | [19, 33] |
| Health state: reduction in menopausal symptoms | ||||
| General practitioner | € 45.38 | ± 20% | Gamma | [19, 33] |
| Medical specialist | € 129.28 | ± 20% | Gamma | [19, 33] |
| Psychologist or psychiatrist | € 43.37 | ± 20% | Gamma | [19, 33] |
| Social worker | € 9.47 | ± 20% | Gamma | [19, 33] |
| Physiotherapist | € 158.05 | ± 20% | Gamma | [19, 33] |
| Lymphedema therapist | € 93.75 | ± 20% | Gamma | [19, 33] |
| Dietitian | € 4.99 | ± 20% | Gamma | [19, 33] |
| Alternative medicine | € 19.11 | ± 20% | Gamma | [19, 33] |
| Health state: recurrence | ||||
| Frist year: in- and outpatient costs | € 10,263.00 | ± 20% | Gamma | [55] |
| First year: drug costs | € 1918.00 | ± 20% | Gamma | [55] |
| Second year: in- and outpatient costs | € 2294.00 | ± 20% | Gamma | [55] |
| Second year: drug costs | € 65.00 | ± 20% | Gamma | [55] |
iCBT Internet-based cognitive behavioral therapy
aAssumption that 600 patients enroll in iCBT
bOnline platform costs are dependent on the therapists in the guided format, whereas these costs are dependent on the number of patients in the self-managed format
Statistical analyses
Markov model
We adapted a previously developed and validated Markov model in Excel (Microsoft, Redmond, WA) in accordance with the Dutch guideline for health economic evaluations and international guidelines for modelling (ISPOR-SMDM guidelines) [22, 33, 34]. A Markov model is a stochastic approach to modelling different states and the probabilities of transitions among them (Appendix A). The following four health states were defined in the current study: (1) experience of menopausal symptoms (based on inclusion criteria of the RCT); (2) reduction in menopausal symptoms; (3) cancer recurrence (local, regional or distant); and (4) death. Transition probabilities are displayed in Table 1. The transition probabilities between the first two health states were based on the percentage of women with a clinically significant improvement per trial arm on the FACT-ES as reported by Atema et al. [19]. Transition probabilities for local, regional, and distant metastases and corresponding increased mortality rate (MR), using age- and sex-specific mortality data, were based on data from Dutch registries [35, 36].
A hypothetical cohort of 1000 patients was used in the model with an average baseline age of 47, mirroring the mean age of participants at the start of the RCT. They were analyzed over ten consecutive 6-month cycles in which the first cycle reflected the costs and effects of the iCBT as derived from the RCT [19]. The 5-year time horizon corresponds to the average duration of bothersome vasomotor symptoms of menopause [37]. The transition from health state ‘menopausal symptoms’ to ‘reduction in menopausal symptoms’ derived from the trial was only applied in the first cycle of the model. All other transitions remained applicable during consecutive cycles (Appendix A).
Cost-utility analysis
Incremental cost-utility ratios (ICURs) of both formats of the iCBT were calculated as follows:
The diversity of willingness-to-pay (WTP) thresholds among countries shows that there is no uniformly accepted value. However, the World Health Organization has proposed a WTP threshold of one to three times the annual GDP per capita [38, 39]. Therefore, we estimated a WTP ceiling ratio of €30,000 per QALY for this study. Effects were discounted at 1.5% and costs at 4% annually as recommended by the Dutch costing manual [33].
Cost-effectiveness analysis
We also performed a cost-effectiveness analysis using the principles of number needed to treat (NNT). NNT expresses how many patients, on average, need to be treated for one less adverse event or improvement of disease to be observed at a specific point in time [40, 41]. To calculate NNT and associated costs, we used the following formulas:
The incremental costs to treat one patient reflect the costs per person of guided or self-managed iCBT over a 5-year period multiplied by the NNT to obtain one clinically significant reduction on the FACT-ES or HFRS problem rating scale.
Budget impact analysis
We performed the budget impact analysis (BIA) in accordance with ISPOR principles of good practice [22, 42]. The incremental costs were calculated using the same assumptions and model that we developed for the cost-utility analysis. We then multiplied the incremental costs by the target population in the Netherlands, which we based on previous studies [35, 43–45]. We calculated that approximately 20% of the target population (3000 invasive BC cases in women aged ≤ 50 years) will start to use the iCBT program when offered in routine care, which corresponds to 600 patients per year in the Netherlands [35]. Therefore, the current BIA reflects the annual budget impact on the Dutch healthcare system.
