Brief summary:
Mast cells are innate immune effector cells that reside in the healthy synovial sublining and expand in number with inflammation. These cells can play an important role in initiation of arthritis, but much about their biology and importance remains obscure. This chapter reviews the use of animal models for the study of mast cells in arthritis, with a particular focus on the K/BxN serum transfer model. We discuss tissue preparation and histological analysis for the assessment of joint inflammation, injury, and for the presence and phenotype of synovial mast cells, as well as the use of bone marrow-derived mast cell (BMMC) engraftment into W/Wv mice as a tool to isolate the role of mast cells in joint inflammation and injury.
Keywords: K/BxN, arthritis, synovium, synovial mast cells
1. Introduction
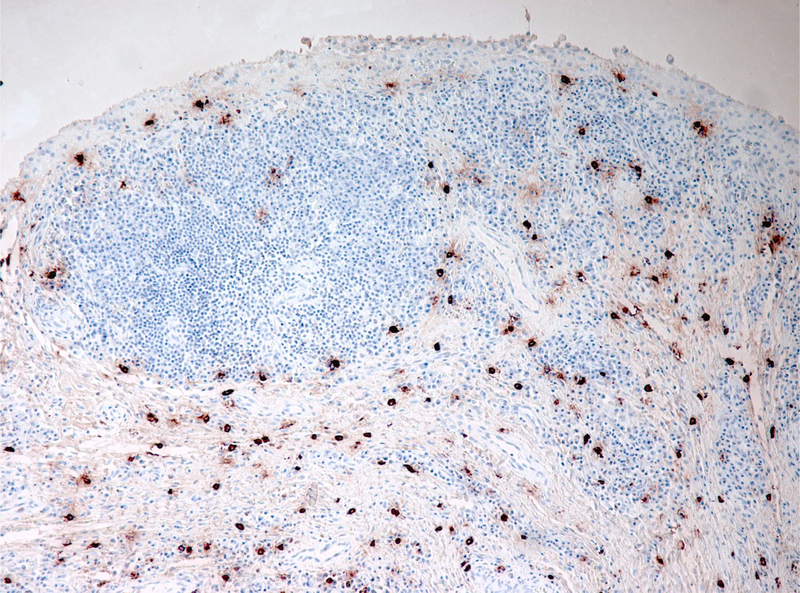
In the normal human joint, mast cells represent approximately 3% of nucleated cells residing within 70 μm of the joint lumen (1). These cells do not co-compact directly with the fibroblasts and macrophages that make up the synovial lining, but rather cluster beneath it in the loose connective tissue of the synovial sublining, where they reside near blood vessels, fascial planes, and within nerves (2). In autoimmune inflammatory arthritis and osteoarthritis, mast cells can expand in number by ten-fold or more, most likely via maturation of resident or newly-recruited mast cell progenitors originating from the bone marrow (2, 3). Mast cells thereby become an impressive histological feature of the inflamed synovium (Figure 1), and identifying their role is an important task for the synovial biologist.
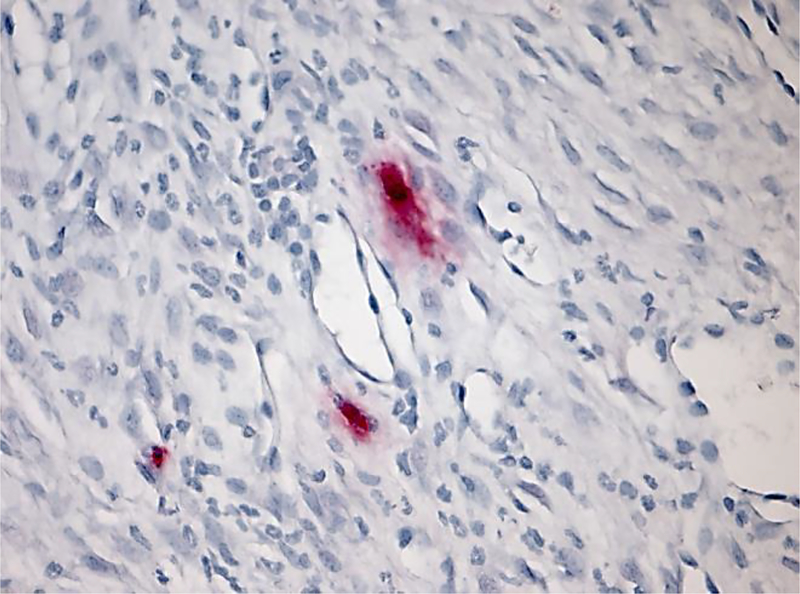
Figure 1.
Human RA synovium stained for tryptase (red) highlights abundance of mast cells in chronically inflamed joint tissue; reproduced from reference (2).
Multiple systems are available for induction of experimental arthritis (4). Most of these can be grouped into two categories. The first category consists of models in which mice develop systemic autoimmunity that then translates into joint inflammation. One example is K/BxN arthritis, in which KRN mice on the C57Bl/6 genetic background (therefore “K/B”) bearing a transgenic T cell receptor are crossed to NOD mice expressing a specific MHC II (I-Ag7). F1 mice from this cross (“K/BxN”) spontaneously develop high-titer IgG antibodies against the glycolytic enzyme glucose-6-phosphate isomerase (GPI), and arthritis develops principally through the action of these autoantibodies (5–7). A similar sequence of events occurs in collagen-induced arthritis (CIA), a model in which DBA/1 mice immunized with type II collagen develop joint-specific autoimmunity (8). Arthritis in these systems reflects both the formation of the adaptive immune response and the subsequent effector-phase of joint inflammation. In most such models, antibodies represent a major pathogenic actor, the exception being arthritis in the SKG mouse strain, which can develop through the activity of autoimmune T cells in the absence of antibody (9).
