Abstract
Purpose
To compare the efficacy of chemoradiotherapy or surgery for limited-stage small cell lung cancer (SCLC).
Patients and methods
A retrospective analysis was performed on 138 patients with limited-stage SCLC who received surgery (69 patients) or chemoradiotherapy (69 patients) between January 2000 and September 2016 in Zhejiang Cancer Hospital. Patients of the chemoradiotherapy group were selected by using “pair-matched case-control” methodology from a cohort of 503 patients who received chemoradiotherapy.
Results
The major prognostic factors, including T, N stage, treatment duration, age, gender, and whether or not they received prophylactic cranial irradiation were well balanced between two groups. The median overall survival (OS) time and 5-year OS rate were 37.1 months and 45.0% in the surgical group vs 45.0 months and 45.0% in the chemoradiotherapy group (P=0.846). The median progression-free survival (PFS) time and 5-year PFS rate were 27.1 months and 37.8% vs 36.2 months and 40.0%, respectively, in the two groups (P=0.610). The 5-year OS rate (62.3% vs 40.1%, P=0.038) and 5-year PFS rate (80.1% vs 40.1%, P=0.048) in the surgical group were significantly higher than those of the chemoradiotherapy group in patients with stage I disease. The 5-year OS rate (41.2% vs 50.6%, P=0.946) and 5-year PFS rate (64.7% vs 42.1%, P=0.280) of surgery for stage II SCLC were comparable to chemoradiotherapy. As for stage III SCLC, compared with the surgical group, the chemoradiotherapy group had a better 5-year OS trend (25.1% vs 47.6%, P=0.220), but the difference did not reach statistical significance.
Conclusion
Surgery could confer survival benefits in patients with p-stage I disease, but not in patients with p-stage II and III disease.
Keywords: SCLC, survival, prognosis, lung cancer, surgery, chemoradiotherapy
Introduction
Small cell lung cancer (SCLC) represents approximately 14–20% of all types of lung tumors, it is characterized by a poor prognosis, rapid growth, and early dissemination.1,2 Surgery is recommended in the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of patients with stage I SCLC.3 However, some patients with stage II/III SCLC also received surgery under the following situations: no definite preoperative histopathologic diagnosis, preoperative diagnoses of non-small cell lung cancer (NSCLC), and combined SCLC.4 What’s the role of surgery in the treatment of stage II/III SCLC patients? Whether patients in the surgery group would demonstrate a higher distant metastasis rate compared with chemoradiotherapy group? Currently, little research has been reported on these issues. The role of surgery in patients with stage II/III SCLC is undefined. Our study was conducted to compare the long-term results of chemoradiotherapy with surgical treatment for limited stage SCLC.
Materials And Methods
Patients
We retrospectively reviewed patients who were diagnosed as limited-stage SCLC and received a definitive operation in Zhejiang Cancer Hospital between January 2000 and December 2016. The chemoradiotherapy group was selected using “pair-matched case-control” methodology, in a cohort of 503 limited-stage patients who received chemoradiotherapy. TNM stage was defined according to the 7th American Joint Committee on Cancer (AJCC) staging criteria. The major prognostic factors including T, N stage, treatment duration, age, gender, and whether or not they received prophylactic cranial irradiation (PCI) were well balanced between these two groups. The study was approved by the Institutional Review Board and Ethics Committee of Zhejiang Cancer Hospital. This study was conducted in accordance with the Declaration of Helsinki. Written informed consent for the collection of medical information was obtained from all patients. All procedures performed in the study were in accordance with the ethical standards of the institutional research committee.
