Abstract
BACKGROUND
ABO-incompatible and ABO-compatible kidney transplantation are equivalent in terms of short-term graft and patient survival. This is thought to be the result of ABO-incompatible graft accommodation, which occurs when anti-blood group antibodies re-occur after transplantation but somehow do not yield their detrimental effect. The underlying mechanism is unclear, but one of the hypotheses is that this is the result of complement inhibition. Since virtually all ABO-incompatible graft biopsies are C4d positive, this complement inhibition must occur somewhere in the complement cascade after the formation of C4d has already taken place, but where exactly is unclear. It is also unclear whether complement inhibition is complete. Incomplete accommodation could explain why recent studies have shown that long-term graft function in ABO-incompatible transplantation is somewhat inferior to ABO-compatible kidney transplantation.
AIM
To unravel the relationship between pre-transplant anti-ABO antibodies, complement activation, and long-term graft function.
METHODS
We included all 27 ABO-incompatible transplantations that were performed between 2008 and 2013 at the Academic Medical Center Amsterdam and the University Medical Center Groningen. For each ABO-incompatible transplantation, we included four ABO-compatible controls matched by age, sex, and transplantation date.
RESULTS
Graft and patient survival were not significantly different. The slope of kidney function during five-year follow-up was also not significantly different, but ABO-incompatible recipients did have a lower kidney function at three months (creatinine clearance 58 vs 69 mL/min, P = 0.02, Modification of Diet in Renal Disease 46 vs 52 mL/min/1.73 m2, P = 0.08), due to a high rate of early rejection (33% vs 15%, P = 0.03), mostly T-cell mediated. Pre-transplant anti-ABO IgG titers were positively correlated with C5b-9 staining, which itself was positively correlated with the occurrence of T-cell mediated rejection. This may be the result of concurrent C5a formation, which could function as a costimulatory signal for T-cell activation.
CONCLUSION
Co-stimulation of T-cell activation by ongoing complement activation by anti-ABO antibodies may be responsible for an impaired long-term graft function in ABO-incompatible kidney transplantation.
Keywords: ABO-incompatible, Kidney transplantation, Complement, Graft function, Rejection
Core tip: This retrospective case-control study was designed to unravel the relationship between complement activation, pre-transplant anti-ABO antibodies, and renal graft function. In this study, the slope of kidney function during a five-year follow up was not significantly different compared to matched ABO-compatible transplant recipients, but ABO-incompatible kidney transplant recipients did have a lower kidney function at three months, due to a high rate of early T-cell mediated rejection. Based on several complement staining results, we argue that this may be due to co-stimulation of T-cell activation by ongoing complement activation by anti-ABO antibodies.
INTRODUCTION
To combat the long waiting times of kidney transplantation there is a push to increase deceased donation, for example by using organs from marginal donors, as well as living donation[1]. Based on the prevalence of ABO blood groups of donors and recipients, approximately 30% of willing and otherwise appropriate kidney donor-recipient pairs are estimated to be blood type incompatible and do not proceed to living donor transplantation[2]. Paired kidney exchange is a strategy that helps some ABO-incompatible patients to find a suitable donor, although the probability of a match varies greatly depending on the blood type combination and sensitization status[3]. For example, the chances for ABO-incompatible and sensitized non-O recipients to find a possible match are around 60%, whereas ABO-incompatible O recipients only have a 15% chance for a match[2]. It is estimated that an additional 10%-20% of living donor kidney transplantations can be performed through the implementation of an ABO-incompatible kidney transplantation program[4,5].
ABO-incompatible kidney transplantation is precluded by the presence of isohemagglutinins –antibodies formed against blood group A and/or B- leading to hyperacute rejection and allograft loss. Blood group antigens are present not only in blood, but are also expressed on renal tubular cells and endothelium[6]. As first described by Alexandre et al[7], removal of isohemagglutinins through plasmapheresis prior to ABO-incompatible kidney transplantation can prevent hyperacute rejection. Their work on ABO-incompatible kidney transplantation in the early 1980s was greatly expanded on in Japan, a country with very low rates of postmortal kidney donation due to religious objections[8]. In Japan, it was customary to perform a splenectomy prior to ABO-incompatible transplantation, but this practice has mostly been abandoned after the introduction of rituximab in the last decade[9]. The introduction of rituximab has also led to a more widespread adoption of ABO-incompatible kidney transplant programs outside Japan. In a large series of 101 European, Australian and New Zealand centers 1420 ABO-incompatible transplantations were compared to 1:1 matched ABO-compatible transplantations[10]. Three-year death-censored graft survival (89.9%) and three-year patient survival (95.6%) were not significantly different, but there was a slightly lower one-year ABO-incompatible patient survival due to a higher rate of infections. In a large American series comparing 738 ABO-incompatible kidney transplantations to 1:5 matched ABO-compatible transplantations, a lower ten-year death-censored graft survival (76.1% vs 72.9% was found in ABO-incompatible kidney transplantations[11]. Ten-year patient survival was comparable for both groups (75.1% vs 74.5%).
ABO-incompatible kidney transplantation results in superior long-term graft survival when compared to kidney transplantation in HLA-sensitized recipients[12-15]. This is thought to be caused by accommodation, which occurs when anti-blood group antibodies re-occur after transplantation but somehow do not yield their detrimental effect[16]. The pathophysiology of accommodation is poorly understood. Proposed mechanisms can be divided into adaptations in the graft and in the host. Possible adaptations in the graft include diminished blood type expression in the graft[17] and expression of protective genes[18]. Possible adaptations in the host include IgG subclass switching[19], an increase in regulatory B and/or T cells[20], and complement inhibition[21]. The complement inhibition hypothesis is especially intriguing in light of the well-known fact that almost all ABO-incompatible kidney biopsies are C4d positive, but that C4d-positivity – in contrast to ABO-compatible kidney transplantation – is not a surrogate marker of antibody-mediated rejection[22].