The second category of arthritis model reflects only the effector phase of disease, bypassing the generation of systemic autoimmunity via the adoptive transfer of arthritogenic autoantibodies. Examples are arthritis induced by anti-type II collagen antibodies (anti-collagen antibody-induced arthritis, CAIA), or by injection of autoantibody-containing serum from K/BxN mice (K/BxN serum transfer arthritis). In each of these experimental systems, a single monoclonal antibody is insufficient to cause disease. Rather, a cocktail of several different autoantibodies is required, likely reflecting the need for immune complex formation – i.e. both systems model IgG immune complex arthropathy (10). These systems have several advantages. First, formation of the autoimmune response can be taken out of the equation, affording a discrete focus on the effector phase of disease. Second, they are rapid, evolving within days of autoantibody transfer; an important corollary is that they may not model the pathogenic processes occurring in chronic, established disease such as human rheumatoid arthritis. Third, they can be induced in most strains of mice, enabling study of informative mutants, though since the intensity of resulting arthritis depends on the genetic background, care must be taken to match the background of experimental strains.
Given strong evidence implicating IgG immune complexes in human autoimmune arthritis, in particular rheumatoid-factor positive rheumatoid arthritis, our studies have employed K/BxN serum transfer arthritis (11). In this model, anti-GPI antibodies are believed to target the joints either through deposition of circulating immune complexes or by formation of immune complexes in situ on GPI deposited on the cartilage surface (12). This chapter describes methods for the evaluation of the role of mast cells in murine K/BxN arthritis.
Distinct mast cell (MC) subpopulations are situated at specific microanatomic locations. MCs have historically been divided into 2 subpopulations based on histochemical staining properties: chondroitin sulfate proteoglycan–rich mucosal mast cells (MMCs) and heparan sulfate proteoglycan–rich connective tissue mast cells (CTMCs). As their names suggest, MMCs are generally found in mucosal tissue, while CTMCs are localized in connective tissue (e.g., skin, peritoneum, and synovium).
Subsequent analyses have refined the phenotypic characterization of murine MCs based on the proteases found in their cytoplasmic secretory granules. Although more than a dozen proteases are expressed in murine MCs, limited subsets are useful for defining MC subtypes. Specifically, murine MMCs express the chymases murine mast cell protease 1 (mMCP-1) and mMCP-2, whereas CTMCs express a different combination of the proteases, including the chymases mMCP-4 and mMCP-5 and the tryptases mMCP-6 and mMCP-7 (13). Analyses using protease expression to define tissue MC subsets have revealed that MC subpopulations are quite variable, with identifiable distinctions in phenotype within the MMC and CTMC subsets at separate anatomic locations.
Murine synovial mast cells have been identified as CTMCs, therefore expressing mMCP-4, −5, −6 and −7 (13). In contrast, human MCs can be divided into 2 subsets: MCs that are positive for tryptase only (MCT) and more MMC-like, and MCs that are positive for both tryptase and chymase (MCTC) and more CTMC-like. Healthy human synovial MCs are heterogeneously populated with both subsets, although MCTC outnumber MCT by 5:1 (2).
In 2002, Lee at al. demonstrated that mast cell deficient W/Wv mice are relatively resistant to K/BxN serum transfer arthritis, and that this resistance may be overcome by engraftment with cultured done marrow-derived mast cells (BMMC) (14). Analogous engraftment experiments have been conducted using Pretty2 mice, which like W/Wv animals lack mast cells through mutation in Kit and can be engrafted with BMMC (15). Other mice deficient in mast cells are susceptible to antibody-mediated arthritis, including Wsh and CPA3-Cre (“CreMaster”) animals (16–18). These conflicting results have been interpreted as demonstrating the shortcomings of Kit and KitL mutant mice as a model for mast cell deficiency, since mast cells are not the only lineage affected by these gene defects (19). Yet such divergent findings could represent an interesting opportunity to understand conditions under which mast cells play a key role in joint inflammation. For example, the role of mast cells in arthritis may depend on the susceptibility of the background strain to disease and the strength of the arthritogenic stimulus. Thus, the wild-type control strain for W/Wv (WBB6) achieves a far lower intensity of arthritis than B6 or Balb/c, suggesting one explanation why mast cells might be particularly important in this background (14, 16). Arthritis resistance in mMCP6−/− mice emerges only at submaximal doses of K/BxN serum (20), while induction of arthritis in Wsh mice using lower serum doses also exposes partial arthritis resistance (PAN, unpublished data). To dissect the role of mast cells in the acute phase of K/BxN arthritis, we have used two approaches: 1) reconstitution of W/Wv mice with BMMC, and 2) study of mice genetically deficient in mediators specific for mast cells.
Mast cells are multifaceted effectors capable of both pro- and anti-inflammatory activity. Genetic deficiency ablates both facets of mast cell activity, as well as any potential effect of mast cells on neighboring cells such as fibroblasts and endothelial cells. Thus, while absence of mast cells helps to assess the “net” effect of mast cells upon a given system, alternate approaches are superior at identifying the specific contributions of individual mediators, for example as therapeutic targets. As examples of such studies, we have explored the role of mast cell mediators by inducing arthritis in animals deficient for the mast cell protease mMCP6, and more recently in mice lacking Ras guanyl nucleotide-releasing protein 4 (RasGRP4), expressed predominantly in mast cells and implicated in the modulation of signal transduction (20, 21).
2. Materials
Part I: K/BxN serum transfer arthritis
2.1. Mouse strains and K/BxN serum
KRN (K/B) mice (see Note 1)
NOD mice bearing H2 haplotype IAg7 (e.g., NOD/ShiLtJ or NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ)
Experimental mouse strains
Non-heparinized glass pipettes (Fischer 22-260-943)
1.5 mL microfuge tubes and microfuge
Sterile syringes (0.3–1 cc volume) and needles (28–30G, 3/8” or 1/2” length)
2.2. Arthritis assessment
Spring-loaded thickness gauge with range at least 10 mm with a resolution ~0.01 mm and accuracy of ~15 μm. (e.g., Kafer Model J15 with 6 mm flat anvils, SPI Model 21-790-1, Long Island Indicator, Inc.).
2.3. ELISA for anti-GPI IgG quantitation
ELISA plate carbonate coating buffer: 0.1 M sodium bicarbonate buffer (pH 7.0). Dissolve 8.4 g sodium bicarbonate (NaHCO3) in 1 L water adjust pH to 7.0
Recombinant glucose-6-phosphate isomerase (GPI) standard: 5 μg/mL in carbonate coating buffer
High-binding ELISA plates (96-well flat bottom).