Treatment
The surgical procedures consisted of lobectomy, or pneumonectomy with ipsilateral hilar and mediastinal lymphadenectomy. Patients of the surgery group received two to three cycles of induction chemotherapy (EP: etoposide, 100 mg/m2 on days 1–3 and cisplatin, 30 mg/m2 on days 1–3) and two to four cycles of adjuvant chemotherapy (EP: etoposide, 100 mg/m2 on days 1–3 and cisplatin, 30 mg/m2 on days 1–3 or EC: etoposide, 100 mg/m2 on days 1–3 and carboplatin, AUC 5 on day 1). Patients of the chemoradiotherapy group received four to six cycles of chemotherapy (EP: etoposide, 100 mg/m2 on days 1–3 and cisplatin, 75 mg/m2 on days 1–3 or EC: etoposide, 100 mg/m2 on days 1–3 and carboplatin, AUC 5 on day 1). Three-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiation therapy (IMRT) was used in thoracic radiotherapy (TRT). The clinical target volume (CTV) of postoperative TRT included bronchial stump, ipsilateral hilar, and adjacent mediastinal lymph nodes. The planning target volume (PTV) included CTV with a 0.5–1 cm margin. The total dose of postoperative TRT was 50–60 Gy with 1.8–2 Gy per fraction for 5 days a week. The gross tumor volume (GTV) of radical TRT included the primary tumor (GTV-T) and pre-chemotherapy positive lymph nodes (GTV-N), the clinical target volume-tumor (CTV-T) included post-chemotherapy GTV-T with a margin of 0.8 cm. Planning target volumes (PTV) involved CTVs with a margin of 1–1.5 cm. The total dose of radical TRT was 50–60 Gy with 1.8–2 Gy per fraction for 5 days a week or 45 Gy with 1.5 Gy bid in 30 fractions over a 3-week period. Patients who achieved complete-remission (CR) or partial-remission (PR) of the tumor after the completion of chemoradiotherapy received PCI, which was delivered to a total dose of 25 Gy over 2 weeks.
Follow-Up
Patients were followed up every 3 months for the first year, and every 4 months for the next year, then every 6 months for the following years, and annually over 5 years. Follow-up imaging included enhanced chest and abdominal CT, and other necessary examination as clinically indicated. The follow-up schedule started from the time of first treatment. Overall survival (OS) was calculated from the time of first treatment to the date of death or censored at the date of last follow-up (if the patient was alive). Brain metastasis-free survival (BMFS) was calculated from the time of first treatment to the date of first diagnosis of brain metastases. Progression-free survival (PFS) was calculated from the time of first treatment to the date of first diagnosis of relapse or metastasis or death.
Statistical Analysis
Statistical analysis was carried out with SPSS 22.0 software. Chi-square test was used to compare baseline characteristics between the two groups. The Kaplan-Meier method was used to estimate survival rates, median survivals, and survival curves. The Log rank test was used to compare the survival curves. Multivariate analysis for OS was performed using Cox proportional hazards model. Hazard ratios (HRs) and 95% CIs were calculated using Cox’s proportional-hazard model. The statistical significance level was set at 0.05.
Results
Patient Characteristics
Between January 2000 and September 2016, 69 patients with limited-stage SCLC received surgery. Sixty-nine patients in the chemoradiotherapy group were selected using “pair-matched case-control” methodology. Major prognostic factors including T, N stage, treatment duration, age, gender, and whether or not they received PCI were well balanced between these two groups. Patients’ characteristics are summarized in Table 1.
Table 1.
Patient Characteristics
| Variable | Total | Surgical Group | Chemoradiotherapy Group | P |
|---|---|---|---|---|
| Gender | 1.0 | |||
| Male | 113 | 57 | 56 | |
| Female | 25 | 12 | 13 | |
| Age (years) | 0.726 | |||
| ≥60 | 53 | 28 | 25 | |
| <60 | 85 | 41 | 44 | |
| KPS | 0.399 | |||
| ≥90 | 124 | 60 | 64 | |
| <90 | 14 | 9 | 5 | |
| PCI | 0.336 | |||
| Yes | 69 | 35 | 34 | |
| No | 69 | 38 | 31 | |
| T-stage | 0.894 | |||
| T1 | 58 | 30 | 28 | |
| T2 | 51 | 25 | 26 | |
| T3 | 9 | 3 | 6 | |
| T4 | 20 | 11 | 9 | |
| N-stage | 0.699 | |||
| N0 | 58 | 30 | 28 | |
| N1 | 33 | 17 | 16 | |
| N2 | 47 | 22 | 25 |
Abbreviations: KPS, Karnofsky performance status; PCI, prophylactic cranial irradiation; HR, hazard ratio; CI, confidence interval.