Sensitivity analyses
We used probabilistic sensitivity analysis (PSA) to estimate the uncertainty of the input parameters of the model using 5000 Monte Carlo simulations. We used Dirichlet, gamma, and beta distributions to estimate the uncertainty around transition probabilities, costs, and utilities, respectively. Uncertainty surrounding the ICURs was explored by plotting bootstrapped incremental cost-utility pairs on cost-effectiveness planes (CE-planes). A summary measure of the joint uncertainty of costs and effects for different thresholds was presented using cost-effectiveness acceptability curves (CEACs). CEACs indicate the intervention’s probability of being cost-effective compared with the waiting list control group at different values of WTP. Additionally, we examined deterministic one-way sensitivity and structural uncertainty by addressing various assumptions regarding the model such as the duration of the treatment effects (from 5 to 3 years), different healthcare costs, QALYs, and intervention costs. Corresponding parameters were based on the trial data (e.g., standard errors), ranging between two extreme yet plausible values. These analyses are displayed in tornado diagrams for both guided and self-managed iCBT separately.
We also conducted a scenario analysis in which we calculated cost-utility, cost-effectiveness, and budget impact from an intervention perspective by using only the intervention costs, meaning that we did not take into account healthcare utilization (e.g., general practitioner visits).
Results
Costs and QALYs
Total intervention costs for guided and self-managed iCBT were €226 and €48 per patient, respectively (Table 1). At longer-term follow-up of the RCT, healthcare costs were higher in the ‘Reduction in Menopausal’ state as compared to the state ‘Menopausal Symptoms’ (Table 1). For a 5-year time horizon, total healthcare costs were €5315.55, €5118.22, and €4993.90 for guided iCBT, self-managed iCBT, and the waiting list control group, respectively (Table 3).
Table 3.
Incremental cost-effectiveness results using NNT
| Guided iCBT | Self-managed iCBT | |
|---|---|---|
| Significant reduction on the FACT-ESa | ||
| Number needed to treat (NNT) | 4.74 | 6.06 |
| Incremental intervention costs | € 226.09 | € 47.92 |
| Incremental total costs (total healthcare) | € 321.65 | € 124.32 |
| Total incremental costs to treat one patient (intervention perspective) | € 1071.51 | € 290.39 |
| Total incremental costs to treat one patient (healthcare perspective) | € 1524.62 | € 753.38 |
| Significant reduction on the HFRS problem rating scalea | ||
| Number needed to treat (NNT) | 4.54 | 4.02 |
| Incremental intervention costs | € 226.09 | € 47.92 |
| Incremental total costs (total healthcare) | € 321.65 | €124.32 |
| Total incremental costs to treat one patient (intervention perspective) | € 1026.45 | € 192.64 |
| Total incremental costs to treat one patient (healthcare perspective) | € 1460.29 | € 499.77 |
NNT number needed to treat, FACT-ES functional assessment of cancer treatment-endocrine symptoms, HFRS hot flush rating scale
aWaiting list control group is reference category
The average 5-year QALY score was 4.119, 4.117, and 4.106 for guided iCBT, self-managed ICB, and the waiting list control group, respectively (Table 3).
Cost-utility analyses
The results indicated ICURs of €23,331/QALY and €11,277/QALY for guided and self-managed iCBT, respectively (Table 2). Descriptive CEACs and iCE planes are displayed in Figs. 1 and 2, and described in the ‘sensitivity analyses’ section. For the intervention scenario, the ICURs were €16,399/QALY and €4346/QALY for guided and self-managed iCBT, respectively.
Table 2.
Deterministic incremental cost-utility results and budget impact analyses for the base-case (FACT-ES)
| Costs | QALY | Incremental costs | Incremental QALYs | ICER | BIAb | |
|---|---|---|---|---|---|---|
| Healthcare perspective | ||||||
| Guided iCBT | €5315.55 | 4.119 | €321.65 | 0.0138 | €23,330.50 | €192,990 |
| Self-managed iCBT | €5118.22 | 4.117 | €124.32 | 0.01102 | €11,277.63 | €74,592 |
| Waiting list controla | €4993.90 | 4.106 | n/a | n/a | n/a | n/a |
| Scenario analysis: intervention perspective | ||||||
| Guided iCBT | €226.09 | 4.119 | €226.09 | 0.0138 | €16,399.45 | €135,654 |
| Self-managed iCBT | €47.92 | 4.117 | €47.92 | 0.01102 | €4,346.58 | €28,752 |
| Waiting list controla | €0.00 | 4.106 | n/a | n/a | n/a | n/a |
BIA budget impact analysis, iCBT Internet-based cognitive behavioral therapy, ICER incremental cost-utility ratio, QALY quality-adjusted life year, n/a not applicable
aGuided and self-managed interventions are compared with waiting list control group
bEstimated that 600 patients per year will use the intervention in the Netherlands
Fig. 1.