ELISA wash buffer: phosphate buffered saline (PBS), 1% Tween-20
Super Block: 4% whey (w/v), 10% fetal bovine serum (FBS), 0.5% Tween-20, 0.05% sodium azide (NaN3) (w/v) in PBS. Add 40 g whey, 100 mL FBS, 5 mL Tween-20 and 0.5 g NaN3 to 1 L PBS.
Detection antibody: donkey anti-mouse IgG conjugated to horse radish peroxidase (HRP).
ELISA substrate: TMB (3,3′,5,5′-tetramethylbenzidine) commercial stock (e.g. BD OptEIA®).
K/BxN serum
Normal mouse serum (negative control)
Spectrophotometer capable of absorbance detection at 650 nm
Part II: Histological assessment of K/BxN Serum transfer arthritis
2.4. Tissue harvest and preparation
Scissors, serrated or toothed forceps and scalpel
4% (w/v) paraformaldehyde (PFA): 40 g PFA, 1 L of PBS. Heat PFA and 900 mL PBS in large Erlenmeyer flask with magnetic agitation. Solution will go from cloudy to clear when ready. Do not allow solution boil over. If PFA does not dissolve, add a few chips of sodium hydroxide (NaOH). When PFA has dissolved, switch off heat and leave to stir while solution cools. Adjust pH to 7.2–7.4 using HCL. Add remaining PBS to 1 L volume. Store at 4°C.
- Kristenson’s decalcification solutions:
- Stock “A” (40.5% formic acid): add 410 mL of 99% formic acid to 590 mL distilled water
- Stock “B” (1 M sodium formate): Dissolve 68 g sodium formate in 1 L distilled water
Kristenson’s Decalcification Buffer (20%:80%) : 20 mL Stock “A”, 80 mL Stock “B”
2.5. Frozen sections
O.C.T. freezing medium (TissueTek®)
Tissue histology cryo-molds
2.6. Histological and immunohistological staining
2.6.1. Histological stains
Microscope slides and coverslips
- Toluidine Blue
- Toluidine Blue stock solution (1%): 5g Toluidine blue “O” (powder) in 500 ml dH2O
- Toluidine Blue working solution (0.1%): dilute stock solution 1:10 in dH2O
Freshly filtered Harris’ Hematoxylin (commercial)
Hematoxylin/eosin stain (commercial)
Xylene
Naphthol AS-D chloroacetate (CAE) solution: napthol AS-D chloroacetate 100mg + N-N dimethyl formamide 50 ml (store @ −20 degrees C)
- Phosphate buffer (0.1M, pH 7.6): 6.5 ml of stock A + 43.5 ml of stock B, dilute with 50 ml H2O
- Stock A. 0.2 M monobasic sodium phosphate (13.9g NaH2PO4 in 500ml H2O)
- Stock B. 0.2 M dibasic sodium phosphate (14.2g Na2HPO4 in 500 ml H2O)
4% sodium nitrite: 1g sodium nitrite + 25ml dH20
New Fuchsin solution: New Fuchsin 1g, 2N hydrochloric acid (HCI) 25 ml
Lithium carbonate buffer: lithium carbonate 1.54 g, dH20 100 ml
Absolute ethanol
Non-aqueous mounting medium (e.g., CytoSeal 60) – for H+E, CAE and toluidine blue stains
2.6.2. Immunohistological staining reagents
Hoechst #33258 (Sigma): Prepare 0.5mg/ml in dH20, store frozen aliquots. Dilute to 1:10,000 for inclusion in secondary antibody stain to visualize DNA.
VECTASTAIN® kit (Vector Labs): Contains Vectastain ABC-AP and FastRed.
Zenon® IgG labeling kit (Invitrogen)
Citrate buffer (pH 6.0) (e.g. Dako S236984–2)
Primary antibody to detect protein of interest
Aqueous mounting medium (e.g. Biomeda Crystal/Mount) – for VECTASTAIN®
- Immunofluorescence mounting medium (“Vinol”): 15% w/v polyvinyl alcohol, 33% v/v glycerol, 0.1% azide
- 5 g of Fisher “cold soluble” polyvinyl alcohol, P-8136–250G in 20 ml of PBS in a 50 mL tube. Mix by sonication, followed by tumbling overnight at room temperature.
- Add 5 ml (12.7 gm) of absolute glycerol and 0.2 ml of 20% sodium azide. Continue tumbling for 16 h at room temperature.
- Remove undissolved material by centrifugation at 20,000 g for 20 minutes.
- Decant the syrupy supernatant and store in airtight vials at −20C; frozen aliquots last indefinitely.
Part III: In vivo assessment of the role of mast cells in K/BxN serum transfer arthritis
2.7. Adoptive transfer of BMMCs into recipient mice
Donor mast cell strain
Recipient mice, e.g., W/Wv (WBB6F1/J-KitW/KitW-v)
BMMC media: IMDM, 15% FBS, 10 ng/mL interleukin 3 (IL-3) and 10–25 ng/mL Kit-L (cKit ligand, stem cell factor) with 150 μM monothioglycerol and antibiotics.
3. Methods
Part I: K/BxN serum transfer arthritis
3.1. Generation of K/BxN serum.
Generate donor arthritic mice by crossing the KRN strain with NOD mice bearing I-Ag7. All offspring develop arthritis, typically by 4–6 weeks. Both male and female mice are acceptable donors.
Harvest donor serum from mice between 9–11 weeks old.
Bleed animals by cardiac puncture immediately after CO2 euthanasia. Eject blood gently (to avoid hemolysis) into 1.5 mL microfuge tubes into which small non-heparinized glass pipettes have been placed to nucleate the clot.
Allow blood to clot at room temperature (RT), typically 30 minutes.
Remove glass pipettes with adherent clot from the serum
Centrifuge serum at maximum speed for 10 min in a microfuge.
Pool all serum and then split into 1 mL fractions. Store frozen at −20°C.
Serum remains active for at least 1 year and probably much longer.
3.2. K/BxN serum transfer and assessment of arthritis
Inspect paws and record baseline clinical scores and paw measurements of mice before administering K/BxN serum (see section 3.3).
Inject experimental mice (intraperitoneal (i.p.)) with desired volume of K/BxN serum on Day 0 (see Notes 2,3 & 4).
Inject mice with second K/BxN serum dose on Day 2 if desired.