In the surgical group, 26.1% (18/69) of patients received two to three cycles of induction chemotherapy, 81.2% (56/69) of patients received adjuvant chemotherapy; and 55.1% (38/69) of patients received postoperative radiotherapy.
Survival
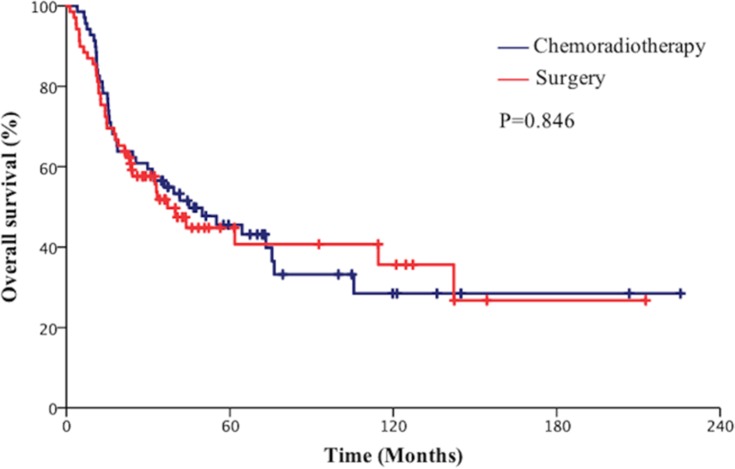
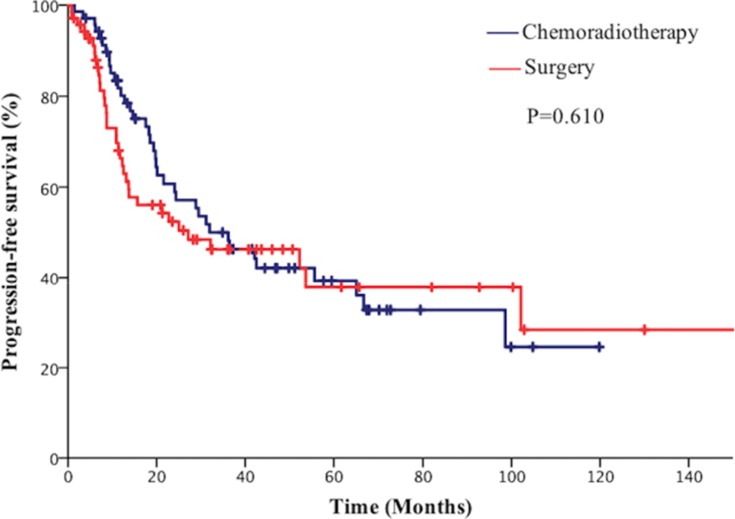
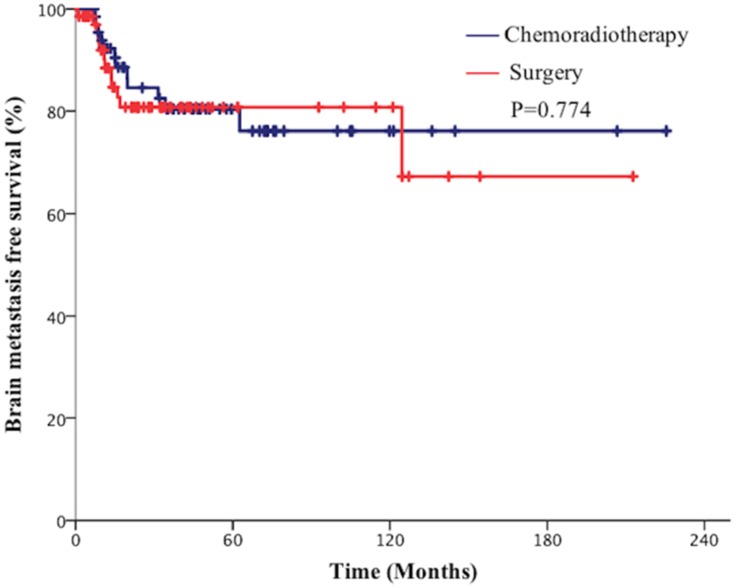
The median follow-up for all patients was 66 months. The median OS time was 37.1 months (95% CI=24.1–50.2) in the surgical group and 45.0 months (95% CI=15.8–74.2) in the chemoradiotherapy group. The 2-year and 5-year OS rates were 60.1% and 45.0% in the surgical group vs 63.8% and 45.0%, respectively, in the chemoradiotherapy group (P=0.846, Figure 1). The median PFS time was 27.1 months (95% CI=0.00–60.3) in the surgical group and 36.2 months (95% CI=20.9–51.4) in the chemoradiotherapy group. The 2-year and 5-year PFS rates were 52.3% and 37.8% in the surgical group vs 55.9% and 40.0% in the chemoradiotherapy group, respectively (P=0.610, Figure 2). The 2-year and 5-year BMFS rates were 81.0% and 76.2% in the surgical group vs 84.3% and 80.0%, respectively, in the chemotherapy group (P=0.774, Figure 3).