C4d is a split product without known biological function that is produced when activation of the classical or the lectin pathway results in the conversion of C4 into C3. Because it forms a stable bond to the cells in the tissue where it is deposited, it remains visible as a footprint while antibodies dissociate over time[23]. Complement inhibition in ABO-incompatible transplantation is thought to occur more distally in the complement cascade, i.e., after the formation of C4d has already taken place, but the exact mechanism is unknown.
The case-control study presented here was designed to elucidate the relationship between the rate of kidney function decline, pre-transplant anti-ABO antibodies, and complement activation in ABO-incompatible renal transplantation. Firstly, we investigated whether ABO-incompatible kidney transplantation is equivalent to ABO-compatible kidney transplantation not only in terms of graft and patient survival, but also in terms of kidney function and long-term rate of kidney function decline. Full accommodation would imply that both groups are indistinguishable. Secondly, we studied various markers of complement activation (C1q, C3c, C4d, and C5b-9) in ABO-incompatible kidney biopsies.
MATERIALS AND METHODS
Study design
We conducted a retrospective case-control study in which we included all ABO-incompatible transplantations performed at the Amsterdam University Medical Centers, location AMC, and the University Medical Center Groningen between 2008 and 2013. For each ABO-incompatible transplantation, we included four matched ABO-compatible controls. The matching procedure was done as follows: from all 469 ABO-compatible kidney transplantations that were performed between 2008 and 2013, we selected the transplantations scheduled six months prior or after an ABO-incompatible transplantation in the same transplantation center. Within this group, we selected the four kidney transplant recipients of the same gender who had the smallest age difference with their ABO-incompatible counterpart.
All patients were treated according to standard clinical practice. All patient data were obtained and analyzed retrospectively after being anonymized. Patient treatment and clinical outcomes were not in any way affected by their retrospective inclusion in this study, implying that Institutional Review Board approval was not required according to Dutch law.
ABO-compatible transplantation immunosuppression
ABO-compatible kidney transplant recipients received basiliximab (20 mg on day 0 and day 4) and intravenous prednisolone (50 mg twice daily on day 0 and day 1) as induction therapy. Post-transplantation maintenance immunosuppression consisted of tacrolimus (target levels 10-15 ng/mL in the first six weeks and 6-10 ng/mL thereafter), prednisolone 10 mg once daily, and mycophenolic acid (1000 mg twice daily), with graduate tapering of immunosuppression after one year.
ABO-incompatible transplantation requirements and procedure
In order to be eligible for ABO-incompatible transplantation, patients had to have a negative (HLA)-crossmatch with an ABO-incompatible donor and anti-ABO IgG titers (measured twice) not exceeding 1:256. All ABO-incompatible kidney transplant recipients received a single dose of rituximab of 375 mg/m2 30 d prior to their scheduled transplantation. Maintenance immunosuppression consisted of prednisolone, tacrolimus and mycophenolic acid, in identical dosages as in ABO-compatible transplantation. However, whereas ABO-compatible recipients started maintenance immunosuppression on the day of transplantation, ABO-incompatible recipients did so two weeks before kidney transplantation. Immuno-adsorptions were started one week before transplantation using an antigen-specific carbohydrate column (Glycosorb®). At least four immuno-adsorption sessions were scheduled, with the option of additional sessions until the target anti-ABO titer ≤ 1:8 was reached. Intravenous immunoglobulins (500 mg/kg) were given just before transplantation. After transplantation, three more immuno-adsorption sessions were performed.
Infection prophylaxis
Patients with a cytomegalovirus mismatch (recipient IgG negative and donor IgG positive) received six months of valgancyclovir prophylaxis. In addition, all patients were prescribed at least four months of Pneumocystis Jirovecii prophylaxis (trimethoprim/sulfamethoxazole).
Complement activation in ABO-incompatible indication and protocol biopsies
Protocol biopsies were performed one year after ABO-incompatible kidney transplantation according to standard clinical practice. Indication biopsies were performed at the discretion of the treating physician. Immunofluorescence staining for C4d, C1q, C3c and C5b-9 on fresh frozen sections was made available for all ABO-incompatible indication and protocol biopsies (15 each).
Endpoints
The primary endpoint of this study was rate of kidney function decline, measured as the slope of estimated glomerular filtration (eGFR) by means of the Modification of Diet in Renal Disease (MDRD) equation and the slope of creatinine clearance. Secondary endpoints were proteinuria, graft and patient survival, biopsy proven rejections, infections, malignancies and complement activation on all indication and protocol biopsies of ABO-incompatible transplants.
Statistical analysis
For the primary endpoint (rate of kidney function decline), linear mixed models were used to estimate the slope of the MDRD and creatinine clearance for both ABO-incompatible and ABO-compatible kidney transplant recipients. In case of graft loss, an eGFR of 10 mL/min/1.73 m2 and a creatinine clearance of 10 mL/min were imputed. Sensitivity analyses were run both with and without these imputations. Graft and patient survival and rejection episodes were compared using Kaplan-Meier analyses. Infections and malignancies were compared using chi-square tests. The statistical methods of this study were reviewed by H. Peters Sengers, senior epidemiologist at Amsterdam University Medical Centers, location AMC.