3.3. Assessment of arthritis
K/BxN arthritis principally affects the paws, and is typically assessed in all four paws at each reading using both subjective (clinical index) and objective (paw swelling) measurements. In experienced hands, assessment can be done and recorded in less than 1 min per mouse.
Grade each mouse daily by scoring clinical index and measuring paw swelling. We typically grade mice daily starting with observations on Day 0 before injecting the first serum dose.
Clinical index: Grade each paw on an arbitrary scale of 0–3 with a total possible of score is 12. Normal ankles are scored 0; a point is given for swelling affecting the dorsal surface, ventral surface, toes, and ankle/wrist itself, capping at a maximum of 3 (Table 1) (see Note 5)(14, 21).
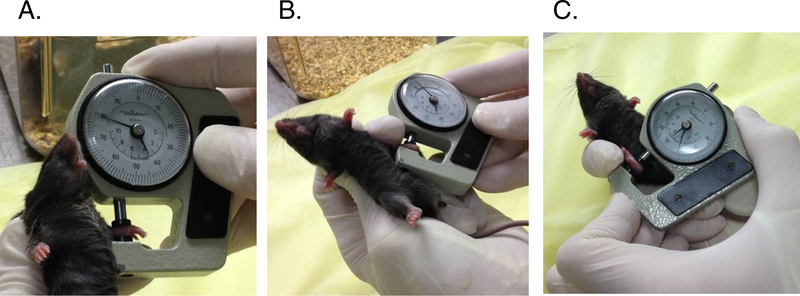
Paw swelling: Paws are measured in a standardized way at each reading using a spring-loaded thickness gauge (Figure 2). Ankles are measured across the malleoli with the joint in dorsiflexion; the degree of dorsiflexion varies somewhat from examiner to examiner depending on preferred grip technique, but should be consistent among measurements. We prefer to measure wrists as well. These are measured with the joint in neutral (straight) position (Note 6).
The “flare”: Several groups have described the rapid entry of K/BxN serum into joints after injection (22, 23). Binstadt et al. found that this entry reflected transient paw-restricted vascular leak that was dependent upon both mast cells and neutrophils (23). We find that this acute edema can be readily assessed as transient swelling in the paws, peaking 20–30 min after i.p. injection (24) (Note 7).
Table 1.
Assessment of arthritis (clinical index)
| Score for Each Paw | Description |
|---|---|
| 0 | Normal |
| 1 | Swelling isolated to one aspect of joint (toes, dorsal surface, ventral surface, or ankle/wrist). |
| 2 | Swelling evident in two aspects of joint (toes, dorsal surface, ventral surface, or ankle/wrist). |
| 3 | Swelling evident in three or more aspects of joint (toes, dorsal surface, ventral surface, or ankle/wrist). |
Clinical index = sum of each paw score. Maximum score = 12.
Figure 2.
Measurement of paw thickness in arthritis. A. Forepaw measurement in dorsal-ventral axis. B. Left hindpaw measurement. C. Right hindpaw measurement. Note in B and C that a finger in the restraining hand is used to maintain the measured joint in a degree of flexion that must be maintained constant from measurement to measurement. The caliper must be held in each hand alternately.
3.4. ELISA for anti-GPI IgG quantitation
In some situations, it may be useful to quantify anti-GPI IgG in treated mice, for example to justify exclusion of an extreme experimental outlier. Another situation in which this is useful is in the evaluation of new murine strains, where unusual susceptibility or resistance can occasionally result from aberrant clearance of administered IgG (25).
Coat high-binding ELISA plate overnight at 4°C with 100 μl of 5 μg/mL recombinant GPI in 0.1M sodium bicarbonate buffer (pH 7.0)
Wash plate 2X with ELISA wash buffer, 5 min per wash at room temperature (RT).
Add 100 μl Super Block and incubate for 1 h at RT.
Wash plate 2X with ELISA wash buffer, 5 min per wash at RT
Add 50 μl/well of K/BxN or normal mouse serum diluted in Super Block to plates and incubate for 1 h at RT (see Note 8). Positive control, KBN serum (diluted). Negative control, normal mouse serum.
Wash plate 4X with ELISA wash buffer, 5 min per wash at RT (Note 9)
Add 50 μL per well HRP-conjugated detection antibody (1:400 in PBS). Incubate for 1 h at RT.
Wash plate 4X with ELISA wash buffer, 5 min per wash at RT.
Add 100 μl of TMB substrate per well and incubate 5 min at RT.
Read absorbance using spectrophotometer with λ=650 nm.
Part II: Histological assessment of K/BxN Serum transfer arthritis
3.5. Tissue harvest and preparation
Following euthanasia, wet the skin with 70% ethanol spray to control fur.
Make a single longitudinal incision through skin with a sharp scalpel from mid-shin to dorsal mid-foot.
Grasp edges of skin with toothed forceps and gently pull up and down to remove skin from deeper tissues (Note 10).
Use scissors to cut lower leg just distal to the knee and cut off distal foot and attached skin.
The sample can be trimmed further after fixation by the histotechnologist to fit the cassette prior to embedding.
3.5.1. Paraffin.
Paraffin sections are most generally used for standard histological analysis as well as for immunohistochemical study.
3.5.2. Frozen sections.
Frozen sections are used where antigens are unavailable for staining after paraffin or where immunofluorescence studies are desired.
Embed whole ankles in O.C.T. freezing medium (Tissue-Tek®) in specially designed plastic receptacles (e.g., Histology cryo-molds) (Note 13).
Samples are stored at −80°C in Bitran® freezer bags to avoid desiccation.
3.6. General histological assessment of joint injury.
Multiple aspects of arthritis pathophysiology can be assessed by histology using standard hematoxyln and eosin (HE) stained slides of joint synovial sections. Typically, we begin by grading HE-stained paraffin sections of ankle tissue for inflammation, bone erosion, and cartilage injury using the system described in Table 2. The importance of blinding the investigator to the sample identifications during assessment to guarantee objectivity cannot be overstated
Table 2.