Figure 1.
Comparison of overall survival (OS) of patients with limited-stage small cell lung cancer (SCLC) between the surgical group and chemoradiotherapy group.
Figure 2.
Comparison of progression free survival (PFS) of patients with limited-stage small cell lung cancer (SCLC) between the surgical group and chemoradiotherapy group.
Figure 3.
Comparison of brain metastasis free survival (BMFS) of patients with limited-stage small cell lung cancer (SCLC) between the surgical group and chemoradiotherapy group.
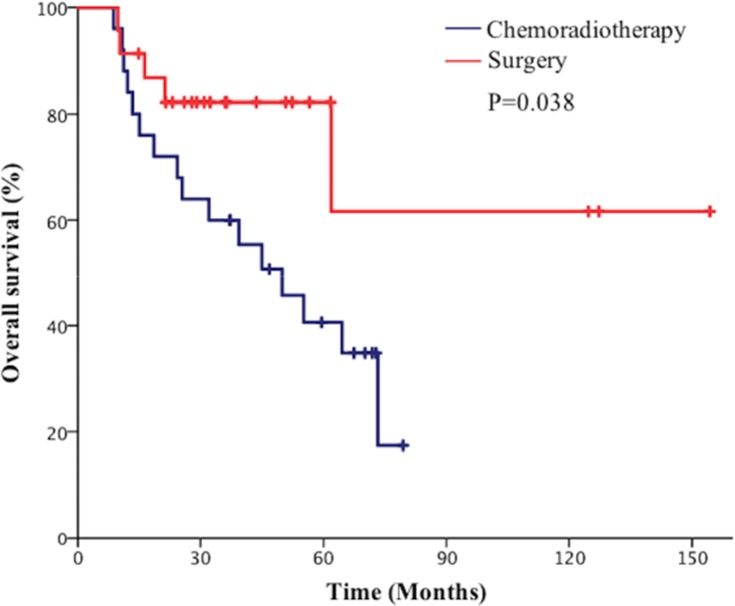
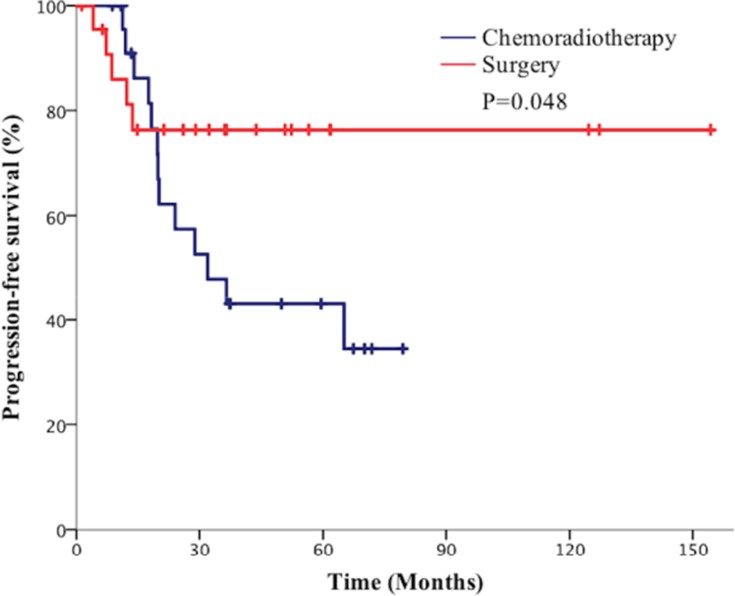
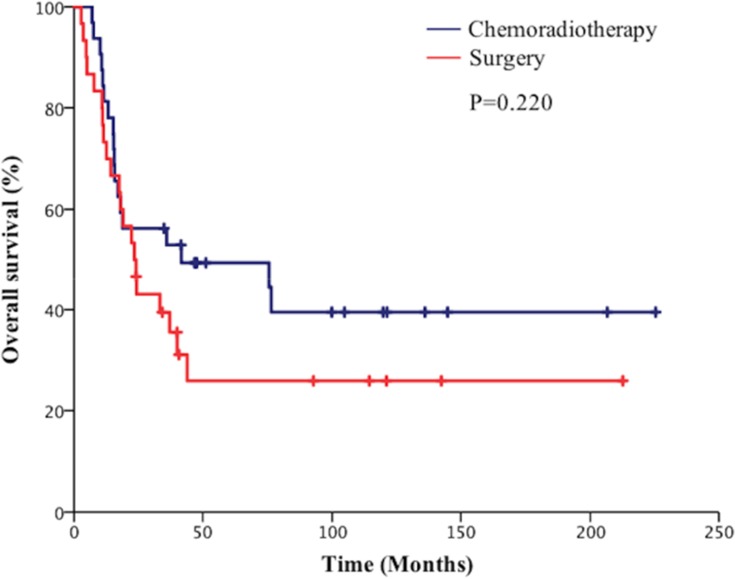
Subgroup analysis showed that the 5-year OS rate in the surgical group was 62.3% vs 40.1% in the chemoradiotherapy group in stage I patients (P=0.038, Figure 4). Besides the 5-year PFS rate in the surgical group was significantly higher than the chemoradiotherapy group in patients with stage I disease (80.1% vs 40.1%, P=0.048, Figure 5). However, the 5-year BMFS rate difference of the patients with stage I disease was not statistically significant (91.7% vs 95.1%, P=0.816). While in patients with stage II disease, the differences in 5-year OS rate (41.2% vs 50.6%, P=0.946), 5-year PFS rate (64.7% vs 42.1%, P=0.280), and 5-year BMFS rate (74.6% vs 78.1%, P=0.720) between the surgical group and chemoradiotherapy group were not statistically significant. As for stage III SCLC, compared with the surgical group, the chemoradiotherapy group had a better 5-year OS trend, but the difference did not reach statistical significance (25.1% vs 