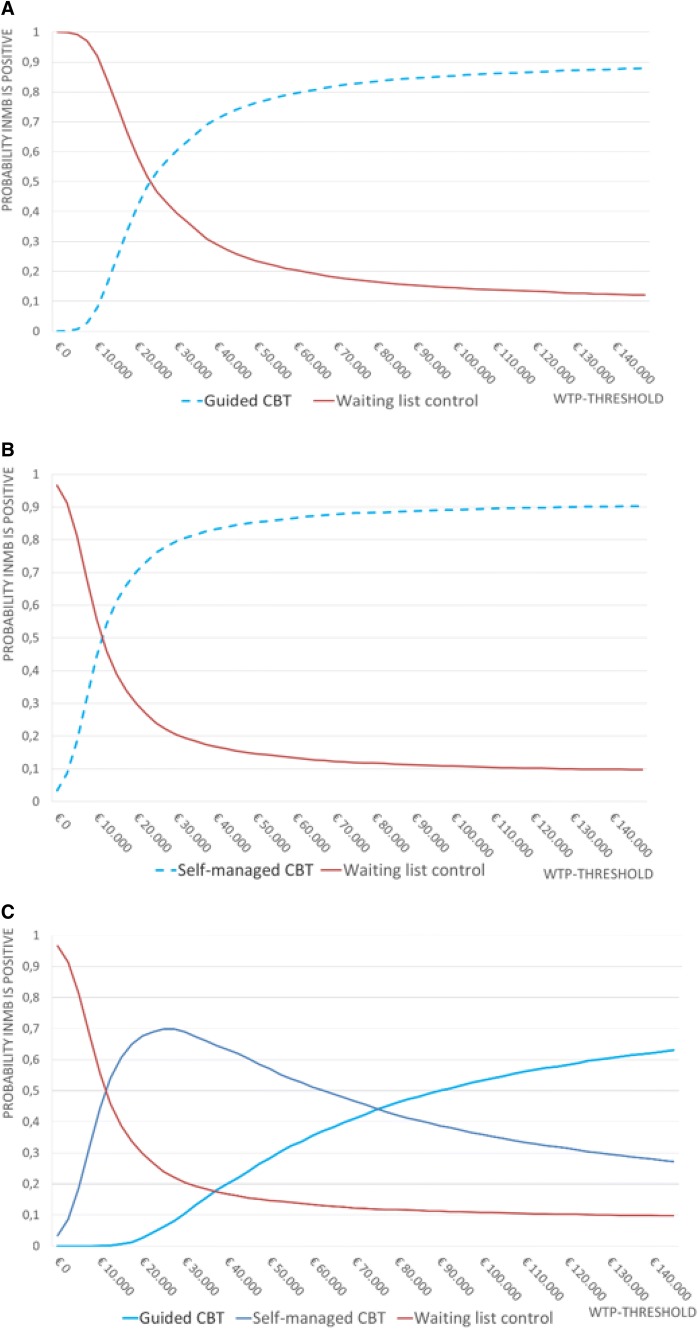
Cost-effectiveness Acceptability Curves (CEAC); presenting the probability of cost-effectiveness for a range of willingness-to-pay (WTP) thresholds of guided iCBT compared to waiting list control group (a), self-managed iCBT compared to waiting list control group (b), and self-managed versus guided versus waiting list control group (c). For a WTP threshold of 30,000 per QALY, guided and self-managed iCBT are superior over waiting list control group with a probability of 60.5% and 79.5%, respectively (a, b), and the self-managed variant is superior when comparing to both waiting list control group and guided iCBT simultaneously with a probability of 68.9% (c)
Fig. 2.
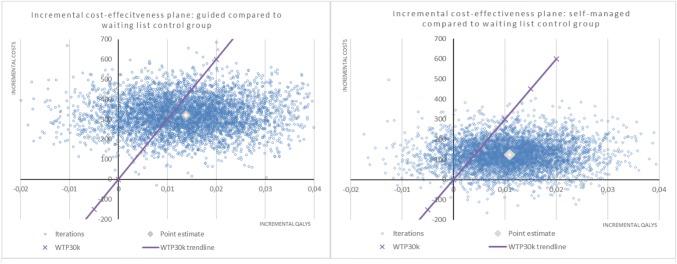
Incremental cost-effectiveness planes of the quality-adjusted life years (QALYs) per costs of the self-managed and guided iCBT intervention groups compared to a waiting list control group. The scatter plots are showing the mean differences in costs and outcomes from the data using 5000 bootstrap replicates. Ninety-two and eighty-nine percent of the dots are in the North-East quadrant of the plane for the guided and self-managed iCBT interventions, respectively. This indicates that there is a high probability that both treatments are more effective and more expensive compared to a waiting list control group
Cost-effectiveness analyses
NNT calculations indicated that relatively fewer patients needed to be treated to obtain a significant reduction in menopausal symptoms (FACT-ES) in the guided iCBT format compared to the self-managed format (4.74 vs. 6.06) (Table 3). The associated costs were higher for the guided iCBT than for the self-managed iCBT (€322 vs. €124). Therefore, total incremental treatment costs to obtain a significant decrease in menopausal symptoms were smaller for the self-managed format than for the guided (€753 vs. €1,525). The same trend was observed for the intervention scenario in which incremental costs were lower for the self-managed than the guided iCBT (€290 vs. €1072).