Histological scoring of arthritis severity
| Score | Inflammation | Bone erosion | Cartilage damage |
|---|---|---|---|
| 0 | Normal | Normal | Normal |
| 1 | Minimal infiltration of inflammatory cells and/or mild edema | Small areas of resorption, not readily apparent on low magnification, in trabecular or cortical bone. | Synovial adherence to margins of cartilage in <3 sites |
| 2 | Mild infiltration | More numerous areas of resorption, not readily apparent on low magnification, in trabecular or cortical bone. | Synovial adherence to margins of cartilage in 3 or more sites |
| 3 | Moderate infiltration | Obvious resorption of trabecular and cortical bone, without full thickness defects in the cortex; loss of some trabeculae; lesions apparent on low magnification. | Synovial adherence to cartilage not limited to margins but no full-thickness |
| 4 | Marked infiltration | Full thickness defects in the cortical bone and marked trabecular bone loss, without distortion of the profile of the remaining cortical surface. | Full-thickness injury in <3 sites |
| 5 | Severe infiltration | Full thickness defects in the cortical bone and marked trabecular bone loss, with distortion of the profile of the remaining cortical surface. | Full-thickness injury in 3 or more sites. |
3.7. Specific stains and protocols for synovial tissue staining
3.7.1. Proteoglycan staining of paraffin embedded sections
Proteoglycans in the cartilage can be stained using Toluidine Blue or by immunohistochemical staining using an anti-aggrecan polyclonal antibody (20) (Note 14).
3.7.2. Toluidine Blue
Deparaffinize and hydrate slide to distilled water.
Expose tissue for 15 to 20 seconds to 0.1% toluidine blue solution (adjusted according to desired staining intensity)
Rinse briefly with distilled water (too long will “decolor” extensively) - this step is empiric and tissue dependent.
Immediately place in 95% ethanol followed by 3 to 4 quick changes of 100% ethanol
Allow to air dry completely
Coverslip with non-aqueous mounting medium (e.g. Cytoseal 60)
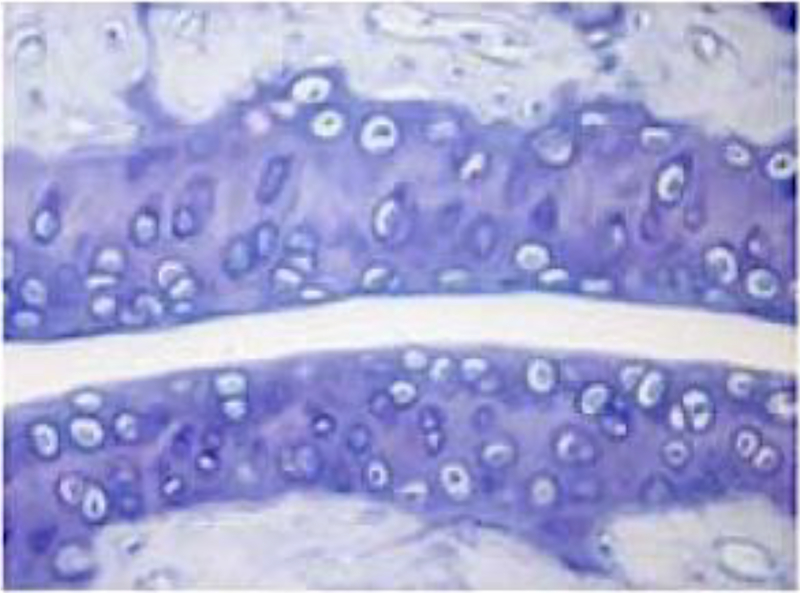
Mast cells and cartilage proteoglycan stain violet (Figures 3 & 4)
Figure 3.
Cartilage proteoglycan staining with toluidine blue. This murine tibotalar joint was sectioned and stained with toluidine blue. Proteoglycan within cartilage is stained as darker bluish-purple.
Figure 4.
Toluidine blue staining of synovial mast cells. Note purple color of mast cells compared to blue background.
3.7.3. Chloroacetate esterase (CAE) staining
Deparaffinize and hydrate slide to distilled water.
Mix 5 μL 4% sodium nitrite solution and 5 μL New Fuchsin solution: wait for ~1 min
Add 2 mL phosphate buffer (0.1 M, pH 7.6) and mix with (2). Wait for ~3 min (turns to pinkish color).
Add 100 μL naphthol AS-D chloroacetate to (3) (gives red turbid color)
Add 100–150 μL of mixture per slide and stain for 10–15 min. Wash with dH20.
Counterstain with hematoxylin for approximately 20–60s (adjust hematoxylin staining duration to desired color intensity). Wash with dH20.
Wash with lithium carbonate
Dehydrate in ethanol series: 95% ethanol x 2 changes, absolute ethanol x 2 changes, then xylene x 2 changes.
Coverslip with non-aqueous mounting medium (e.g. Cytoseal 60).
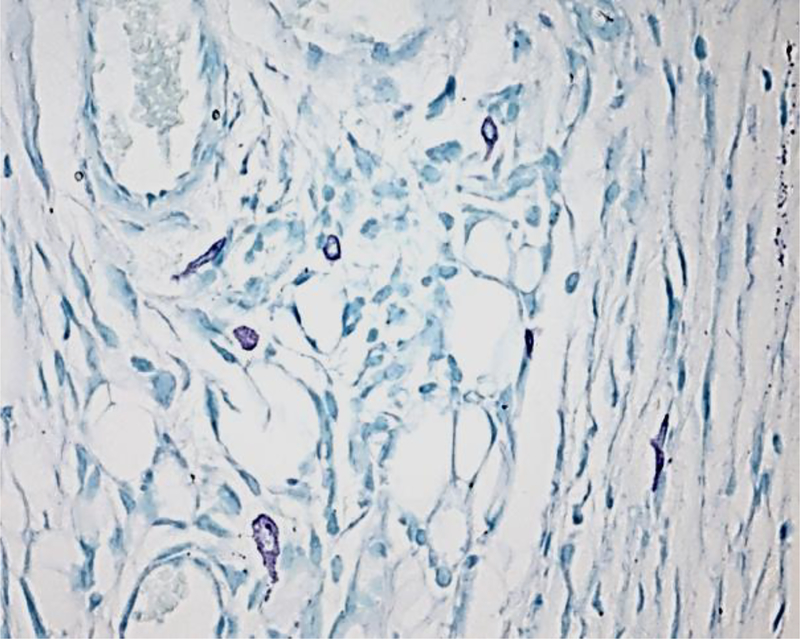
A sample CAE stain of synovial tissue is shown in Figure 5. This stain has the advantage of rendering mast cells bright and easy to identify and photograph.