47.6%, P=0.220, Figure 6). Similarly, there was no statistically significant difference in 5-year PFS rate (27.6% vs 36.2%, P=0.333) and 5-year BMFS rate (76.2% vs 74.3%, P=0.84) between the two groups. The results of subgroup analysis are listed in Table 2.
Figure 4.
Comparison of overall survival (OS) of patients with p-stage I small cell lung cancer (SCLC) between the surgical group and chemoradiotherapy group.
Figure 5.
Comparison of progression free survival (PFS) of patients with p-stage I small cell lung cancer (SCLC) between the surgical group and chemoradiotherapy group.
Figure 6.
Comparison of overall survival of patients with p-stage III small cell lung cancer (SCLC).
Table 2.
Results Of Subgroup Analysis
| Stage | 5-Year OS Rate (%) | P | 5-Year PFS Rate (%) | P | 5-Year BMFS Rate (%) | P | |
|---|---|---|---|---|---|---|---|
| I | Surgical group | 62.3 | 0.038 | 80.1 | 0.048 | 91.7 | 0.816 |
| Chemoradiotherapy group. | 40.1 | 40.1 | 95.1 | ||||
| II | Surgical group | 41.2 | 0.946 | 64.7 | 0.280 | 78.1 | 0.720 |
| Chemoradiotherapy group. | 50.6 | 42.1 | 74.6 | ||||
| III | Surgical group | 25.1 | 0.220 | 27.6 | 0.333 | 76.2 | 0.842 |
| Chemoradiotherapy group. | 47.6 | 36.2 | 74.3 |
Relapse
By the last follow-up, 21.0% (29/138) of patients developed local relapse in primary tumor sites, 15.9% (11/69) in the surgical group, and 26.1% (18/69) in the chemoradiotherapy group (P=0.105). A total of 37.0% (51/138) of patients developed distant metastases, 40.6% (28/69) in the surgical group, and 33.3% (23/69) in the chemoradiotherapy group (P=0.240).