RESULTS
Between 2008 and 2013, 27 ABO-incompatible transplantations were performed at both centers. For each ABO-incompatible transplantation, we included 4 matched ABO-compatible transplantations at both centers, so that the total number of patients included in our analysis equals 135.
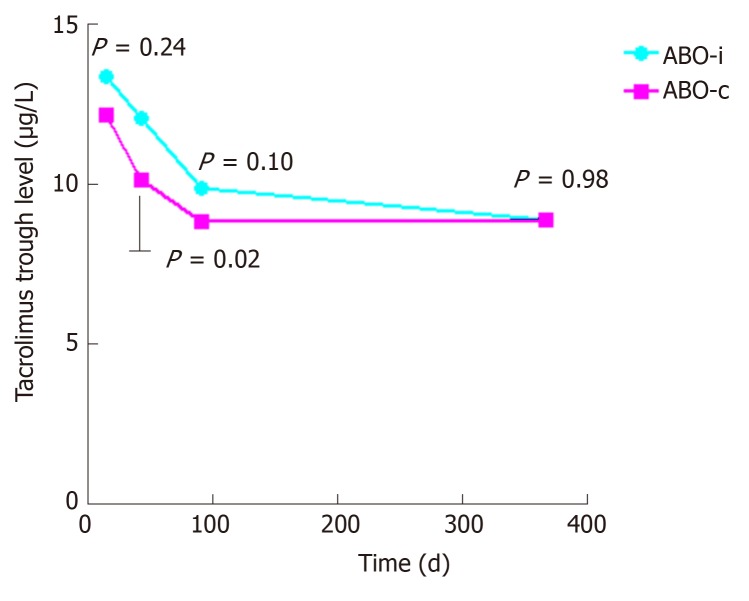
The baseline characteristics are described in Table 1. The groups were well-matched, except for a lower percentage of related donors in the ABO-incompatible group, which did not result in a higher number of HLA-mismatches. Panel-reactive antibodies were low and not significantly different between both groups. Tacrolimus trough levels were also not significantly different between the two groups except for a slightly higher trough level at week six (Figure 1).
Table 1.
Patient and donor characteristics
| ABO-incompatible (n = 27) | ABO-compatible (n = 108) | P value | ||
| Patient and donor characteristics | ||||
| Recipient age (yr) | 51.6 ± 12.3 | 51.7 ± 11.6 | 0.95 | |
| Donor age (yr) | 53.6 ± 12.1 | 52.7 ± 12.4 | 0.73 | |
| Recipient gender (% male) | 63 | 63 | 1.00 | |
| Donor gender (% male) | 63 | 44 | 0.07 | |
| Related/unrelated donors (% related) | 26 | 51 | 0.02 | |
| Donor MDRD (mL/min/1.73m2) | 96.6 ± 20.5 | 93.3 ± 18.4 | 0.42 | |
| HLA mismatches (A/B/DR) | 3.5 ± 1.6 | 3.2 ± 1.7 | 0.48 | |
| Mean follow-up patient (yr) | 3.7 ± 1.7 | 3.7 ± 1.6 | 0.98 | |
| Underlying renal disease | 0.57 | |||
| Glomerulonephritis, (%) | 30 | 27 | ||
| Hypertensive disease, (%) | 7 | 18 | ||
| Diabetic nephropathy, (%) | 4 | 7 | ||
| Cystic kidney disease, (%) | 33 | 19 | ||
| Urological disease, (%) | 7 | 8 | ||
| Other, (%) | 19 | 21 | ||
| Blood type combination | ||||
| A→O, (%) | 59 | |||
| B→O, (%) | 15 | |||
| AB → O, (%) | 0 | |||
| B →A, (%) | 11 | |||
| A→ B, (%) | 7 | |||
| AB→ A, (%) | 4 | |||
| AB → B, (%) | 4 | |||
| Median pre-treatment IgG anti-A/B titer | 1:64 (1:6-1:128) | |||
| Median pre-treatment IgM anti-A/B titer | 1:8 (1:2-1:28) | |||
| Panel reactive antibodies (% positive) | 9 | 7 | 0.60 | |
| Previous renal replacement therapy | ||||
| First transplantation (%) | 89 | 94 | 0.30 | |
| Pre-emptive transplantation (%) | 44 | 53 | 0.44 | |
| Non pre-emptive (%) | 56 | 47 | 0.83 | |
| Haemodialysis (%) | 34 | 26 | ||
| Peritoneal dialysis (%) | 22 | 21 | ||
| Months on dialysis | 22 (9-26) | 16 (11-27) | 0.41 | |
| Ischemia times | ||||
| Cold ischemia time (min) | 161 ± 84 | 155 ± 59 | 0.49 | |
| Second warm ischemia time (min) | 41 ± 27 | 40 ± 22 | 0.77 | |
| Delayed graft function (%) | 8 | 4 | 0.37 | |
All values as percentages, mean ± standard deviation or median and interquartile range. P values calculated with t-test, Mann-Whitney U test or chi-square test where applicable. Panel reactive antibodies > 8% were considered positive. HLA: Human leukocyte antigen; IgG: Immunoglobulin G; IgM: Immunoglobulin M; MDRD: Modification of diet in renal disease.
Figure 1.
Tacrolimus trough levels. Tacrolimus trough levels at 2 wk, 6 wk, 3 mo, and 1 year after transplantation.