Figure 5.
CAE staining of synovial mast cells
3.8.1. Immunohistochemical staining of mouse mast cell proteases or human tryptase
Deparaffinize and hydrate slide to distilled water.
- Perform antigen retrieval
- Immerse slide in preheated citrate buffer (pH 6.0) in 86°C water bath for 15 min.
- Cool to room temperature (RT) for 10 min.
- Rinse with water (RT).
- Immerse in PBS for 5 min (RT).
Incubate slide in blocking serum for 30 min, then blot excess serum (Note 15).
Incubate in 150 μL primary antibody diluted in PBS for 60 min at RT (Note 16). Wash 3X with PBS.
Incubate in 500 μL biotinylated secondary antibody for 30 min. Wash 3X with PBS.
Incubate in 500 μL VECTASTAIN ABC-AP reagent (Note 17) for 30 min. Wash 3X with PBS.
Incubate in 200 μL FASTRED until desired stain intensity develops (~15 min) and then rinse with water
Counterstain lightly with hematoxylin for 30 s. Wash 3X with water.
Dip in lithium carbonate. Wash 1X with water.
Coverslip with aqueous mounting media (e.g. Biomeda Crystal/Mount).
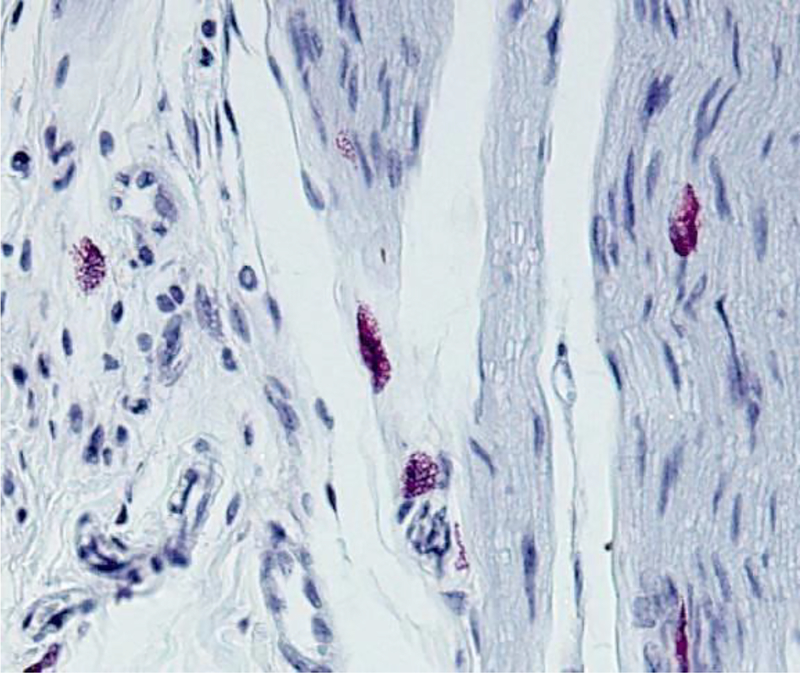
Sample of immunohistology stain of synovial tissue using primary antibody to mMCP6 is shown in Figure 6.
Figure 6.
Immunohistochemistry of murine synovial mast cells for mMCP6.
3.8.2. Immunofluorescence staining of murine mast cell proteases.
Deparaffinize and hydrate slide to distilled water.
- Perform antigen retrieval.
- Immerse slide in preheated citrate buffer (pH 6.0) in 86°C water bath for 15 min.
- Cool to room temperature (RT) for 10 min.
- Rinse with water (RT).
- Immerse in PBS for 5 min (RT).
Incubate slide in blocking serum for 30 min, then blot excess serum (Note 15).
Prepare 1 μg of primary antibody in PBS (volume < 20 μL) (Note 16 & 18).
Add 5 −10 μL (3:1 or 6:1) of Zenon® IgG labeling reagent (Component A) to antibody solution and incubate for 5 min in RT.
Add 5 μL of Zenon® blocking reagent (Component B) to mixture and incubate 5 min in RT (use within 30 min).
Add 100 μL or final mixture diluted in PBS for 60 min.
Wash twice with PBS (or wash once for 10–15 min).
Add 300 μL of 4% PFA and incubate 15 min in RT.
Wash 3 times with PBS.
Counterstain with Hoechst 1:10,000 in PBS for 10 min.
Wash once more with PBS.
Mount with Vinol mounting medium (Note 19).
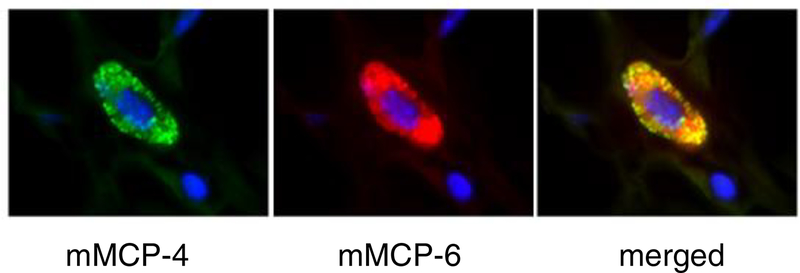
Direct conjugation of antibodies permits multicolor immunofluorescence microscopy (13) (Figure 7).
Figure 7.
Immunofluorescence staining of murine synovial mast cells for mMCP4 (green) and −6 (red). Merged figure (yellow) shows that both proteases are expressed in the synovial mast cell.
Part III: In vivo assessment of the role of mast cells in K/BxN serum transfer arthritis
3.8. Mast cell engraftment
-
Culture bone marrow derived mast cells (BMMC).
- Flush bone marrow from tibias and femurs of a donor mouse and
- Cultured in media supplemented in BMMC media for approximately 4 weeks. Change media weekly.
- After approximately 4 weeks in culture, culture conditions yield a population of 50–80 × 106 cells that is >97% cKit+FcεRI+.
By employing mast cells genetically deficient in specific molecules of interest, the role of these factors can be identified (16, 24).
- Adoptive transfer of BMMCs.
- At 4 weeks, injected 1×107 BMMC (in 200 μL volume) by tail vein into W/Wv mice
- Engraftment can be confirmed using the histological methods described in sections 3.5–3.7.