The NNT to accomplish a significant reduction in the perceived impact of HF/NS (HFRS problem rating scale) favored the self-managed iCBT over the guided iCBT (4.54 vs. 4.02) (Table 3). Again, total incremental costs to obtain a significant decrease in the perceived impact of HF/NS were smaller for the self-managed iCBT than for the guided iCBT (€500 vs. €1460). Results for the intervention scenario indicated a similar pattern in which the incremental costs for the self-managed iCBT were lower than for the guided format (€193 vs. €1026).
Budget impact analyses
The budget impact of treating the Dutch target population (assuming 600 patients) with guided iCBT would result in an annual net increase of €192,990 of additional expenditure from the Dutch healthcare budget to the target population. The budget impact of self-managed iCBT would result in a net increase of €74,592 (Table 2). Additionally, total health expenditure of implementing a 50/50 combination of the guided and self-managed iCBT in the Dutch setting would entail an additional cost of €133,785. Results for the intervention scenario indicated a higher 1-year net increase for the guided and the self-managed iCBT €135,654 and €28,752, respectively, and a net increase of €49,322 when implementing a combination of the guided and self-managed iCBT formats.
Sensitivity analyses
The CEACs indicate that guided iCBT has a 60.5% probability of being cost-effective for a WTP of €30,000 (Fig. 1a). For self-managed iCBT this probability is 79.5% (Fig. 1b). Moreover, the combined CEAC indicates that self-managed iCBT has a 68.9% of being superior over guided iCBT and waiting list control with a WTP of €30,000 (Fig. 1c). For the scenario analyses (intervention perspective), the probability of cost-effectiveness for self-managed and guided iCBT is 88.8% and 72.9%, respectively, when using a WTP of €30,000 (data not shown).
The iCE planes resulted in most iterations being in the North-East quadrant (around 90%), indicating that both guided and self-managed iCBT resulted in higher costs and more QALYs (Fig. 2). Moreover, the point estimates indicated that it is likely that both self-managed and guided iCBT will be below the €30,000/QALY threshold. Results from an intervention perspective indicated a similar pattern, with an average probability of 92% of being in the North-East quadrant (data not shown).
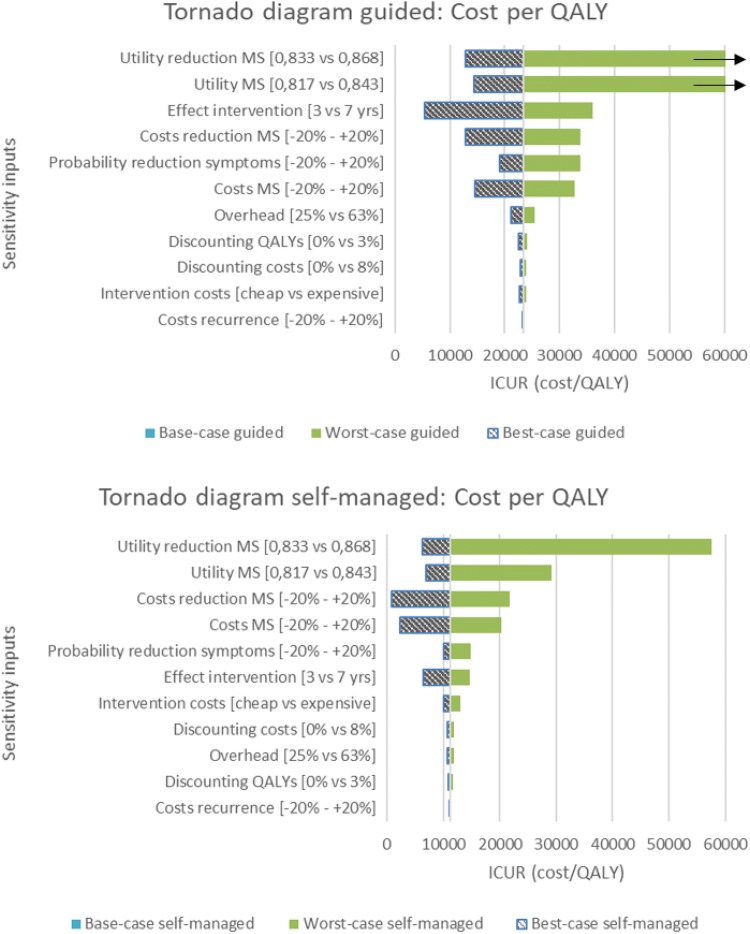
In the sensitivity analysis, the parameters of the costs and utilities associated with the states ‘Menopausal Symptoms’ and ‘Reduction in Menopausal Symptoms’ alongside the duration of intervention effects and transition probabilities showed the greatest influence on the ICER. The tornado diagrams show the impact of the uncertainty per input parameter (Fig. 3).
Fig. 3.