Kidney function
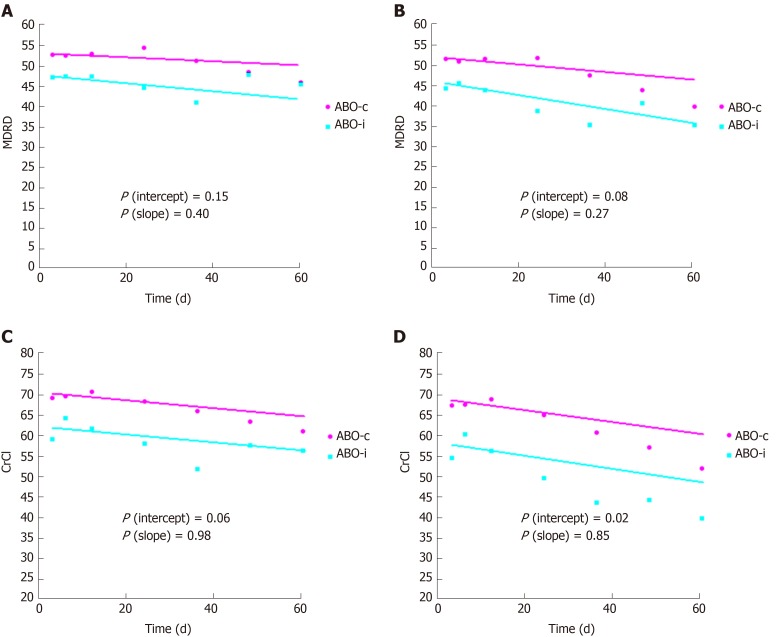
Linear mixed models were used to estimate the slope of eGFR and creatinine clearance with and without imputation of 10 mL/min for graft loss. As Figure 2 indicates, ABO-incompatible kidney transplant recipients had a slightly but significantly lower kidney function at three months, but the slope over time was not significantly different. There was also no significant difference in proteinuria, which was 0.20 g/d at three months and at five years in both groups (P = 0.94 and 0.86, respectively).
Figure 2.
Kidney function. A: Estimated glomerular filtration rate (Modification of Diet in Renal Disease) without imputation in case of graft loss; B: Estimated glomerular filtration rate (Modification of Diet in Renal Disease) with imputation of 10 mL/min/1.73 m2 in case of graft loss; C: Creatinine clearance without imputation in case of graft loss; D: Creatinine clearance with imputation of 10 mL/min/1.73 m2 in case of graft loss. Curves were estimated using linear mixed models. The dots indicate point estimates at 3, 6, 12, 24, 36, 48 and 60 months. CrCl: Creatinine clearance; MDRD: Modification of Diet in Renal Disease.
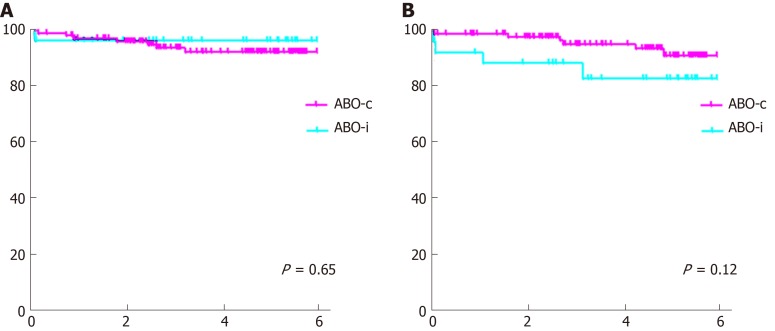
Graft and patient survival
Graft and patient survival (Figure 3) were not significantly different, although there was a trend toward a slightly lower death-censored graft survival in the ABO-incompatible group (one-year graft survival 92% vs 99%, P = 0.65, compared to 83% vs 91% five-year graft survival, P = 0.12).
Figure 3.
Patient and graft survival. A: Patient survival; B: Death-censored graft survival.
Biopsy results
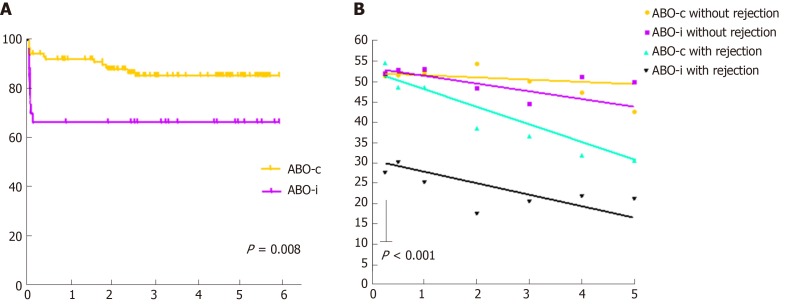
Table 2 shows the results of both the indication and the protocol biopsies. ABO-incompatible kidney transplant recipients had a higher incidence of rejection (33% vs 15%, P = 0.03), mostly T-cell mediated. Antibody-mediated rejection rates were low in both groups. Donor-specific antibodies were low and not significantly different between both groups. Figure 4 (left-hand panel) shows that all ABO-incompatible rejections occurred very early after transplantation, whereas ABO-compatible rejections occurred more gradually over time. In the right-hand panel of Figure 4, ABO-incompatible and ABO-compatible transplantations are split based on whether they experienced a rejection episode or not. This graph clearly indicates that early rejection results in a lower kidney function at three months in ABO-incompatible transplantation (P < 0.001). The slopes of all curves are not significantly different, although there was a trend toward steeper curves for both ABO-compatible and ABO-incompatible recipients with a rejection.
Table 2.