4. Notes
KBN mice are available only by permission of INSERM (Institut National de la Santé et de la Recherche Medicale).
Batch effect. K/BxN serum is a biological product and exhibits batch-to-batch variability in potency. This effect is relatively minor, such that inter-experimental comparison is usually possible. However, if a subtle effect is expected, it is helpful to generate a common serum stock for use in replicate experiments. In every case, all animals within any experiment should receive aliquots of the same serum pool.
Titrate K/BxN serum dose. The intensity of arthritis depends on the background strain (26). For C57BL/6 (B6) mice, we find that a dose of 150 μL K/BxN serum on days 0 and 2 usually elicits a maximal response. Balb/c mice are more susceptible to disease and can be rendered vigorously arthritic at a dose of 50–75 μL x 2. However, maximal arthritis is not always the desired outcome. For example, reduction in arthritis intensity due to deficiency of mouse mast cell protease 6 (mMCP6, B6 background) is seen at 50 μL but not 150 μL/dose (20). Similarly, an increase in the intensity of arthritis cannot be observed if all mice are maximally arthritic. Therefore, arthritis of mid-range intensity (mean clinical index of 6–8) is often optimal. Where such a mid-range of arthritis intensity is required, it can be helpful to prepare a serum pool sufficient for all experimental replicates and titered it to identify an optimal dose.
Serum administration regimen. K/BxN serum may be administered either by intravenous (i.v.) or intraperitoneal (i.p.) routes. For convenience we typically employ the latter. Repeat doses on days 0 and 2 are employed both to achieve maximal arthritis and to provide a safeguard in case one of the doses is inadvertently administered into a hollow viscus such as gut or bladder, which can occur as often as 1 in 5 blind lower quadrant injections. To further minimize this consequence, we commonly divide each injection (e.g., 75 μL into each lower quadrant on both days 0 and 2 for B6 mice).
Where the clinical examination is ambiguous, a change in measured paw thickness (see below) can help at assignment of a final score. Trauma to the toes is fairly common; in particular, swelling of the great toes is of limited specificity, particularly if there is evident nail injury.
Thickness should be noted at the point where the dial gauge first arrests, since continued compression will result from the pressure of the caliper spring, especially in the edematous paw. A typical adult mouse ankle measures around 3 mm and wrist about 2 mm. Inter-observation measurement consistency should be in the range of 0.1 mm. Maximum swelling is typically in the range of 0.5–1.5 mm per paw. Swelling is typically reported as change from baseline summed across all measured joints.
Flare is quantitated by clinical index, caliper measurement of paw swelling, or both. Recognizing that i.p. injections sometimes enter a hollow viscus, where serum is biologically unavailable, we monitor all animals for flare as a proxy measure for satisfactory serum delivery. Note that 1) flare occurs only after the first serum injection and 2) mice lacking mast cells or depleted of neutrophils do not flare (24, 27). Given the role of mast cells in the flare, observing this phenomenon can be informative about the status and behavior of synovial mast cells.
Where large differences in arthritis intensity become evident between test and control animals, it is important to assess serum anti-GPI IgG concentration to determine whether the effect represents accelerated IgG clearance (21, 25). Similarly, absence of detectable anti-GPI IgG is an objective basis to exclude form the analysis a mouse that failed to develop arthritis, since in some cases the serum injected i.p. ends up in a hollow viscus such as gut or bladder. The ELISA is very sensitive. Use multiple serial dilutions (e.g., 1:3 dilution steps) to ensure that the results fall within the linear range of the ELISA.
Extensive washing is required to eliminate potential inactivation of HRP in detection antibody by azide present from Super Block reagent.
Skin removal allows fixative to penetrate the tissues and removes the fur that interferes with processing.
Although a 50% Stock A + 50% Stock B mixture reduces the time necessary to adequately decalcify tissues this is not recommended.
Tissues should remain in working solution for 24 to 48 h as Kristenson’s is slower than commercial decalcification solutions. If bone samples are large, change solution after the first 24 h and continue with new solution for a full 48 h. Decalcification can be repeated or resumed even after tissue dehydration in 70% ethanol.
Be careful to avoid air bubbles and ensure that the specimen floats horizontally (long axis parallel to floor of cassette) in the freezing medium in order to permit optimal sectioning.
Aggrecan (cartilage-specific proteoglycan core protein) is the major protein constituent of proteoglycan in cartilage.
Always use a humidified staining chamber to prevent desiccation of stain onto tissue, and prepare buffers and other reagents immediately before staining.
Primary antibodies against a range of targets can be identified from the published literature (e.g., see ref. (13))
Note that an alkaline phosphatase (AP)-based system is preferable to horseradish peroxidase (HRP) owing to abundant endogenous peroxidase in mast cells. The red-colored substrate also helps to better distinguish mast cells within tissues.
Buffers can affect immunofluorescence staining intensity. While the protocols here are optimized for PBS, some antigens stain better in PHEM buffer (60 mM Pipes, 25 mM Hepes, 10 mM EGTA, 2 mM MgCl2; pH 6.9). Both should be tried when optimizing a new staining protocol.
When applying Vinol, place a small drop of the medium and press down gently without sliding the coverslip. Where feasible, such as with cytospin preparations, consider spinning cells on the coverslip rather than the slide for highest resolution, but this is not possible for tissue sections. . Blot off excess Vinol with laboratory wipes (e.g. Kimwipes) and rinse very briefly with distilled water from a squirt bottle, and immediately re-blot. Slides may be viewed immediately but should be allowed to set overnight (protected from light) at room temperature before viewing with oil immersion. Store fluorescent slides in the dark.
By this time point, mast cells are histologically visible in ankle tissue at levels of approximately 50% that observed in WBB6 controls (16). Mast cells are also abundant in spleen, though splenectomy experiments suggest that these cells are not relevant for the arthritis phenotype (16). Studies in our hands using CD45.1 congenic donors have not identified co-engraftment of hematopoietic lineages aside from mast cells, as determined by flow cytometry of circulating blood, spleen or marrow (PAN, unpublished data). Similar purity of engraftment has been recently found for BMMC transfer into Pretty2 mice (15). Thus, phenotypic changes observed after mast cell transfer can, to the best of our knowledge, justifiably be attributed to the engrafting mast cells. However, limitations include failure to achieve normal numbers of mast cells in joint tissues and, potentially, differences in distribution and effector phenotype compared with endogenous mast cells, though in general engrafted mast cells are believed to assume the phenotype dictated by their new environment (28).