Tornado diagrams. This figure presents several univariate sensitivity analyses for both guided and self-managed iCBT. Parameters are ranked according to impact on incremental cost-utility ratio (ICUR). Results show that the utility attributed to the states ‘Reduction in Menopausal Symptoms (MS)’ and ‘Menopausal Symptoms’, the effect of the intervention lasting shmter/longer, transition probabilities, and the costs of states ‘Reduction in Menopausal Symptoms’ and ‘Menopausal Symptoms’ affect the ICUR the most. Moreover, self-managed iCBT seems to be more resistant to univariate differences in the model compared to guided iCBT
Discussion
This is the first study to investigate the cost-utility, cost-effectiveness, and budget impact of iCBT to alleviate treatment-induced menopausal symptoms in BC survivors. The results show that both the guided and self-managed formats of iCBT are associated with a small gain in QALYs over a 5-year time horizon, a decrease in menopausal symptoms, and a decrease in perceived impact of HF/NS. These improvements were accompanied with an increase in costs due to additional intervention and healthcare costs. However, analyses showed that ICURs are well below the proposed international WTP threshold of €30,000/QALY for both formats [39]. The probability that the ICERs are considered acceptable ultimately depends on the willingness to pay for a clinically significant decrease in menopausal symptoms and/or perceived HF/NS. Our results indicate that, to accomplish a significant reduction in overall levels of menopausal symptoms or perceived impact of HF/NS, an investment between €1026 and €1525 for the guided and €193–€753 for the self-managed iCBT format will be necessary (the range reflects the perspective, i.e., only intervention costs, or intervention and healthcare costs). The annual Dutch budget impact (i.e., treating 600 patients) of implementing this program is estimated to be between €74,592 and €192,990 for the guided and between €28,752 and €74.592 for the self-managed iCBT. Additionally, sensitivity analyses showed that self-managed iCBT remains cost-effective (below the threshold of €30,000/QALY) for all variations in input parameters and assumptions, except when utility in the state ‘Reduction in Menopausal Symptoms’ decreases to its lower extreme value. For guided iCBT, shorter duration of intervention effects, increase in costs, decrease in utilities, and decrease in probability of obtaining a reduction in menopausal symptoms may result in unacceptable cost-effectiveness ratios, i.e., around €35,000/QALY or even higher ratios when utilities decrease unfavorably.
Compared to the economic evaluation of the group-based CBT program for alleviating menopausal symptoms in BC survivors [21], we observed similar costs per QALY outcomes for the guided format, but a reduction of more than €10,000/QALY for self-managed iCBT. We also observed higher incremental costs per clinically significant reduction in overall levels of menopausal symptoms and perceived impact of HF/NS for the guided format (± €500), and lower incremental costs per clinically significant reduction for the self-managed iCBT, when compared with the group-based CBT format [21]. This indicates that an Internet-delivered CBT program, particularly when self-managed, would be a viable alternative to face-to-face group sessions, with the added advantage of decreasing practical barriers as previously reported that hamper attendance at group sessions [16, 21]. In addition, the estimated budget impact is low in comparison with the total healthcare costs associated with the treatment of cancer in the Netherlands [46].
The increase in QALYs observed in our study and that of Mewes et al. [21] are relatively small. We believe this to be inherent to the aim of the current program, which is not primarily focused on improving overall HRQL, but rather on reducing overall levels of menopausal symptoms and perceived impact of HF/NS. When using a generic indicator of HRQL such as the SF-36, important gains in more specific domains are often missed due to the lack of responsiveness of the instrument [47], hence explaining the results from the deterministic sensitivity analysis. Therefore, cost-utility analyses should be supplemented by cost-effectiveness analyses in which the cost per condition-specific outcome are measured and taken into account in reimbursement decisions. Moreover, we encourage the development and testing of condition-specific preference-based instruments which can be used within the QALY framework [47].
Based on our findings, we would recommend implementing the iCBT program according to a stepped care approach [48] in which the self-managed program serves as the primary treatment option. Dependent on available budgets, patient preferences, and support needs, the iCBT program could be supplemented by therapist support. To keep the related costs of this guided format to a minimum, it is advisable to centralize the program within a limited number of treatment centers and have a relatively limited number of trained therapists. Future research is needed to be able to predict which women will benefit most from which format. Finally, as many BC survivors report a range of (interrelated) psychosocial and physical problems [49–51], we would recommend efforts to combine and integrate various iCBT interventions (e.g., for cancer-related fatigue, sleep problems, etc.) to better serve BC survivors and possibly reduce overall costs of psychosocial care in oncology settings.
Limitations and strengths
This study has some limitations that should be noted. First, due to a lack of data, we did not include costs related to medication uptake. However, based on Mewes and colleagues [21], we expect these costs to be relatively low and similar across the intervention and control group. Second, we assessed healthcare consumption via generic questions that did not inquire specifically about the reason for utilization. It is likely that the differences in healthcare costs may not so much reflect the costs associated with the different formats of the iCBT program, but rather other factors. Third, there is increasing interest in conducting economic evaluations from a societal perspective, including costs associated with, among other things, productivity loss [52, 53]. While we had planned to include this perspective, it was evident to us that the productivity losses that were found during the trial could not be attributed to menopausal symptoms, but mainly to comorbid health conditions with which many BC survivors are faced.