Biopsy results
| ABO-incompatible (n = 27) | ABO-compatible (n = 108) | P value | ||
| Treated rejections, (%) | 33 | 15 | 0.03 | |
| Acute antibody mediated rejection, (%) | 4 | 1 | 0.36 | |
| Acute T-cell mediated rejection, (%) | 30 | 14 | 0.05 | |
| Grade IA, (%) | 7 | 7 | ||
| Grade IB, (%) | 4 | 3 | ||
| Grade IIA, (%) | 15 | 3 | ||
| Grade IIB, (%) | 4 | 1 | ||
| Grade III, (%) | 0 | 0 | ||
| Borderline acute T-cell mediated rejection | 4 | 7 | 0.50 | |
| Donor specific antibodies (% positive) | 0 | 6 | 0.17 | |
P values calculated with chi-square test. Donor specific antibodies were measured according to local practice, i.e., at the discretion of the treating physician, and routinely at 8 wk after transplantation for Groningen patients and yearly for Amsterdam patients.
Figure 4.
Rejection-free survival and kidney function split by occurrence of rejection. A: Rejection-free survival; B: Estimated glomerular filtration rate (Modification of Diet in Renal Disease). P value calculated for intercept of ABO-i recipients with rejection (black line) compared to all other groups.
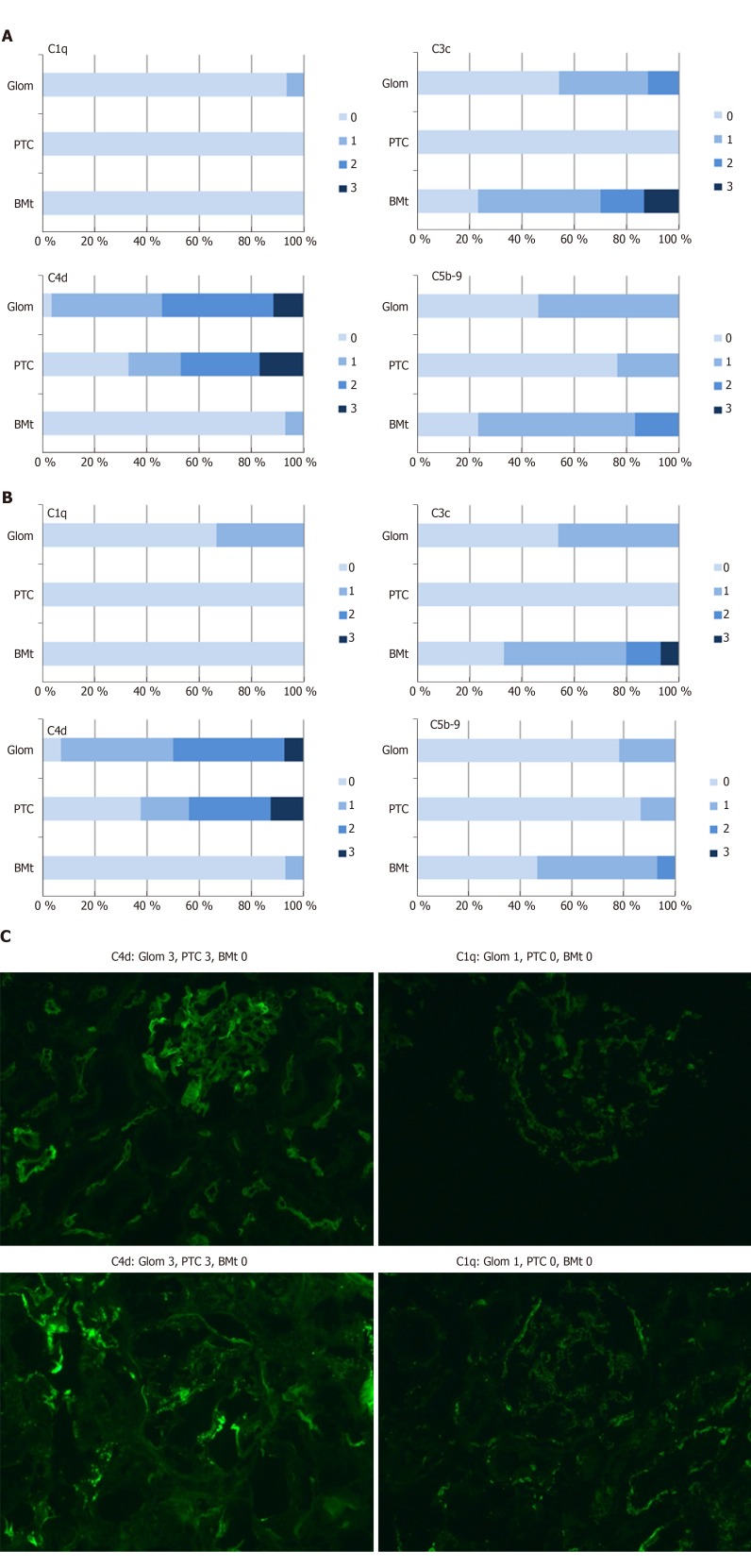
Figure 5A-C contain the results of immunofluorescence staining for C4d, C1q, C3c, and C5b-9. As can be seen by comparing Figure 5A and B, the complement staining results were quite equivocal for indication and protocol biopsies, except for C5b-9 staining, which was significantly more intense in indication biopsies (P = 0.04). In addition, there was a significant correlation between pretransplant anti-ABO IgG titers and peritubular C5b-9 in indication biopsies (correlation coefficient 0.72, P = 0.002), as well as a significant correlation between C5b-9 and the occurrence of rejection (correlation coefficient 0.52, P = 0.016). As expected in ABO-incompatible biopsies, there were no significant correlations between C4d and the occurrence of rejection.
Figure 5.