Prognostic Factors
The results of univariate and multivariate analysis are listed in Table 3. Age ≥60 years (HR=2.210, 95% CI=1.374–3.554, P=0.001) and PCI (HR=0.441, 95% CI=0.224–0.965, P=0.049) were independently prognostic factors for the OS.
Table 3.
Univariate Analysis And Multivariate Analysis Of The Prognostic Factors On OS In Patients With SCLC
| Factors | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| x2 | P | HR | 95% CI | P | |
| Gender | 0.41 | 0.524 | 1.009 | 0.527–1.932 | 0.979 |
| Age (years) | 10.51 | 0.001 | 2.210 | 1.374–3.554 | 0.001 |
| KPS | 2.061 | 0.151 | 2.172 | 0.895–5.500 | 0.101 |
| PCI | 6.022 | 0.014 | 0.441 | 0.224–0.965 | 0.049 |
| Surgery | 0.038 | 0.846 | 0.985 | 0.622–1.560 | 0.949 |
| T-stage | 6.57 | 0.087 | 1.120 | 0.906–1.385 | 0.296 |
| N-stage | 0.80 | 0.670 | 1.109 | 0.839–1.456 | 0.469 |
Abbreviations: KPS, Karnofsky performance status; PCI, prophylactic cranial irradiation; HR, hazard ratio; CI, confidence interval.
Discussion
Lung cancer is one of the most common malignant tumors today. According to the American Cancer Society, the number of new lung cancers in the United States in 2010 was 222,500.5 A study indicated that an estimated 4,292,000 new cancer cases and 2,814,000 cancer deaths would occur in China in 2015, with lung cancer being a major public health problem and the leading cause of cancer death.6
Although surgery is only recommended for clinical stage I (T1-2N0) SCLC patients,3 substantial numbers of patients with stage II/III SCLC also received surgery. A series of recent retrospective study on surgical resection for limited-stage SCLC indicated that the 5-year OS rate was 30–65% in patients with stage I SCLC, 28–57.1% in stage II patients, and 3–19% in stage III patients.7–12 Our study observed a 5-year OS rate of 45% for overall patients who received definitive surgery, and 62.3%, 41.2%, and 25.1% for patients with stage I, II, and III disease, respectively.
Whether surgery improves prognosis in patients with limited-stage SCLC has not been clearly examined. In 1969, a randomized study of surgical resection vs thoracic radiation therapy for limited-stage SCLC with 5-year follow-up was reported by the United Kingdom Medical Research Council. The 5-year OS rate was 1% with surgical resection and 4% with thoracic radiotherapy. Subsequently, the 10-year follow-up data showed a median OS of 6.5 months with resection compared with 10 months with radiotherapy (P=0.04),13,14 The median survival time in the radiotherapy group was not much better than that in the surgery group, as both groups showed poor long-term efficacy. However, there are some limitations in this study, only approximately half of the patients assigned to the surgery group received complete resection, 62% of the patients in the surgery group underwent subsequent chemotherapy or radiation. A multicenter, randomized trial reported 328 patients with limited-stage SCLC received five cycles of cyclophosphamide, doxorubicin, and vincristine, patients who achieved CR or PR of tumor after induction chemotherapy were then randomized to receive either thoracotomy followed by postoperative TRT or definitive TRT alone. Unfortunately, the addition of surgery to induction chemotherapy and adjuvant radiation couldn’t confer improvement in survival. The median overall survival was 15 months with the surgery group vs 19 months with the radiation group alone (P=0.78). Besides, no improvement in local tumor control followed surgery. Similarly, this study also has limitations, patients with peripheral solitary pulmonary nodule (T1N0M0) were specifically excluded. In addition, patients with mediastinal lymphadenopathy were included in the study, only 19% of the patients with early stage (T1-2, N0).15
While several retrospective studies suggest surgical resection may improve the outcome of patients with limited-stage SCLC, Eric et al16 reviewed 59 patients who had complete resection with nodal dissection. The 5-year OS rate was 52% of the patients with stage I–IIIB. Masayoshi et al17 analyzed data from 91 patients with stage I–IIIB SCLC who received surgical resection. The 5-year OS rate was 37.1% of the patients with stage I–IIB. Andrzej et al18 compared survivals in 134 limited-stage SCLC patients treated with either complete resection followed by chemotherapy, or with conventional non-surgical management. The median survival in the surgical group was 22.3 months and 11.2 months in the non-surgical group (P<0.001).