This study also had noteworthy strengths. These included the RCT design, multicenter participation, high response rates, including both a healthcare and intervention perspective, evaluating both cost-utility, cost-effectiveness, and budget impact, and incorporating the intervention specific endpoints.
Conclusion
This economic evaluation of guided and self-managed iCBT supports its cost-effectiveness in three respects. First, the cost-utility analysis indicates a cost per QALY well below frequently used thresholds. Second, the cost to obtain a clinically relevant reduction of menopausal symptoms and/or perceived impact of HF/NS is modest for both formats. Third, the budget impact of both programs is negligible when compared to the total healthcare expenditure for treating cancer in the Netherlands. Additionally, while treatment effects were only slightly greater in the guided format, the self-managed format was associated with substantially lower costs and more stable results when testing various assumptions and/or parameters in sensitivity analyses. Taken together, our results tend to favor the self-managed version of the iCBT program over the guided format, and thus we would favor a stepped care approach in which the self-managed version of the program is the default option, with the guided version being reserved for those situations where women have a strong preference for such support and where sufficient funding is available for the additional costs involved.
Appendix A
Schematic representation of the model structure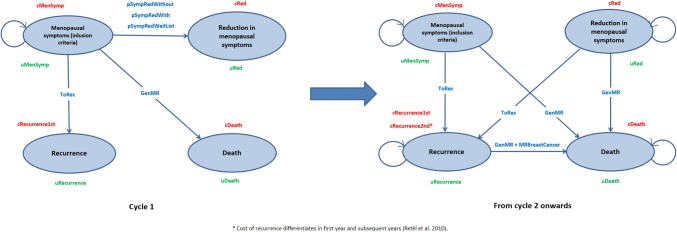
Abbreviations
Costs associated with health states
cMenSympt: costs of group with menopausal symptoms
cRed: costs of group with a clinically significant reduction
cRecuccernce1st: costs of Breast Cancer recurrence in the first year
cRecuccernce2nd*: costs of Breast Cancer recurrence in the second year
cDeath: costs of death
State change probabilities
pSympRedWithout: probability of a clinically significant reduction on FACT-ES for self-managed iCBT
pSympRedWith: probability of a clinically significant reduction on FACT-ES for guided iCBT
pSympRedWaitlist: probability of a clinically significant reduction on FACT-ES for waitlist control group
ToRec: probability of going to recurrence
GenMR: general mortality rate
MRBreastCancer: mortality rate breast cancer
Utilities
uMenSymp: utility for health state menopausal symptoms
uRed: utility for health state reduction in menopausal symptoms
uRecurrence: utility associated with the health state recurrence
uDeath: utility of the health state
Funding
The data used in this study were derived from a recently completed clinical trial (No: NCT02672189). This study was supported by the Dutch Cancer Society (Grant No NKI 2014-6788) and the Netherlands Cancer Institute.
Compliance with ethical standards
Conflict of interest
Myra S. Hunter declares she receives royalties from Routledge and Boom publisher. No other conflicts of interest are present and other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joost G.E. Verbeek and Vera Atema are Shared first author.
References
- 1.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344(26):1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter JS, Andrykowski MA. Menopausal symptoms in breast cancer survivors. Oncol Nurs Forum. 1999;26(8):1311–1317. [PubMed] [Google Scholar]
- 4.Gupta P, Sturdee DW, Palin SL, Majumder K, Fear R, Marshall T, Paterson I. Menopausal symptoms in women treated for breast cancer: the prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric. 2006;9(1):49–58. doi: 10.1080/13697130500487224. [DOI] [PubMed] [Google Scholar]
- 5.Moon Z, Hunter MS, Moss-Morris R, Hughes LD. Factors related to the experience of menopausal symptoms in women prescribed tamoxifen. J Psychosom Obstet Gynaecol. 2016;38(3):226–235. doi: 10.1080/0167482X.2016.1216963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109(5):832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 8.Stan D, Loprinzi CL, Ruddy KJ. Breast cancer survivorship issues. Hematol/Oncol Clin. 2013;27(4):805–827. doi: 10.1016/j.hoc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295(17):2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 10.Pandya KJ, Morrow GR, Roscoe JA, Zhao H, Hickok JT, Pajon E, Sweeney TJ, Banerjee TK, Flynn PJ. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet. 2005;366(9488):818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boekhout AH, Vincent AD, Dalesio OB, van den Bosch J, Foekema-Tons JH, Adriaansz S, Sprangers S, Nuijen B, Beijnen JH, Schellens JH. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2011;29(29):3862–3868. doi: 10.1200/JCO.2010.33.1298. [DOI] [PubMed] [Google Scholar]