Complement activation in ABO-incompatible. A: Complement activation in ABO-incompatible indication biopsies; B: Complement activation in ABO-incompatible protocol biopsies; C: Digital photographs of complement activation. Intensity of staining ranges from 0-3. Figures indicate the percentage of biopsies with each intensity score. Glom: Glomerular; PTC: Peritubular capillaries; BMt: Basal membrane of the tubuli.
Infections and malignancies
Bacterial and viral infections occurred more frequently in the ABO-incompatible group (1.7 compared to 1.0 median infections per patient, P = 0.004, Table 3). As shown in Table 4, malignancies were also more likely to occur, with 22.2% of ABO-incompatible patients diagnosed with a (pre)malignancy compared to 7.4% in the ABO-compatible group (P = 0.01). This number should be interpreted with caution however, as ABO-incompatible patients with a malignancy were on average 10 years older than their counterparts without a malignancy. Also, ABO-compatible patients tended to have more severe malignancies, with 38% of ABO-compatible patients diagnosed with a malignancy dying from their disease compared to 0% in the ABO-incompatible group.
Table 3.
Infectious complications
| ABO-incompatible (n = 27) | ABO-compatible (n = 108) | P value | ||
| Total no. of infectious complications per patient | 1.7 (1-2.3) | 1.0 (0-2) | 0.004 | |
| CMV infections, (%) | 12 | 11 | ||
| EBV infections, (%) | 0 | 3 | ||
| BK infections, (%) | 15 | 6 | ||
| Other viral infections, (%) | 31 | 9 | ||
| First urinary tract infection, (%) | 38 | 30 | ||
| Recurrent urinary tract infections, (%) | 8 | 11 | ||
| Other bacterial infections, (%) | 23 | 7 | ||
| Other infections, not otherwise specified, (%) | 31 | 20 | ||
All values as percentages or median and interquartile range. P values calculated Mann Whitney U test or chi-square test where applicable. CMV: Cytomegalovirus; EBV: Epstein Barr virus.
Table 4.
Malignancies
| ABO-incompatible (n = 27) | ABO-compatible (n = 108) | P value | ||
| % of patients with a (pre)malignancy | 22.2 | 7.4 | 0.01 | |
| Solid organ, (%) | 11 | 2.8 | ||
| Lymphoma, (%) | 0 | 1.9 | ||
| Skin malignancy, (%) | 3.7 | 1.9 | ||
| Melanoma, (%) | 3.7 | 0 | ||
| Premalignant lesion, (%) | 3.7 | 0.9 | ||
| % of patients dying from their malignancy | 0 | 38 | 0.09 | |
P values calculated with chi-square test.
DISCUSSION
In this two-center case-control study, ABO-incompatible kidney transplant recipients had a lower kidney function three months after transplantation compared to ABO-compatible recipients, but the slope of kidney function over time was not significantly different. We have shown that the reason for this lower kidney function at three months was a higher rate of early mostly T-cell mediated rejections, all occurring very early after ABO-incompatible transplantation. Remarkably, antibody-mediated rejection rates were low in both groups. Tacrolimus levels were not significantly different for most of the follow-up period and are thus not responsible for the difference in kidney function.
The high rate of early mostly T-cell mediated rejection in our study is not a common finding in ABO-incompatible studies. It may be due to the fact that our standard protocol for ABO-incompatible transplantation does not include basiliximab, which is widely used as induction therapy to prevent T-cell mediated rejection in ABO-compatible transplantation[24]. There is no conclusive evidence available whether adding basiliximab to ABO-incompatible induction protocols improves outcomes. In the large series published by Opelz et al[10], 39% of centers had added basixilimab, 1% gave anti-thymocyte globulin, and 60% gave neither. Death-censored graft survival was not affected by the addition of basiliximab.
Another explanation for the high rate of early mostly T-cell mediated rejection could be complement activation. As discussed above, higher pre-transplant anti-ABO IgG titers resulted in more intense C5b-9 staining in indication biopsies. C5b-9 is the membrane attack complex, which is formed as a final common pathway of all complement cascades and causes cell lysis. C5b-9 formation implies that complement inhibition, which is one of the mechanisms thought to be responsible for accommodation in ABO-incompatible kidney transplantation, is incomplete. Incomplete complement inhibition also results in C5a still being formed in small quantities. C5a is an anaphylotoxin that has long been known be able to stimulate neutrophils, mast cells and macrophages. There is emerging evidence that C5a can also function as a costimulatory signal for T-cell activation[25-29]. One could postulate therefore that high pre-transplant anti-ABO IgG titers result in high levels of complement activation resulting in C5a-stimulated T-cell activation.
In summary, in our study ABO-incompatible and ABO-compatible renal transplantation have a similar 5-year graft and patient survival, but in ABO-incompatible transplant recipients, kidney function is inferior compared to well-matched ABO-compatible transplant recipients. This may be due to anti-ABO antibody driven ongoing complement activation resulting in a C5a-stimulated increase in early T-cell mediated rejection. In other words, high pretransplant anti-ABO antibody titers could result in incomplete accommodation of the ABO-incompatible graft, especially in the absence of induction therapy with agents like basiliximab. Further studies are needed to confirm this hypothesis.
ARTICLE HIGHLIGHTS
Research background
Short-term graft and patient survival in ABO-incompatible kidney transplantation are equivalent to ABO-compatible kidney transplantation, but in ABO-incompatible kidney transplantation, ongoing activation of complement by anti-ABO antibodies might adversely affect long-term graft function.
Research motivation
We aimed to investigate whether ongoing complement activation in ABO-incompatible kidney transplantation could explain why long-term graft function is impaired in ABO-incompatible kidney transplantation compared to ABO-compatible kidney transplantation.