While W/Wv mice will “accept” mast cells from many B6-derived donor strains, some fail to engraft, perhaps because of rejection of non-B6 elements. Therefore, the success of engraftment must be determined in each experiment, for example by tissue staining of ankle tissue with toluidine blue. Of note, we have been unsuccessful at achieving engraftment of B6 mast cells into ankle tissues of Wsh mice, despite the appearance of mast cells in recipient spleens (PAN, unpublished data).
Acknowledgements
This work was funded in part through the support of the Cogan Family Foundation (to PAN). We are grateful to Dr. Altan Ercan for the K/BxN IgG ELISA protocol, to Ms. Theresa Bowman for histotechnical guidance, and to Dr. Nancy Kedersha for the immunofluorescence mounting medium protocols.
Contributor Information
Peter A. Nigrovic, Harvard Medical School, Division of Rheumatology, Immunology and Allergy, Brigham and Women’s Hospital, Division of Immunology, Boston Children’s Hospital, Smith 516B, One Jimmy Fund Way, Boston MA 02115, USA.
Kichul Shin, Division of Rheumatology, SMG-SNU Borame Medical Center, 20 Boramae-ro-5-gil, Dongjak-gu, Seoul, Korea 156-707.
5. References
- 1.Castor W. The microscopic structure of normal human synovial tissue. Arthritis Rheum 1960;3:140–151. [DOI] [PubMed] [Google Scholar]
- 2.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev 2007;217:19–37. [DOI] [PubMed] [Google Scholar]
- 3.Crisp AJ, Chapman CM, Kirkham SE, Schiller AL, Krane SM. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum 1984;27(8):845–51. [DOI] [PubMed] [Google Scholar]
- 4.Monach PA, Benoist C, Mathis D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv Immunol 2004;82:217–48. [DOI] [PubMed] [Google Scholar]
- 5.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell 1996;87(5):811–22. [DOI] [PubMed] [Google Scholar]
- 6.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity 1999;10(4):451–61. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 1999;286(5445):1732–5. [DOI] [PubMed] [Google Scholar]
- 8.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature 1980;283(5748):666–8. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 2003;426(6965):454–60. [DOI] [PubMed] [Google Scholar]
- 10.Maccioni M, Zeder-Lutz G, Huang H, Ebel C, Gerber P, Hergueux J, et al. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med 2002;195(8):1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigrovic PA, Lee DM. Immune complexes and innate immunity in rheumatoid arthritis In: Firestein GS, Panayi GS, Wollheim FA, editors. Rheumatoid Arthritis: new frontiers in pathogenesis and treatment. 2nd ed. Oxford: Oxford University Press; 2006. p. 135–156. [Google Scholar]
- 12.Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, Brenner M, et al. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol 2002;3(4):360–5. [DOI] [PubMed] [Google Scholar]
- 13.Shin K, Gurish MF, Friend DS, Pemberton AD, Thornton EM, Miller HR, et al. Lymphocyte-independent connective tissue mast cells populate murine synovium. Arthritis Rheum 2006;54(9):2863–71. [DOI] [PubMed] [Google Scholar]
- 14.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 2002;297(5587):1689–92. [DOI] [PubMed] [Google Scholar]
- 15.Guma M, Kashiwakura J, Crain B, Kawakami Y, Beutler B, Firestein GS, et al. JNK1 controls mast cell degranulation and IL-1{beta} production in inflammatory arthritis. Proc Natl Acad Sci U S A 2010;107(51):22122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci U S A 2007;104(7):2325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J Exp Med 2007;204(12):2797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, et al. Cre-Mediated Cell Ablation Contests Mast Cell Contribution in Models of Antibody- and T Cell-Mediated Autoimmunity. Immunity 2011. [DOI] [PubMed] [Google Scholar]
- 19.Katz HR, Austen KF. Mast cell deficiency, a game of kit and mouse. Immunity 2011;35(5):668–70. [DOI] [PubMed] [Google Scholar]
- 20.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, et al. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol 2009;182(1):647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adachi R, Krilis SA, Nigrovic PA, Hamilton MJ, Chung K, Thakurdas SM, et al. Ras guanine nucleotide-releasing protein-4 (RasGRP4) involvement in experimental arthritis and colitis. J Biol Chem 2012;287(24):20047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wipke BT, Wang Z, Kim J, McCarthy TJ, Allen PM. Dynamic visualization of a joint-specific autoimmune response through positron emission tomography. Nat Immunol 2002;3(4):366–72. [DOI] [PubMed] [Google Scholar]
- 23.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol 2006;7(3):284–92. [DOI] [PubMed] [Google Scholar]
- 24.Nigrovic PA, Malbec O, Lu B, Markiewski MM, Kepley C, Gerard N, et al. C5a receptor enables participation of mast cells in immune complex arthritis independently of Fcgamma receptor modulation. Arthritis Rheum 2010;62(11):3322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akilesh S, Petkova S, Sproule TJ, Shaffer DJ, Christianson GJ, Roopenian D. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest 2004;113(9):1328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohmura K, Johnsen A, Ortiz-Lopez A, Desany P, Roy M, Besse W, et al. Variation in IL-1{beta} gene expression is a major determinant of genetic differences in arthritis aggressivity in mice. Proc Natl Acad Sci U S A 2005;102(35):12489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J-X, King S, Bair A, Shnayder R, Hsieh Y-F, Shieh C-C, et al. Ly6G ligation blocks recruitment of neutrophils via a beta 2 integrin-dependent mechanism <in revision>. [DOI] [PMC free article] [PubMed]
- 28.Gurish MF, Pear WS, Stevens RL, Scott ML, Sokol K, Ghildyal N, et al. Tissue-regulated differentiation and maturation of a v-abl-immortalized mast cell-committed progenitor. Immunity 1995;3(2):175–86. [DOI] [PubMed] [Google Scholar]
- 29.Chen M et al. (2006) Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med 203:837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pettit AR et al. (2001) TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol 159:1689–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]