- 12.Hunter MS, Grunfeld EA, Mittal S, Sikka P, Ramirez AJ, Fentiman I, Hamed H. Menopausal symptoms in women with breast cancer: prevalence and treatment preferences. Psychooncology. 2004;13(11):769–778. doi: 10.1002/pon.793. [DOI] [PubMed] [Google Scholar]
- 13.Hunter MS, Liao KLM. Evaluation of a four-session cognitive-behavioural intervention for menopausal hot flushes. Br J Health Psychol. 1996;1:113–125. [Google Scholar]
- 14.Mann E, Smith MJ, Hellier J, Balabanovic JA, Hamed H, Grunfeld EA, Hunter MS. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol. 2012;13(3):309–318. doi: 10.1016/S1470-2045(11)70364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayers B, Smith M, Hellier J, Mann E, Hunter MS. Effectiveness of group and self-help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): a randomized controlled trial. Menopause. 2012;19(7):749–759. doi: 10.1097/gme.0b013e31823fe835. [DOI] [PubMed] [Google Scholar]
- 16.Duijts SFA, Van Beurden M, Oldenburg HSA, Hunter MS, Kieffer JM, Stuiver MM, Gerritsma MA, Menke-Pluymers MBE, Plaisir PW, Rijna H, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized controlled multicenter trial. J Clin Oncol. 2012;30(33):4124–4133. doi: 10.1200/JCO.2012.41.8525. [DOI] [PubMed] [Google Scholar]
- 17.Atema V, van Leeuwen M, Oldenburg HSA, van Beurden M, Hunter MS, Aaronson NK. An Internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors: results of a pilot study. Menopause. 2017;24(7):762–767. doi: 10.1097/GME.0000000000000836. [DOI] [PubMed] [Google Scholar]
- 18.Atema V, van Leeuwen M, Oldenburg HS, Retel V, van Beurden M, Hunter MS, Aaronson NK. Design of a randomized controlled trial of Internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors. BMC Cancer. 2016;16(1):920. doi: 10.1186/s12885-016-2946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atema V, van Leeuwen M, Kieffer JM, Oldenburg HSA, van Beurden M, Gerritsma MA, Kuenen MA, Plaisier PW, Lopes Cardozo AMF, van Riet YEA, et al. Efficacy of internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors: results of a randomized controlled trial. J Clin Oncol. 2019;37(10):809–822. doi: 10.1200/JCO.18.00655. [DOI] [PubMed] [Google Scholar]
- 20.Brennan A, Akehurst R. Modelling in health economic evaluation. What is its place? What is its value? PharmacoEconomics. 2000;17(5):445–459. doi: 10.2165/00019053-200017050-00004. [DOI] [PubMed] [Google Scholar]
- 21.Mewes JC, Steuten LM, Duijts SF, Oldenburg HS, van Beurden M, Stuiver MM, Hunter MS, Kieffer JM, van Harten WH, Aaronson NK. Cost-effectiveness of cognitive behavioral therapy and physical exercise for alleviating treatment-induced menopausal symptoms in breast cancer patients. J Cancer Surviv. 2015;9(1):126–135. doi: 10.1007/s11764-014-0396-9. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, Orlewska E, Penna P, Rodriguez Barrios JM, Shau WY. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14. doi: 10.1016/j.jval.2013.08.2291. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 24.Aaronson NK, Muller M, Cohen PDA, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MAG, Velde AT, Verrips E. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055–1068. doi: 10.1016/s0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 25.Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available) Value Health. 2008;11(7):1131–1143. doi: 10.1111/j.1524-4733.2008.00352.x. [DOI] [PubMed] [Google Scholar]
- 26.Rowen D, Brazier J, Roberts J. Mapping SF-36 onto the EQ-5D index: how reliable is the relationship? Health Qual Life Outcomes. 2009;7:27. doi: 10.1186/1477-7525-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55(2):189–199. doi: 10.1023/a:1006263818115. [DOI] [PubMed] [Google Scholar]
- 28.Hunter MS, Liao KLM. A psychological analysis of menopausal hot flushes. Br J Clin Psychol. 1995;34:589–599. doi: 10.1111/j.2044-8260.1995.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 29.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 30.Streiner DL, Norman GR, Cairney J. Health measurement scales: a practical guide to their development and use. 5. Oxford, UK: Oxford University Press; 2014. [Google Scholar]
- 31.Bouwmans C, De Jong K, Timman R, Zijlstra-Vlasveld M, Van der Feltz-Cornelis C, Tan Swan S, Hakkaart-van Roijen L. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P) BMC Health Serv Res. 2013;13:217. doi: 10.1186/1472-6963-13-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oostenbrink JB, Koopmanschap MA, Rutten FF. Standardisation of costs: the Dutch manual for costing in economic evaluations. PharmacoEconomics. 2002;20(7):443–454. doi: 10.2165/00019053-200220070-00002. [DOI] [PubMed] [Google Scholar]