A large sample retrospective study based on the SEER (Surveillance, Epidemiology, and End Results) database also reported the results of surgical treatment for patients with SCLC. Weksler et al19 identified 3,566 patients with stage I or II SCLC between 1988 and 2007, lung resection was performed in 895 patients. The median survival time was 34.0 months with surgery vs 16.0 months without surgery (P<0.001). This study showed that surgical resection is associated with significantly improved survival for stage I–II SCLC. A similar study examined the SEER database for patients diagnosed with limited-stage SCLC between 1988 and 2003. In total, 863 received surgical resection, the 5-year OS rate was approximately 45% in patients who were classified as localized disease (T1–2N1–3M0) and received surgical resection, compared with 14% in patients with localized disease treated with conventional therapy (P<0.001). In addition, patients with regional disease (T3-4NxM0 or T1–4N1–2M0) who received surgery showed a 5-year OS rate of 26%, compared with 9% among those treated with conventional therapy (P<0.001).20 Despite the large sample size of the SEER database, the nature of the retrospective study determined its own inevitable shortcomings, for example, it did not provide data on patient performance status, the surgical margin status, or multimodality therapy, all of which may influence patient outcome, and no further subgroup analysis was performed in these studies.
The survival of the surgical group is comparable to the chemoradiotherapy group in our study. The median survival time was 37.1 months in the surgical group and 45.0 months in the chemoradiotherapy group. The 5-year survival rates of both groups were 45.0%. (P=0.846). In addition, there were no statistically significant differences between the two groups in PFS (P=0.610) and BMFS (P=0.774).
Subgroup analysis of our study indicated that the 5-year OS rate in the surgical group was significantly higher than those of the chemoradiotherapy group in patients with stage I disease (62.3% vs 40.1%, P=0.038). In addition, surgery conferred 5-year PFS improvement (80.1% vs 43.1%, P=0.048), but there was no significant difference of BMFS rate (91.7% vs 95.1%, P=0.816) between the two groups. For stage II SCLC, the 5-year OS rate, 5-year PFS rate, and 5-year BMFS rate of surgery is comparable to chemoradiotherapy. Notably, although the difference was not statistically significant, the chemoradiotherapy group indicated a trend of a better 5-year OS rate compared with the surgical group in patients with stage III disease (25.1% vs 47.6%, P=0.220).
In this study, 15.9% of patients in the surgical group developed local relapses, while 26.1% were observed in the chemoradiotherapy group (P=0.105). The surgical group had a trend of better local control, but the difference was not statistically significant.
There are several limitations in our study; the retrospective study determines its own inevitable shortcomings, and the sample size of the subgroup analysis is not big enough, which might influence the statistical analysis. While “pair-matched case-control” methodology was used in our study, this method made the known clinical factors that may affect the prognosis of the two groups close to equilibrium, and made the conclusion more reliable. in addition, few research has been reported on these issues currently. Final conclusions require a larger sample or a prospective randomized controlled trial.
Conclusion
In conclusion, surgery in limited-stage SCLC is still a controversial topic. In future, large-scale, multi-center prospective studies are warranted to screen out patients who are truly suitable for surgical treatment.