Research objectives
To measure long-term graft function in all ABO-incompatible kidney transplantation recipients at the Academic Medical Center Amsterdam and the University Medical Center Groningen, compare this to long-term graft function in matched ABO-compatible kidney transplantation recipients and relate this to various markers of complement activation.
Research methods
We used linear mixed models to estimate long-term graft function decline in both ABO-incompatible and matched ABO-compatible kidney transplantation recipients. Matching criteria were age, sex, and transplantation date. AB O-incompatible kidney biopsies were stained for various markers of complement activation.
Research results
The slope of kidney function during five-year follow-up was not significantly different between ABO-incompatible and ABO-compatible kidney transplantation, but ABO-incompatible kidney transplant recipients did have a lower function at three months after transplantation due to a high rate of early mostly T-cell mediated rejection. Rejection and C5b-9 activation were positively correlated.
Research conclusions
Ongoing complement activation adversely affects long-term graft function in ABO-incompatible kidney transplantation. We hypothesize that this may be due to concurrent C5a formation, which functions as a costimulatory signal for T-cell activation.
Research perspectives
Further studies are needed to confirm whether ongoing C5a formation is responsible for T-cell mediated rejection in ABO-incompatible kidney transplantation.
ACKNOWLEDGEMENTS
The authors would like to thank Kers J (Department of Pathology, Amsterdam University Medical Centers, location AMC) for his assistance with scoring the biopsies, Ranzijn C (Sanquin Blood Supply, Amsterdam) and Hepkema BG (Faculty of Medical Sciences, University Medical Center Groningen) for their assistance in providing donor-specific antibodies, and Peters Sengers H, senior epidemiologist (Department of CEMM, University Medical Centers Amsterdam, location AMC) for his assistance with the statistical analysis of the data.
Footnotes
Institutional review board statement: In this study, anonymized patient information was retrospectively obtained and analyzed. This information was available from the Dutch Organ Transplantation Registry. All subjects consented to being included in this Registry at the time of transplantation. All subjects of this study were treated according to standard clinical practice, and their treatment or clinical outcomes were not in any way affected by their retrospective inclusion in this study. As the subjects of this study were not subjected to procedures nor were required to follow rules of behaviour, Institutional Review Board approval was not required according to Dutch law.
Informed consent statement: Patient consent was not obtained but the presented data are anonymized and risk of identification is low.
Conflict-of-interest statement: All authors declare no potential conflicts of interest.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at m.s.vansandwijk@amsterdamumc.nl.
STROBE statement: The authors have read the STROBE Statement – checklist of items, and the manuscript was prepared and revised according to the STROBE Statement – checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: May 23, 2019
First decision: August 1, 2019
Article in press: October 18, 2019
Specialty type: Urology and nephrology
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Markic D, Trimarchi H S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
Contributor Information
Marit S van Sandwijk, Department of Nephrology, Amsterdam University Medical Centers, Amsterdam NL-1105 AZ, Netherlands. m.s.vansandwijk@amsterdamumc.nl; Dianet Dialysis Center, Amsterdam NL-1105 AZ, Netherlands.
Astrid Klooster, Department of Pathology and Laboratory Medicine, University Medical Center Groningen, Groningen NL-9700 RB, Netherlands; Department of Pathology, Pathology Friesland, Leeuwarden NL-8917 EN, Netherlands.
Ineke JM ten Berge, Department of Nephrology, Amsterdam University Medical Centers, Amsterdam NL-1105 AZ, Netherlands.
Arjan Diepstra, Department of Pathology and Laboratory Medicine, University Medical Center Groningen, Groningen NL-9700 RB, Netherlands.
Sandrine Florquin, Department of Pathology, Amsterdam University Medical Centers, Amsterdam NL-1105 AZ, Netherlands.
Joris J Hoelbeek, Department of Pathology, Amsterdam University Medical Centers, Amsterdam NL-1105 AZ, Netherlands.
Frederike J Bemelman, Department of Nephrology, Amsterdam University Medical Centers, Amsterdam NL-1105 AZ, Netherlands.
Jan-Stephan Sanders, Department of Internal Medicine, Division of Nephrology, University Medical Center Groningen, Groningen NL-9700 RB, Netherlands.