- 33.Tan SS, Bouwmans CA, Rutten FF, Hakkaart-van Roijen L. Update of the Dutch manual for costing in economic evaluations. Int J Technol Assess Health Care. 2012;28(2):152–158. doi: 10.1017/S0266462312000062. [DOI] [PubMed] [Google Scholar]
- 34.Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, Kuntz KM. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force–3. Value Health. 2012;15(6):812–820. doi: 10.1016/j.jval.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Netherlands Comprehensive Cancer Organisation: Dutch Cancer Figures https://www.cijfersoverkanker.nl/
- 36.Statistics Netherlands: Mortality statistics https://statline.cbs.nl/Statweb/
- 37.Davis SR, Panjari M, Robinson PJ, Fradkin P, Bell RJ. Menopausal symptoms in breast cancer survivors nearly 6 years after diagnosis. Menopause. 2014;21(10):1075–1081. doi: 10.1097/GME.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 38.Schwarzer R, Rochau U, Saverno K, Jahn B, Bornschein B, Muehlberger N, Flatscher-Thoeni M, Schnell-Inderst P, Sroczynski G, Lackner M, et al. Systematic overview of cost-effectiveness thresholds in ten countries across four continents. J Comp Eff Res. 2015;4(5):485–504. doi: 10.2217/cer.15.38. [DOI] [PubMed] [Google Scholar]
- 39.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organization. 2015;93(2):118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg V, Shen X, Cheng Y, Nawarskas JJ, Raisch DW. Use of number needed to treat in cost-effectiveness analyses. Ann Pharmacother. 2013;47(3):380–387. doi: 10.1345/aph.1R417. [DOI] [PubMed] [Google Scholar]
- 41.Chatellier G, Zapletal E, Lemaitre D, Menard J, Degoulet P. The number needed to treat: a clinically useful nomogram in its proper context. BMJ (Clin Res ed) 1996;312(7028):426–429. doi: 10.1136/bmj.312.7028.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauskopf JA, Sullivan SD, Annemans L, Caro J, Mullins CD, Nuijten M, Orlewska E, Watkins J, Trueman P. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health. 2007;10(5):336–347. doi: 10.1111/j.1524-4733.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 43.Willems RA, Bolman CA, Mesters I, Kanera IM, Beaulen AA, Lechner L. Cancer survivors in the first year after treatment: the prevalence and correlates of unmet needs in different domains. Psychooncology. 2016;25(1):51–57. doi: 10.1002/pon.3870. [DOI] [PubMed] [Google Scholar]
- 44.Thorsen L, Gjerset GM, Loge JH, Kiserud CE, Skovlund E, Flotten T, Fossa SD. Cancer patients’ needs for rehabilitation services. Acta Oncologica (Stockholm, Sweden) 2011;50(2):212–222. doi: 10.3109/0284186X.2010.531050. [DOI] [PubMed] [Google Scholar]
- 45.Plass A, Koch U. Participation of oncological outpatients in psychosocial support. Psychooncology. 2001;10(6):511–520. doi: 10.1002/pon.543. [DOI] [PubMed] [Google Scholar]
- 46.Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–1174. doi: 10.1016/S1470-2045(13)70442-X. [DOI] [PubMed] [Google Scholar]
- 47.Wijnen BFM, Mosweu I, Majoie M, Ridsdale L, de Kinderen RJA, Evers S, McCrone P. A comparison of the responsiveness of EQ-5D-5L and the QOLIE-31P and mapping of QOLIE-31P to EQ-5D-5L in epilepsy. Eur J Health Econ. 2018;19(6):861–870. doi: 10.1007/s10198-017-0928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. Narrative literature review. Br J Psychiatry. 2005;186:11–17. doi: 10.1192/bjp.186.1.11. [DOI] [PubMed] [Google Scholar]
- 49.Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: quality of life of young breast cancer survivors. Psychooncology. 2004;13(3):147–160. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt ME, Wiskemann J, Steindorf K. Quality of life, problems, and needs of disease-free breast cancer survivors 5 years after diagnosis. Qual Life Res. 2018;27(8):2077–2086. doi: 10.1007/s11136-018-1866-8. [DOI] [PubMed] [Google Scholar]
- 51.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 52.Krol M, Brouwer W, Rutten F. Productivity costs in economic evaluations: past, present, future. PharmacoEconomics. 2013;31(7):537–549. doi: 10.1007/s40273-013-0056-3. [DOI] [PubMed] [Google Scholar]
- 53.Guideline for conducting economic evaluations in healthcare. https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare
- 54.Lidgren M, Wilking N, Jonsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16(6):1073–1081. doi: 10.1007/s11136-007-9202-8. [DOI] [PubMed] [Google Scholar]
- 55.Retel VP, Joore MA, Knauer M, Linn SC, Hauptmann M, Harten WH. Cost-effectiveness of the 70-gene signature versus St Gallen guidelines and Adjuvant Online for early breast cancer. Eur J Cancer. 2010;46(8):1382–1391. doi: 10.1016/j.ejca.2010.02.035. [DOI] [PubMed] [Google Scholar]