Acknowledgment
This work was supported by National Natural Science Foundation of China (grant numbers 81402540, 81672972) and the National Health and Family Planning Commission Scientific Research Funds-Zhejiang Province Major Science and Technology Project on Medicine (grant number WKJ-ZJ-1701).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Goninan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. dio: 10.1200/JCO.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 2.Bayman NA, Sheikh H, Kularatne B, et al. Radiotherapy for small-cell lung cancer – where are we heading? Lung Cancer. 2009;63(3):307–314. doi: 10.1016/j.lungcan.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN): Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer (version 1.2016). Washington:NCCN, 2016. [Google Scholar]
- 4.Gong L, Wang QI, Zhao L, et al. Factors affecting the risk of brain metastasis in small cell lung cancer with surgery: is prophylactic cranial irradiation necessary for stage I-III disease? Int J Radiat Oncol Biol Phys. 2013;85(1):196–200. doi: 10.1016/j.ijrobp.2012.03.038 [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Cancer Facts & Gures 2010. Atlanta (GA): American Cancer Society; 2010. [Google Scholar]
- 6.Chen WQ, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya R, Suzuki K, Ichinose Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan clinical oncology lung cancer study group trial (JCOG9101). J Thorac Cardiovasc Surg. 2005;129(5):977–983. doi: 10.1016/j.jtcvs.2004.05.030 [DOI] [PubMed] [Google Scholar]
- 8.Shields TW, Higgins GA Jr, Matthews MJ, Keehn RJ. Surgical resection in the management of small cell carcinoma of the lung. J Thorac Cardiovasc Surg. 1982;84(4):481–488. [PubMed] [Google Scholar]
- 9.Chi-Fu JY, Derek Y, Paul J, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol. 2016;34(10):1057–1064. doi: 10.1200/JCO.2015.63.8171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bischof M, Debus J, Herfarth K, et al. Surgery and chemotherapy for small cell lung cancer in stages I-II with or without radiotherapy. Strahlenther Onkol. 2007;183(12):679–684. doi: 10.1007/s00066-007-1740-z [DOI] [PubMed] [Google Scholar]
- 11.Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: its time has come. J Thorac Cardiovasc Surg. 2005;129(1):64–72. doi: 10.1016/j.jtcvs.2004.08.022 [DOI] [PubMed] [Google Scholar]
- 12.Shepherd FA, Ginsberg RJ, Feld R, Johansen E. Surgical treatment for limited small-cell lung cancer. J Thorac Cardiovasc Surg. 1991;101(3):385–393. [PubMed] [Google Scholar]
- 13.Miller AB, Fox W, Tall R. Five-year follow-up of the medical research council comparative trial of surgery and radiotherapy for the primary treatment of small-celled or oat-celled carcinoma of the bronchus. Lancet. 1969;2(7619):501–505. doi: 10.1016/s0140-6736(69)90212-8 [DOI] [PubMed] [Google Scholar]
- 14.Fox W, Scadding JG. Medical research council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet. 1973;2(7820):63–65. [DOI] [PubMed] [Google Scholar]
- 15.Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest. 1994;106(6):320S–323S. [DOI] [PubMed] [Google Scholar]
- 16.Eric L, Elizabeth B, Yoon KY, Nicholson AG, Goldstraw P. The role of surgery in the treatment of limited disease small cell lung cancer time to reevaluate. J Thorac Oncol. 2008;3(11):1267–1271. doi: 10.1097/JTO.0b013e318189a860 [DOI] [PubMed] [Google Scholar]
- 17.Masayoshi I, Shinichiro M, Tsutomu Y, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Ann Thorac Surg. 2000;70(5):1615–1619. doi: 10.1016/s0003-4975(00)01401-6 [DOI] [PubMed] [Google Scholar]
- 18.Andrzej B, Krzysztof K, Hanna KM, Jassem J. A retrospective comparative study of surgery followed by chemotherapy vs. non-surgical management in limited-disease small cell lung cancer. Eur J Cardiothorac Surg. 2004;26(1):183–188. doi: 10.1016/j.ejcts.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 19.Weksler B, Nason KS, Shende M, Landreneau RJ, Pennathur A. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg. 2012;94(3):889–893. doi: 10.1016/j.athoracsur.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 20.Schreiber D, Rineer J, Weedon J, et al. Survival outcomes with the use of surgery in limited stage small cell lung cancer: should its role be re-evaluated? Cancer. 2010;116(5):1350–1357. doi: 10.1002/cncr.24853 [DOI] [PubMed] [Google Scholar]