References
- 1.Maggiore U, Oberbauer R, Pascual J, Viklicky O, Dudley C, Budde K, Sorensen SS, Hazzan M, Klinger M, Abramowicz D ERA-EDTA-DESCARTES Working Group. Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol Dial Transplant. 2015;30:217–222. doi: 10.1093/ndt/gfu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilpert J, Fischer KG, Pisarski P, Wiech T, Daskalakis M, Ziegler A, Neumann-Haefelin E, Drognitz O, Emmerich F, Walz G, Geyer M. Long-term outcome of ABO-incompatible living donor kidney transplantation based on antigen-specific desensitization. An observational comparative analysis. Nephrol Dial Transplant. 2010;25:3778–3786. doi: 10.1093/ndt/gfq229. [DOI] [PubMed] [Google Scholar]
- 3.Gentry SE, Montgomery RA, Segev DL. Kidney paired donation: fundamentals, limitations, and expansions. Am J Kidney Dis. 2011;57:144–151. doi: 10.1053/j.ajkd.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Stegall MD, Dean PG, Gloor JM. ABO-incompatible kidney transplantation. Transplantation. 2004;78:635–640. doi: 10.1097/01.tp.0000136263.46262.0d. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery RA, Locke JE, King KE, Segev DL, Warren DS, Kraus ES, Cooper M, Simpkins CE, Singer AL, Stewart ZA, Melancon JK, Ratner L, Zachary AA, Haas M. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87:1246–1255. doi: 10.1097/TP.0b013e31819f2024. [DOI] [PubMed] [Google Scholar]
- 6.Breimer ME, Mölne J, Nordén G, Rydberg L, Thiel G, Svalander CT. Blood group A and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and secretor status. Transplantation. 2006;82:479–485. doi: 10.1097/01.tp.0000231697.15817.51. [DOI] [PubMed] [Google Scholar]
- 7.Alexandre GP, Squifflet JP, De Bruyère M, Latinne D, Reding R, Gianello P, Carlier M, Pirson Y. Present experiences in a series of 26 ABO-incompatible living donor renal allografts. Transplant Proc. 1987;19:4538–4542. [PubMed] [Google Scholar]
- 8.Tanabe K. Japanese experience of ABO-incompatible living kidney transplantation. Transplantation. 2007;84:S4–S7. doi: 10.1097/01.tp.0000296008.08452.4c. [DOI] [PubMed] [Google Scholar]
- 9.Macklin PS, Morris PJ, Knight SR. A systematic review of the use of rituximab for desensitization in renal transplantation. Transplantation. 2014;98:794–805. doi: 10.1097/TP.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 10.Opelz G, Morath C, Süsal C, Tran TH, Zeier M, Döhler B. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation. 2015;99:400–404. doi: 10.1097/TP.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93:603–609. doi: 10.1097/TP.0b013e318245b2af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couzi L, Manook M, Perera R, Shaw O, Ahmed Z, Kessaris N, Dorling A, Mamode N. Difference in outcomes after antibody-mediated rejection between abo-incompatible and positive cross-match transplantations. Transpl Int. 2015;28:1205–1215. doi: 10.1111/tri.12621. [DOI] [PubMed] [Google Scholar]
- 13.Ko EJ, Yu JH, Yang CW, Chung BH Korean Organ Transplantation Registry Study Group. Clinical outcomes of ABO- and HLA-incompatible kidney transplantation: a nationwide cohort study. Transpl Int. 2017;30:1215–1225. doi: 10.1111/tri.12979. [DOI] [PubMed] [Google Scholar]
- 14.Marfo K, Lu A, Ling M, Akalin E. Desensitization protocols and their outcome. Clin J Am Soc Nephrol. 2011;6:922–936. doi: 10.2215/CJN.08140910. [DOI] [PubMed] [Google Scholar]
- 15.Pankhurst L, Hudson A, Mumford L, Willicombe M, Galliford J, Shaw O, Thuraisingham R, Puliatti C, Talbot D, Griffin S, Torpey N, Ball S, Clark B, Briggs D, Fuggle SV, Higgins RM. The UK National Registry of ABO and HLA Antibody Incompatible Renal Transplantation: Pretransplant Factors Associated With Outcome in 879 Transplants. Transplant Direct. 2017;3:e181. doi: 10.1097/TXD.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K. A new concept of accommodation in ABO-incompatible kidney transplantation. Clin Transplant. 2005;19 Suppl 14:76–85. doi: 10.1111/j.1399-0012.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- 17.Tanabe T, Ishida H, Horita S, Yamaguchi Y, Toma H, Tanabe K. Decrease of blood type antigenicity over the long-term after ABO-incompatible kidney transplantation. Transpl Immunol. 2011;25:1–6. doi: 10.1016/j.trim.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Park WD, Grande JP, Ninova D, Nath KA, Platt JL, Gloor JM, Stegall MD. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003;3:952–960. doi: 10.1034/j.1600-6143.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- 19.Ishida H, Tanabe K, Ishizuka T, Furusawa M, Miyamoto N, Ishikawa N, Shirakawa H, Shimmura H, Ishii D, Nozaki D, Setoguchi K, Toma H. Differences in humoral immunity between a non-rejection group and a rejection group after ABO-incompatible renal transplantation. Transplantation. 2006;81:665–671. doi: 10.1097/01.tp.0000185193.77929.96. [DOI] [PubMed] [Google Scholar]
- 20.Dehoux JP, Gianello P. Accommodation and antibodies. Transpl Immunol. 2009;21:106–110. doi: 10.1016/j.trim.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 21.King KE, Warren DS, Samaniego-Picota M, Campbell-Lee S, Montgomery RA, Baldwin WM., 3rd Antibody, complement and accommodation in ABO-incompatible transplants. Curr Opin Immunol. 2004;16:545–549. doi: 10.1016/j.coi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Setoguchi K, Ishida H, Shimmura H, Shimizu T, Shirakawa H, Omoto K, Toki D, Iida S, Setoguchi S, Tokumoto T, Horita S, Nakayama H, Yamaguchi Y, Tanabe K. Analysis of renal transplant protocol biopsies in ABO-incompatible kidney transplantation. Am J Transplant. 2008;8:86–94. doi: 10.1111/j.1600-6143.2007.02036.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen D, Colvin RB, Daha MR, Drachenberg CB, Haas M, Nickeleit V, Salmon JE, Sis B, Zhao MH, Bruijn JA, Bajema IM. Pros and cons for C4d as a biomarker. Kidney Int. 2012;81:628–639. doi: 10.1038/ki.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, Chapman JR, Craig JC. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev. 2010;(1):CD003897. doi: 10.1002/14651858.CD003897.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant. 2013;13:2530–2539. doi: 10.1111/ajt.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunkelberger JR, Song WC. Role and mechanism of action of complement in regulating T cell immunity. Mol Immunol. 2010;47:2176–2186. doi: 10.1016/j.molimm.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217:216–224. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]